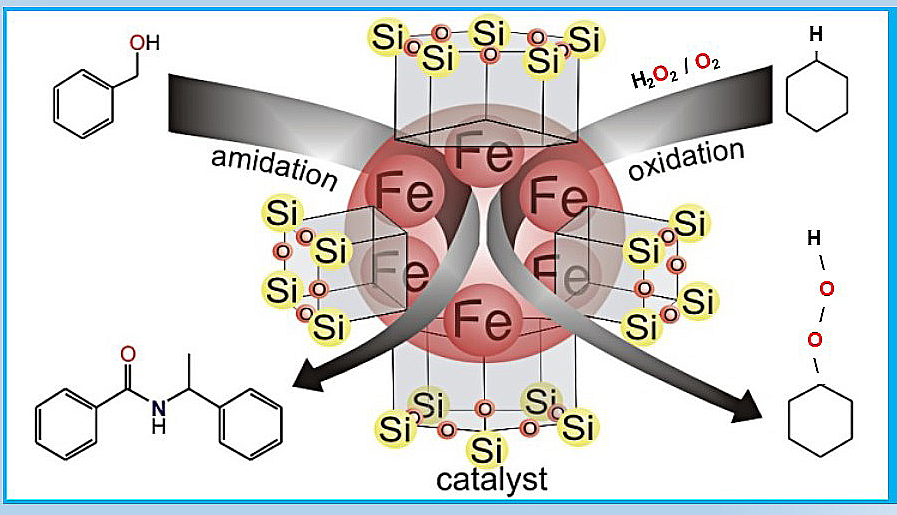
Two types of heterometallic (Fe(III),Na) silsesquioxanes [Ph5Si5O10]2[Ph10Si10O21]Fe6(O2‒)2Na7(H3O+)(MeOH)2(MeCN)4.5.1.25(MeCN), I, and [Ph5Si5O10]2[Ph4Si4O8]2Fe6Na6(O2‒)3(MeCN)8.5(H2O)8.44, II, were obtained and characterized. X-Ray studies established distinctive structures of both products, with pair of Fe(III)-O-based triangles surrounded by siloxanolate ligands, giving fascinating cage architectures. Complex II proved to be catalytically active in the formation of amides from alcohols and amines, thus becoming a rare example of metallasilsesquioxanes performing homogeneous catalysis. Benzene, cyclohexane and other alkanes, as well as alcohols, can be oxidized in acetonitrile solution to phenol, the corresponding alkyl hydroperoxides and ketones, respectively, by hydrogen peroxide in air in the presence of catalytic amounts of complex II and trifluoroacetic acid. Thus, the cyclohexane oxidation at 20 °C gave oxygenates in very high for alkanes yield (48% based on alkane). The kinetic behaviour of the system indicates that the mechanism includes the formation of hydroxyl radicals generated from hydrogen peroxide in its interaction with diiron species. The latter are formed via monomerization of starting hexairon complex with further dimerization of the monomers.