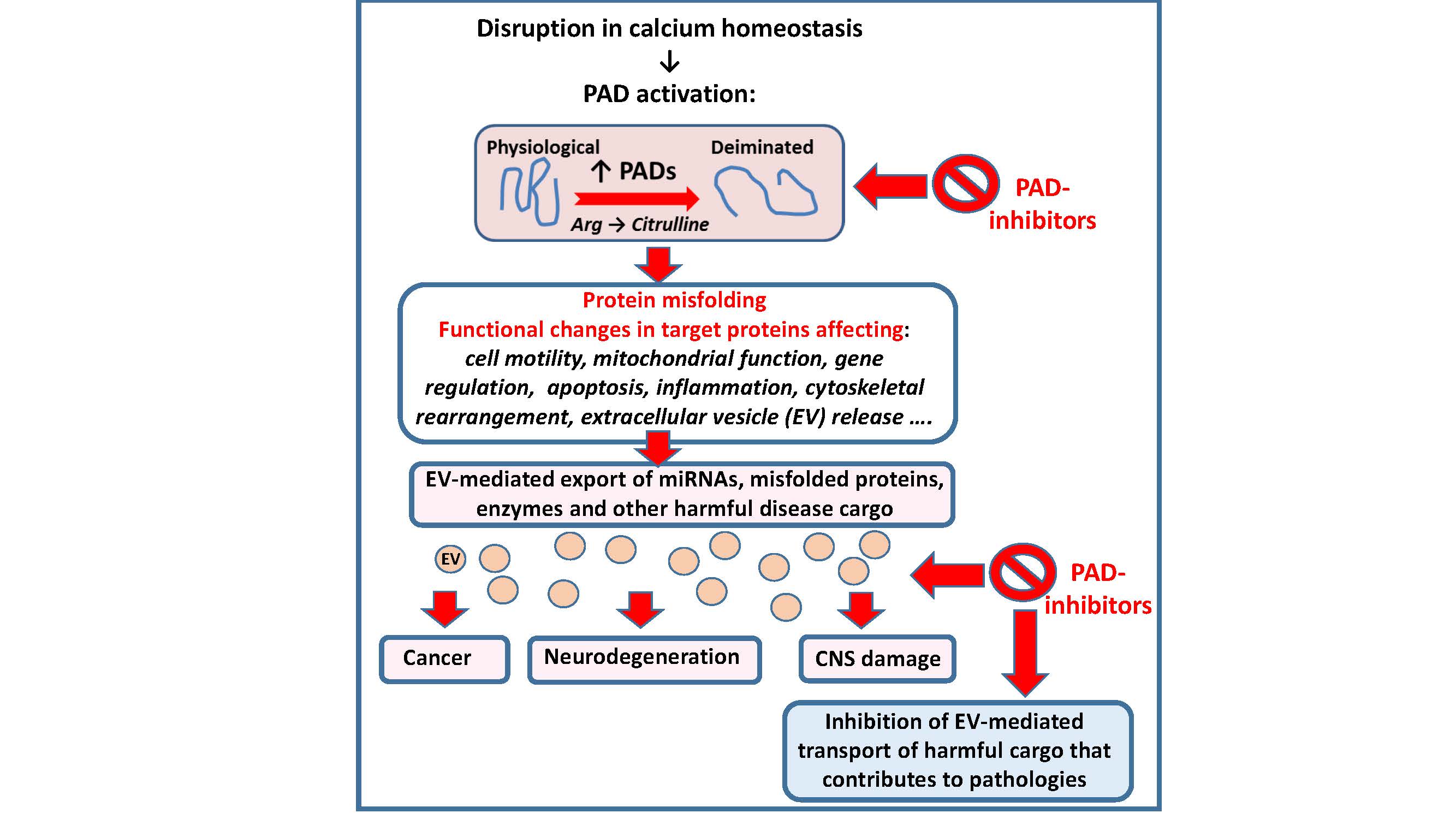
Extracellular vesicle (EV) release, which occurs in most eukaryotic cells, has recently been associated with peptidylarginine deiminase (PAD)-driven protein deimination. Evidence points to the involvement of deiminated cytoskeletal proteins and changes in histone deimination. Both PADs and EVs are associated with various pathologies including cancers, autoimmune and neurodegenerative diseases. The elevated PAD expression observed in cancers may contribute to increase in EV shedding observed from cancer cells, contributing to cancer progression. Similarly, elevated PAD expression observed in neurodegenerative diseases may cause increased EV shedding and spread of neurodegenerative EV cargo, contributing to disease progression and pathologies. Pharmacological inhibition of PAD-mediated deimination using pan-PAD inhibitor Cl-amidine, reduced cellular EV release in prostate cancer cells, rendering them significantly more susceptible to chemotherapeutic drugs. Studies on models of central nervous system damage have demonstrated critical functional roles for PADs and neuroprotective effects using PAD inhibitors in vivo, while human neurodegenerative iPSC in vitro models showed evidence of increased protein deimination. Besides using refined PAD inhibitors to selectively manipulate EV biogenesis for novel combination therapies in cancer treatment, we also speculate how EV biogenesis could be targeted via the newly identified PAD-pathway to ameliorate neurodegenerative disease progression.