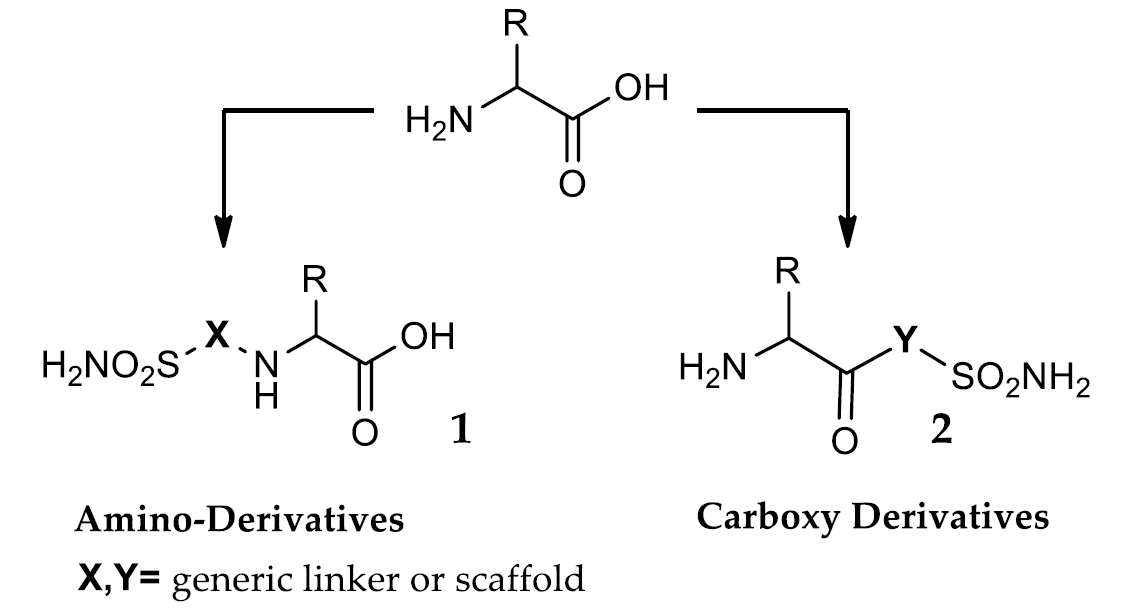
Carbonic Anhydrases (CAs) are a superfamily of metalloenzymes widespread in all life kingdoms, classified into seven genetically different families (α-θ). These enzymes catalyse the reversible hydration of carbonic anhydride (CO2), generating bicarbonate (HCO3-) and protons (H+). Fifteen isoforms of human CA (hCA I-XV) have been isolated, their presence being fundamental for the regulation of many physiological processes. In addition, overexpression of some isoforms has been associated with the outbreak or the progression of several diseases. For this reason, for a long time CA inhibitors (CAIs) are used in the control of glaucoma and as diuretics. Furthermore, the search for new potential CAIs for other pharmacological applications is a very active field. Amino acids constitute the smallest fundamental monomers of protein and, due to their useful bivalent chemical properties, are widely used in organic chemistry. Both proteinogenic and non-proteinogenic amino acids have been extensively used to synthesize CAIs. This article provides an overview of the different strategies that have been used to design new CAIs containing amino acids, and how these bivalent molecules influence the properties of the inhibitors.