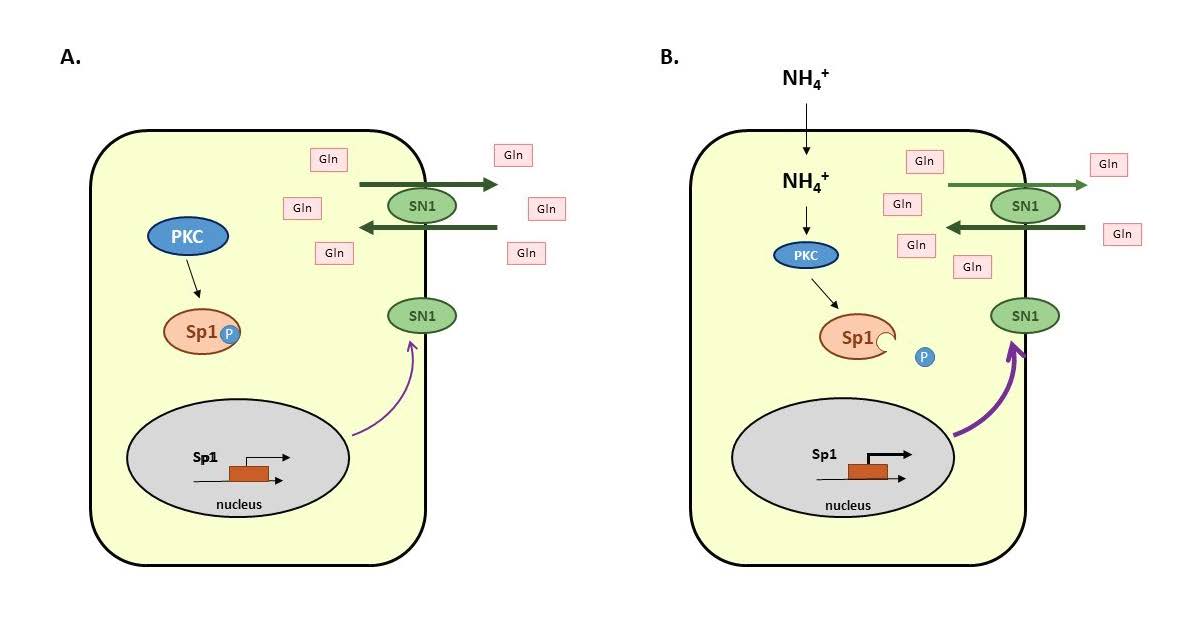
The involvement of astrocytic SN1 (SNAT3) transporter in ammonia-induced L-glutamine retention was recently documented in mouse cultured astrocytes. Here we investigated the involvement of specificity protein 1 (Sp1) transcription factor in SN1 regulation in ammonium chloride (“ammonia”)-treated astrocytes. Sp1 expression and its cellular localization were determined using real-time qPCR, Western blot and confocal microscopy, respectively. Sp1 binding to Snat3 promoter was analyzed by chromatin immunoprecipitation. Ammonia-induced Sp1 regulatory role in SN1-mediated [3H]glutamine transport was verified using siRNA and mithramycin A. The involvement of protein kinase C (PKC) isoforms in Sp1 level/phosphorylation status was verified using siRNA technology. Sp1 translocation to the nuclei and its enhanced binding to Snat3 promoter, along with Sp1 dependence of system N-mediated [3H]glutamine transport were observed in astrocytes upon ammonia exposure. Ammonia decreased the level of phosphorylated Sp1, and the effect was reinforced by long-term incubation with PKC modulator, phorbol 12-myristate 13-acetate, a treatment likely to dephosphorylate Sp1. Furthermore, silencing of PKCδ isoform abolished the increase of Sp1 level by ammonia. Collectively, the results demonstrate the regulatory role of Sp1 in regulation of SN1 expression and activity in ammonia-treated astrocytes and implicate altered Sp1 phosphorylation status in this capacity.