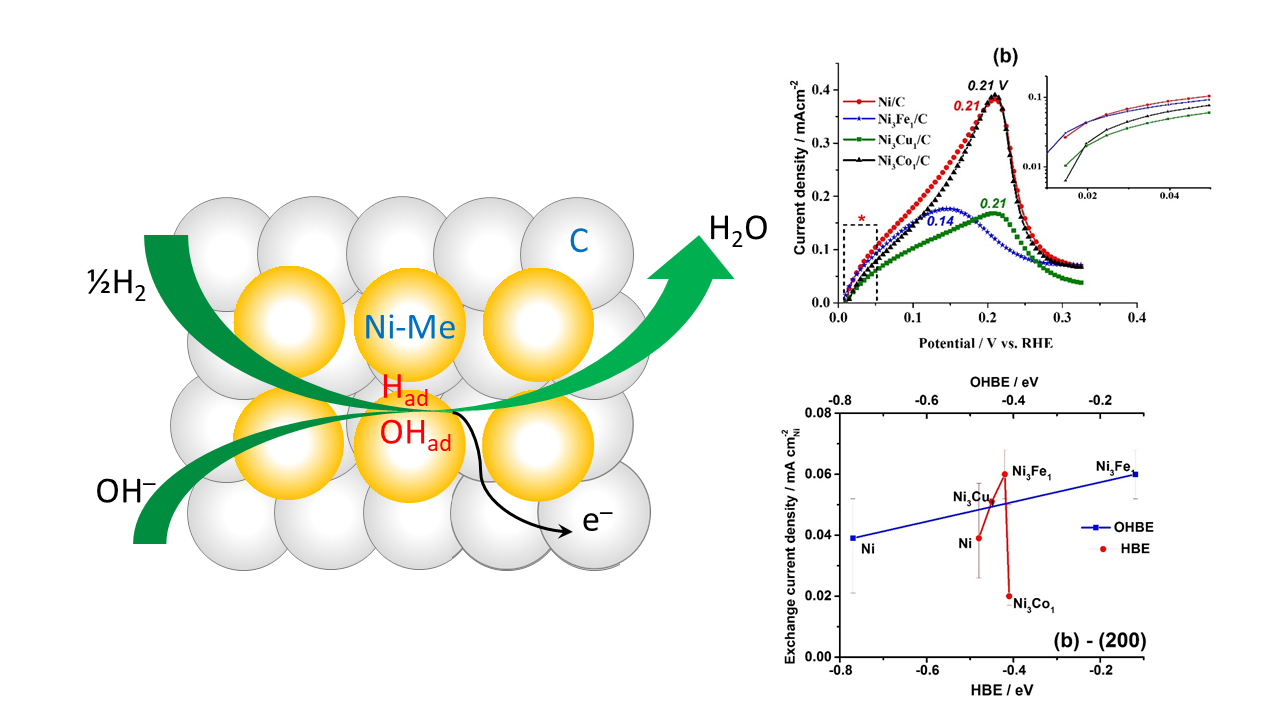
Carbon supported nanoparticles of monometallic Ni catalyst and binary Ni-Transition Metal (Ni-TM/C) electrocatalytic composites were synthesized via chemical reduction method, where TM stands for the doping elements Fe, Co, and Cu. The chemical composition, structure and morphology of the Ni-TM/C materials were characterized by X-ray photoelectron spectroscopy (XPS), X-ray diffractometry (XRD), transmission electron microscopy (TEM), scanning transmission electron microscopy (STEM) and energy-dispersive X-ray spectroscopy (EDS). The electrochemical properties towards hydrogen oxidation reaction in alkaline medium were studied using the rotating disc electrode and cycling voltammetry methods. A significant role of the TM dopant in the promotion of the hydrogen electrooxidation kinetics of the binary Ni-TM/C materials were revealed. A record-high in exchange current density value of 0.060 mA cm2Ni was measured for Ni3Fe1/C, whereas the monometallic Ni/C counterpart has only shown 0.039 mA cm2Ni. In order to predict the feasibility of the electrocatalysts for hydrogen chemisorption, density functional theory was applied to calculate the hydrogen binding energy and hydroxide binding energy values for bare Ni and Ni3TM1.