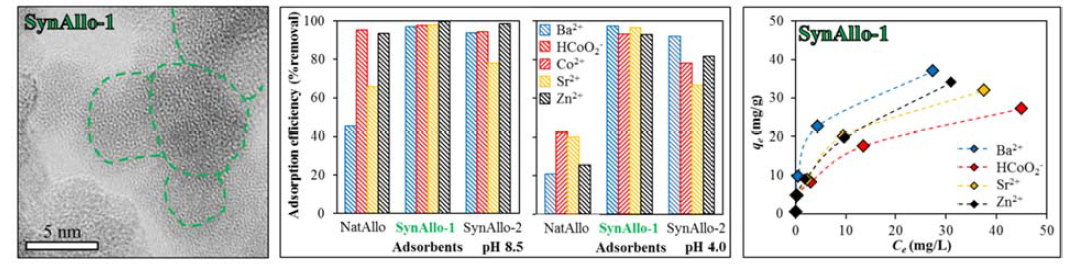
The capacity and the mechanism of the adsorption of aqueous barium (Ba), cobalt (Co), strontium (Sr) and zinc (Zn) by Ecuadorian (NatAllo) and synthetic (SynAllo-1 and SynAllo-2) allophanes were studied as a function of contact time, pH and metal ion concentration using kinetic and equilibrium experiments. The mineralogy, nano-structure and chemical composition of the allophanes were characterized by X-ray diffraction, Fourier transform infrared spectroscopy, transmission electron microscopy and specific surface area analyses. The evolution of adsorption fitted to a pseudo-first-order reaction kinetics, where equilibrium between aqueous metal ions and allophane was reached within < 10 min. The metal ion removal efficiencies varied from 0.7 to 99.7 % at pH 4.0 to 8.5. At equilibrium, the adsorption behavior is better described by the Langmuir model than by the Dubinin-Radushkevich model, yielding sorption capacities of 10.6, 17.2 and 38.6 mg/g for Ba^(2+), 12.4, 19.3 and 29.0 mg/g for HCoO_2^-, 7.2, 15.9 and 34.4 mg/g for Sr^(2+) and 20.9, 26.9 and 36.9 mg/g for Zn^(2+), respectively, by NatAllo, SynAllo-2 and SynAllo-1. The uptake mechanism is based on a physical adsorption process. Allophane holds great potential to remove aqueous metal ions and could be used instead of zeolites, montmorillonite, carbonates and phosphates for wastewater treatment.