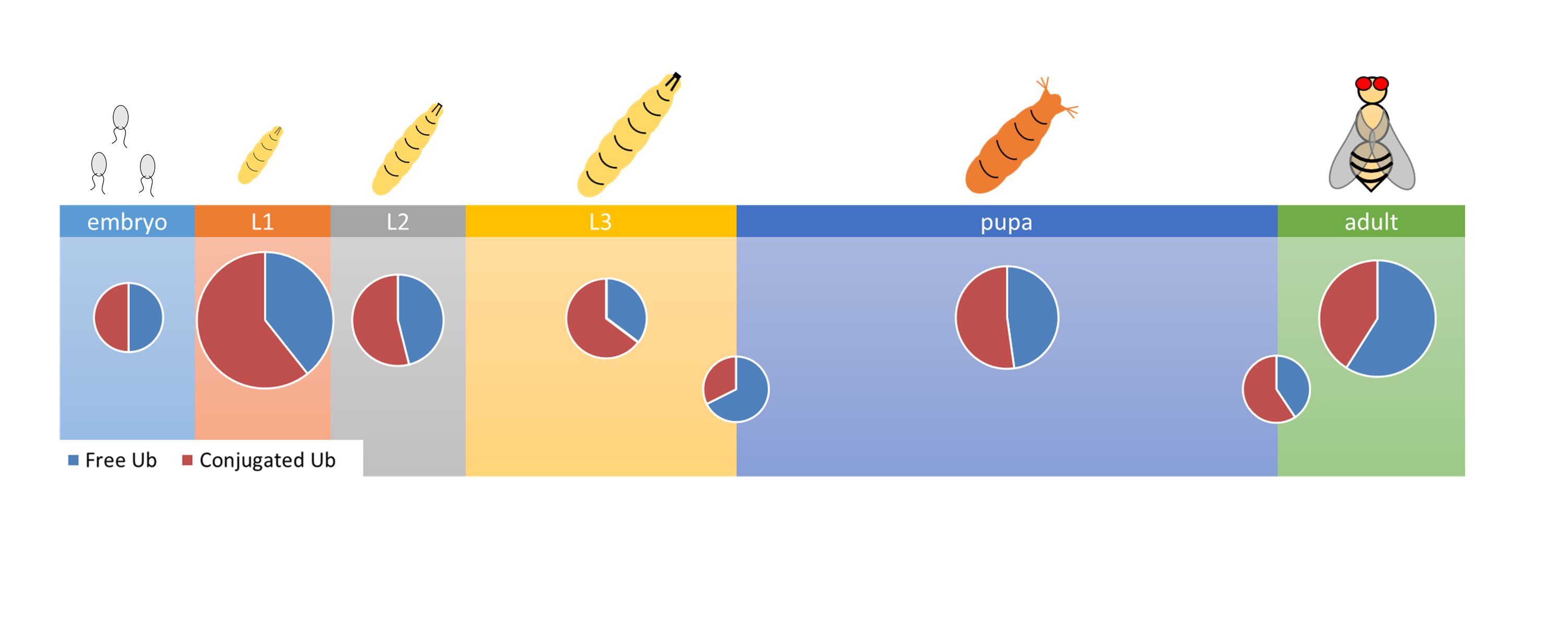
In most Eukaryotes, ubiquitin either exists as free monoubiquitin or as a molecule that is covalently linked to other proteins. These two forms cycle between each other and due to the concerted antagonistic activity of ubiquitylating and deubiquitylating enzymes, an intracellular ubiquitin equilibrium is maintained that is essential for normal biological function. However, measuring the level and ratio of these forms of ubiquitin has been difficult and time consuming. In this paper, we have adapted a simple immunoblotting technique to monitor ubiquitin content and equilibrium dynamics in different developmental stages and tissues of Drosophila. Our data show that the level of total ubiquitin is distinct in different developmental stages, lowest at the larval-pupal transition and in three days old adult males, and highest in first instar larvae. Interestingly, the ratio of free mono-ubiquitin remains within 30-50% range of the total throughout larval development, but peaks to 70-80% at the larval-pupal and the pupal-adult transitions. It stays within the 70-80% range in adults. In developmentally and physiologically active tissues, the ratio of free ubiquitin is similarly high, most likely reflecting a high demand for ubiquitin availability. We also used this method to demonstrate the disruption of the finely tuned ubiquitin equilibrium by the abolition of proteasome function or the housekeeping deubiquitylase, Usp5. Our data support the notion that the ubiquitin equilibrium is regulated by tissue- and developmental stage-specific mechanisms.