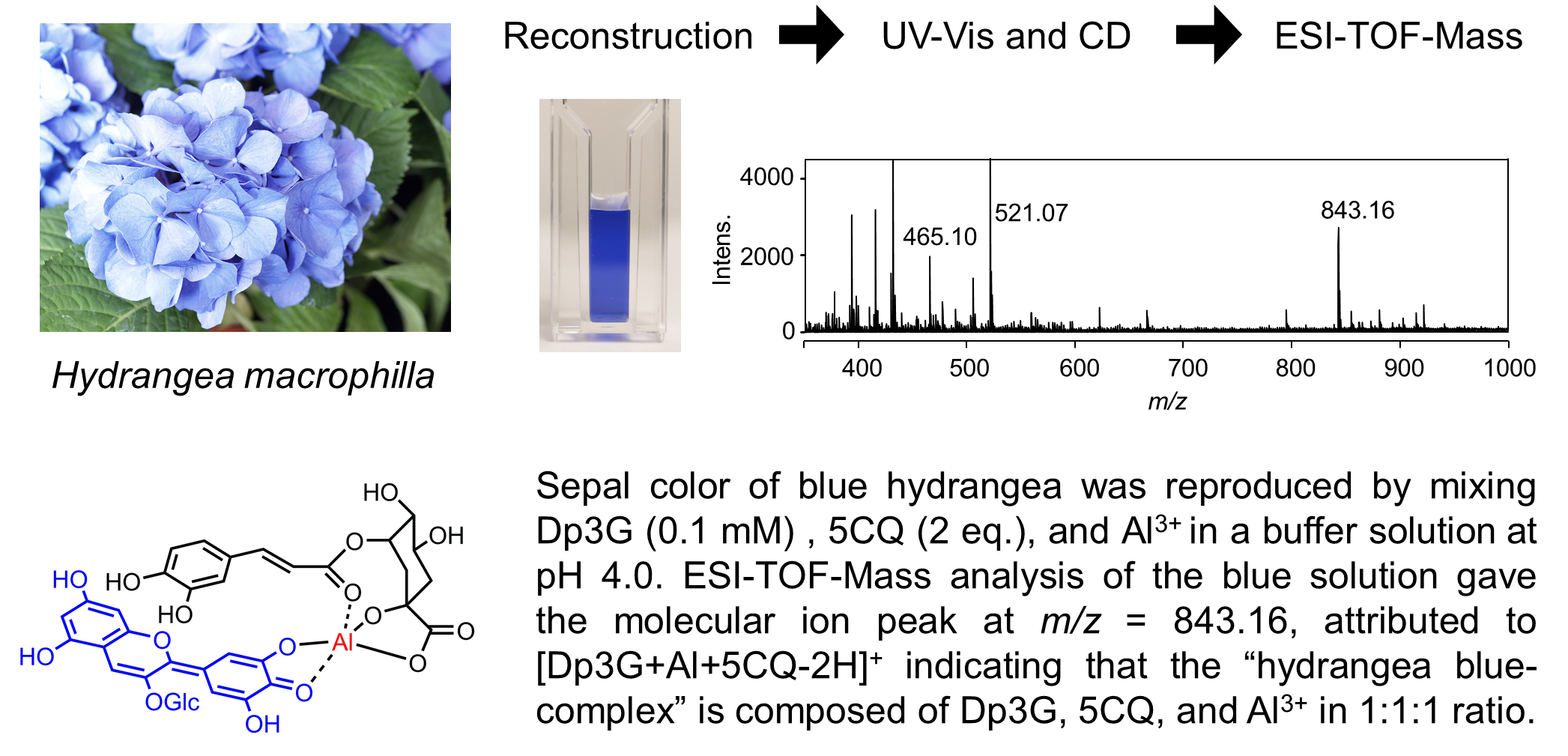
The blue sepal color of hydrangea is due to a metal complex anthocyanin composed of 3-O-glucosyldelphinidin (1) and an aluminum ion with the co-pigments 5-O-caffeoylquinic acid (2) and/or 5-O-p-coumaroylquinic acid (3). The three components, namely, anthocyanin, Al3+ and 5-O-acylquinic acids, are essential for blue color development, but the complex is unstable and only exists in aqueous solution. Furthermore, the complex did not give analyzable NMR spectra or crystals. Therefore, many trials to determine the detailed chemical structure of the hydrangea-blue complex have failed to date. Instead, via experiments mixing 1, Al3+ and 2 or 3 in a buffered solution at pH 4.0, we obtained the same blue solution derived from the sepals. However, the ratio was not stoichiometric but fluctuated. To determine the composition of the complex, we tried direct observation of the molecular ion of the complex using electrospray-ionization mass spectrometry. In a very low-concentration buffer solution (2.0 mM) at pH 4.0, we reproduced the hydrangea-blue color by mixing 1, 2 and Al3+ in ratios of 1:1:1, 1:2:1 and 1:3:1. All solution gave the same molecular ion peak at m/z = 843, indicating that the blue solution has a ratio of 1:1:1 for the complex. By using 3, the observed mass number was m/z = 827 and the ratio of 1, 3 and Al3+ was also 1:1:1. A mixture of 1, 3-O-caffeoylquinic acid (4) and Al3+ did not give any blue color but instead was purple, and the intensity of the molecular ion peak at m/z = 843 was very low. These results strongly indicate that the hydrangea blue-complex is composed of a ratio of 1:1:1 for 1, Al3+ and 2 or 3.