Introduction
Emergence of AICs
Anomalous intravascular casts (AICs) are elongated, elastic intravascular structures increasingly reported by embalmers and clinicians since early 2021. (Hirschman, 2022; Haviland, 2023) These casts differ from conventional post-mortem or antemortem thrombi in both gross morphology and mechanical behavior, often exhibiting rubber-like elasticity and resistance to fragmentation and are now frequently being reported by embalmers worldwide. (Haviland, 2024).
Initial morphological, histological, and elemental investigations suggested that AICs represent a discrete clot phenotype rather than a variant of ordinary coagulum. These findings necessitate molecular-level characterization to determine whether their protein architecture aligns with or departs from classic fibrin biology.
Morphological and histological analyses established the gross form, internal lamination, and fiber-rich nature of these structures, while elemental analysis defined their bulk biochemical composition. However, neither approach could resolve the internal molecular organization of the matrix itself. Elemental signatures cannot distinguish between intact proteins, fragmented domains, or altered stoichiometries
1, and morphology alone cannot determine whether atypical fibrin architecture is preserved at the subunit level. Proteomic analysis was therefore required to determine whether the observed material represents ordinary fibrin in an unusual physical state, or a fundamentally altered protein assembly with non-physiological chain composition and impaired regulatory machinery. There is a need for molecular characterization.
Why Proteomics?
Proteomic analysis provides a powerful means to resolve fibrin composition, chain stoichiometry, and incorporation of fibrinolytic machinery.
In fibrin-based materials, mechanical behavior and biological persistence are governed not only by protein identity but by subunit stoichiometry and network architecture. Alterations in the relative abundance of fibrinogen chains can profoundly affect fiber thickness, packing density, extensibility, and susceptibility to enzymatic degradation, even when the same nominal proteins are present. Likewise, the incorporation or absence of key regulatory components such as plasminogen determines whether a clot is transient and self-limiting or structurally persistent. Proteomic analysis therefore provides information that cannot be inferred from morphology or elemental composition alone, distinguishing between the mere presence of fibrin-related proteins and a fundamentally altered molecular assembly.
Here, we apply HPLC–MS/MS profiling to characterise the protein composition of anomalous intravascular casts (AICs) and assess whether their molecular architecture can account for their unusual persistence and mechanical properties.
Materials and Methods
Sample Collection and Blinding
AIC specimens were obtained from post-mortem waste streams under conditions of laboratory blinding and anonymization. Samples were processed independently across internationally distributed laboratories using harmonized protocols.
Protein Extraction and Digestion
Proteins were extracted using standard lysis buffers, enzymatically digested with trypsin, and analyzed by liquid chromatography–tandem mass spectrometry (HPLC–MS/MS). Peptide identification was performed using established false discovery rate thresholds. Relative protein abundance was calculated from normalized spectral intensities.
LC-MS/MS Instrumentation
Peptide mixtures were analyzed by high-resolution liquid chromatography–tandem mass spectrometry using a hybrid high-accuracy mass spectrometer operated in data-dependent acquisition mode. Peptides were separated by reverse-phase chromatography using a linear gradient over tens of minutes prior to MS/MS fragmentation. Instrument settings and acquisition parameters were selected to optimize peptide identification depth while maintaining stringent mass accuracy and reproducibility across samples.
Results
Protein Identification Summary
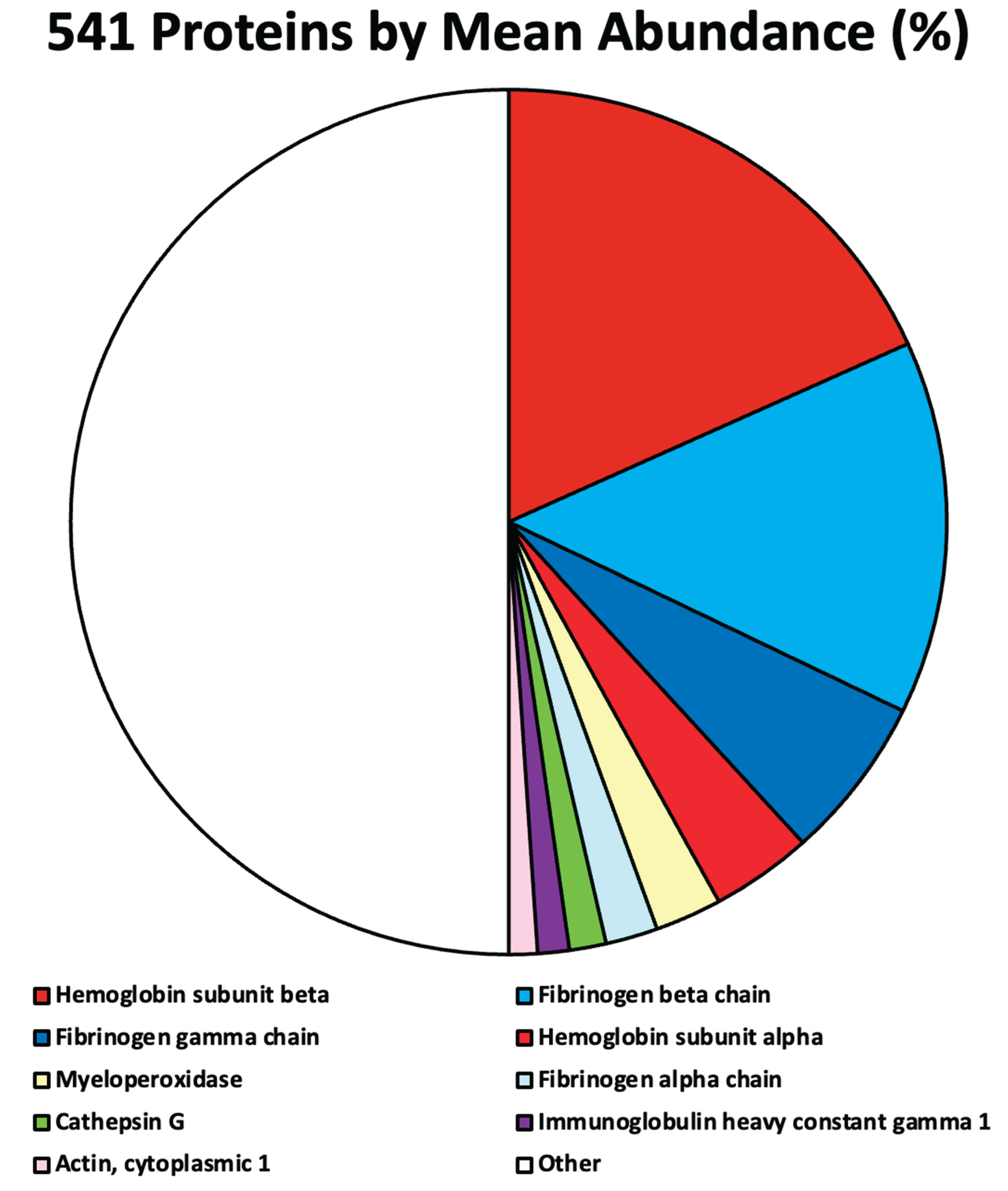
HPLC–MS/MS analysis identified a total of 541 human proteins across four independently processed AIC samples.
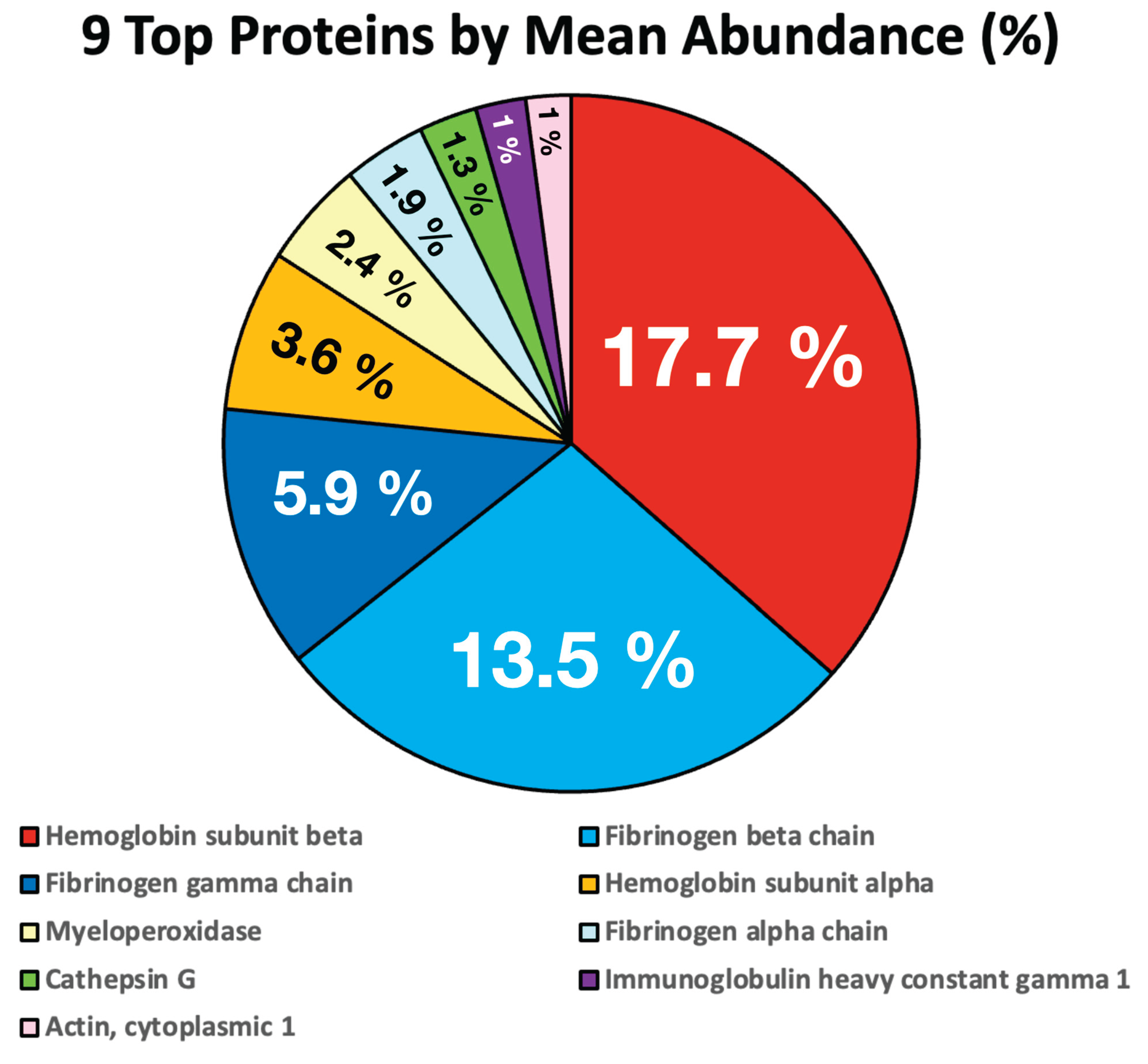
Protein abundance distributions were highly skewed, with a small subset accounting for a substantial fraction of total signal intensity. The values identifying the top 9 proteins are shown in
Table 1 and
Figure 1. All other 532 identified proteins were each present below the 1% threshold and together accounted for approximately 50% of the clot proteome, consistent with non-structural association.
If we ignore the 532 minor proteins that are all present at levels well below 1%, it is easier to visualize the main proteins of interest that make up the AIC
Figure 2.
Fibrinogen Chain Imbalance
Fibrinogen chains were detected in markedly non-physiological proportions in relation to typical fibrin clots. The observed mean α:β:γ ratio of approximately 1:7:3 contrasts sharply with the near-equimolar ratios expected in classical fibrin. This imbalance was consistent across samples.
Structural Implications
The fibrinogen α chain contributes domains essential for lateral aggregation and extensibility. Its depletion implies formation of thinner, densely packed fibrin fibers with reduced compliance, consistent with the observed mechanical rigidity of AICs.
Fibrinolytic Parameters
Plasminogen was detected at only 0.1283% of total protein signal. While there is no recognised standard percentage concentration of plasminogen in typical fibrinclots, it is generally considered to be in line with other proteins that fall in the <0.5% range. This profound deficiency indicates severely impaired intrinsic fibrinolytic capacity.
Summary of Proteomic Phenotype
Together, these findings define a reproducible proteomic signature characterized by fibrin-family dominance, severe fibrinogen chain imbalance, and marked plasminogen depletion.
Integrative Interpretation of Proteomic and Elements Findings
The proteomic phenotype observed in AICs departs fundamentally from traditional fibrin biology.
In typical thrombus formation, fibrin-based clots are transient structures that incorporate both architectural balance and the enzymatic machinery required for timely resolution (lysis). By contrast, the anomalous intravascular casts examined here exhibit a proteomic composition consistent with persistence rather than self-limitation, reflecting both distorted fibrin chain stoichiometry and depletion of fibrinolytic components. This contrast situates the present findings within the broader biological distinction between regulated hemostatic repair and structurally aberrant clot formation.
While fibrin-family proteins dominate the proteomic signal, their internal stoichiometry is severely distorted, and the machinery required for fibrinolysis is largely absent.
The depletion of fibrinogen α-chain material predicts altered fibrin fiber architecture, while plasminogen deficiency provides a parsimonious explanation for clot persistence. Elemental analyses indicating phosphorus enrichment and sulfur depletion further suggest that AICs represent hybrid organic–inorganic matrices rather than purely proteinaceous clots.
Importantly, the proteomic and elemental findings place reciprocal constraints on interpretation. Proteomics establishes the presence and relative organization of fibrin-family proteins, while elemental analysis limits the fraction of bulk mass that can plausibly be accounted for by protein alone. Neither dataset, considered in isolation, is sufficient to define the material properties of these structures; taken together, however, they converge on a coherent description of a fibrin-bearing matrix diluted and mechanically modified by non-protein components. This convergence represents an internal consistency between independent analytical modalities rather than an inferential extension beyond the data.
Non-covalent association of phosphate species, including platelet-derived polyphosphate and calcium–phosphate microdomains, offers a coherent mechanistic model compatible with established fibrin chemistry.
This overall model is advanced as a constrained explanatory framework rather than a definitive causal claim, proposed because it satisfies the combined morphological, elemental, and proteomic constraints observed in these specimens without invoking novel or unsupported biochemical mechanisms.
Importantly, these interpretations yield falsifiable predictions that can be tested using phosphate speciation, chelation, and cross-link–aware proteomics.
A complete list of all 541 identified human proteins, together with their relative abundances, is provided in
Supplementary Table S1.
Discussion: Atypical Fibrin Architecture, Impaired Fibrinolysis, and the Pathological Consequences of Persistent Intravascular Structures
Non-Physiological Fibrinogen Stoichiometry and Structural Consequences
The present proteomic analysis demonstrates that anomalous intravascular casts (AICs) are constructed from a markedly non-physiological fibrinogen composition. The observed mean α:β:γ chain ratio of approximately 1:7:3 contrasts sharply with the near-equimolar (1:1:1) stoichiometry characteristic of conventional fibrin clots. This ratio-metric imbalance was consistent across samples, indicating that it reflects an intrinsic feature of AIC formation rather than stochastic variation.
The fibrinogen α chain contributes domains essential for lateral aggregation, fibre extensibility, and normal clot compliance. Its relative depletion implies the formation of thinner, more densely packed fibrin fibres with reduced elasticity. Such an architecture is consistent with the pronounced mechanical rigidity observed in AICs and suggests that these structures have enhanced persistence and do not serve a normal physiological haemostatic function.
Protein Composition Reveals a Restricted, Structured, and Inflammatory Matrix
Beyond fibrinogen, AICs exhibit a restricted, yet highly structured, proteomic signature. The dominant presence of hemoglobin subunit beta, together with hemoglobin subunit alpha, indicates substantial incorporation of damaged erythrocyte-derived material. Although hemoglobin is not a structural clot protein, its abundance implies close spatial association between red blood cells and the fibrin matrix, consistent with formation under conditions of active blood flow rather than passive post-mortem aggregation. In addition, hemoglobin can participate in redox chemistry, potentially contributing to oxidative modification or cross-linking of matrix proteins.
The prominence of fibrinogen β and γ chains further reinforces fibrinogen as the core structural scaffold of AICs, while their relative dominance over the α chain supports the conclusion that the resulting fibrin architecture is mechanically robust yet atypical. This proteomic pattern aligns with prior morphological and histological observations indicating that AICs differ fundamentally from conventional thrombi.
Inflammatory and Immunothrombotic Contributions
The detection of myeloperoxidase (MPO) and cathepsin G implicates neutrophil involvement during AIC formation. MPO is a potent mediator of oxidative stress, generating reactive species capable of modifying fibrinogen and fibrin, increasing resistance to fibrinolysis and altering mechanical properties. Cathepsin G, a neutrophil-derived serine protease, can cleave fibrinogen, activate platelets, and modulate coagulation pathways. Its presence suggests that proteolytic processing occurred contemporaneously with structural assembly rather than as a secondary degradative process.
The incorporation of immunoglobulin heavy constant gamma 1 (IgG1) further indicates participation of adaptive immune components. While immunoglobulins are abundant plasma proteins, their selective retention within a structured intravascular matrix suggests binding to modified fibrin(ogen), immune complexes, or trapped cellular debris, reinforcing the interpretation that AICs represent an irregular immunothrombotic structure rather than a simple fibrin clot.
Actin, cytoplasmic 1, likely derived from leukocytes, platelets, or damaged endothelial cells, provides additional evidence that AICs form in vivo under dynamic conditions involving cellular participation rather than as acellular fibrin deposits.
Impaired Fibrinolytic Capacity and Aberrant Clot Persistence
A defining feature of physiological clots is the deliberate incorporation of plasminogen throughout the fibrin network, positioning the inactive zymogen for subsequent activation by tissue plasminogen activator (tPA) once vascular repair is complete. In AICs, plasminogen was detected at only 0.13% of total protein signal, indicating a profound deficiency relative to typical fibrin clots. Moreover, tPA was detected in only a single sample at extremely low abundance.
This marked depletion of fibrinolytic components indicates that AICs possess severely impaired intrinsic capacity for enzymatic degradation. Combined with their aberrant fibrin architecture, this deficiency provides a mechanistic explanation for the observed persistence of these structures. Rather than representing an exaggerated physiological clotting response to vascular injury, AICs appear to be formed by an aberrant process that excludes proteins normally required for controlled resolution. Under such conditions, continued accretion and elongation of intravascular material would be expected, consistent with the observation of cast-like structures extending to lengths of several centimetres. Some reports relate to structures as long as 69 cm (27 inches).
Minor Proteins as Secondary “Detritus” of Aberrant Architecture
Although more than 500 additional proteins were detected within AICs, the vast majority were present at extremely low abundance. Rather than indicating functional complexity, this pattern is more parsimoniously explained by nonspecific incorporation. The imbalance in fibrinogen chain composition is likely to leave numerous unsatisfied binding sites within the matrix, capable of interacting with a wide range of circulating plasma proteins through electrostatic or hydrophobic interactions. These minor constituents can therefore be considered biochemical “detritus”, incidental material captured by an aberrant scaffold, rather than deliberate structural or functional components of the cast.
Physiological Implications of Persistent Intravascular Solid Material
At its most fundamental level, any biological process that results in the formation of solid material within the vascular lumen has the potential to obstruct blood flow, impair oxygen and nutrient delivery, trigger inflammation, and damage tissue. From this principle, the adverse health consequences associated with pathological clotting can be understood.
In large vessels, obstruction produces acute and often catastrophic events, including ischaemic stroke, myocardial infarction, pulmonary embolism, and acute limb ischaemia, driven by abrupt loss of perfusion and irreversible tissue necrosis (death) as well as sudden death.
In contrast, obstruction of small vessels and the microvasculature produces a more insidious but equally consequential pattern of injury. Impaired capillary perfusion and diffusion-limited oxygen delivery result in patchy, chronic hypoxia, contributing to multi-organ dysfunction, renal impairment, myocarditis, cognitive dysfunction, fatigue, and exercise intolerance. Importantly, the cumulative impact of numerous microvascular obstructions may exceed that of a single large-vessel event.
Persistence of intravascular material further amplifies pathology. Failure of fibrinolysis is associated with chronic thromboembolic pulmonary hypertension, post-thrombotic syndrome, ongoing inflammation, and delayed tissue healing, allowing biologically active material to remain within the circulation long after its formation.
Clotting is also tightly coupled to inflammation. Dysregulation of this interaction—immunothrombosis—promotes endothelial injury, neutrophil activation, oxidative protein modification, and vascular stiffening, creating a self-reinforcing pathological loop. In addition, fragments of intravascular material may embolise, causing distant injury even when the source structure is clinically silent.
Finally, clots exert mechanical effects independent of their biochemical activity. By distorting vessel geometry, increasing shear stress, and damaging endothelium, persistent intravascular structures promote secondary clot formation, vascular remodelling, and chronic vascular disease. When haemostatic regulation fails globally, as in disseminated intravascular coagulation, the result is systemic organ failure rather than localised thrombosis.
Concluding Perspective
Taken together, these findings demonstrate that AICs are not exaggerated versions of physiological clots but represent a fundamentally atypical intravascular matrix, characterised by aberrant fibrinogen stoichiometry, inflammatory modification, and profound resistance to fibrinolytic clearance.
While physiological clotting is essential for haemostasis and survival, dysregulated intravascular clot formation of this type provides a mechanistically coherent explanation for persistent vascular obstruction, impaired tissue perfusion, inflammation, and a broad spectrum of acute and chronic organ dysfunction.
If spike protein were demonstrated to associate with anomalous intravascular casts, this would raise serious implications not only for viral pathophysiology but also for genetic and molecular platforms that induce sustained host manufacture of spike protein, making it imperative that this potential association be rigorously investigated and either confirmed or ruled out.
Conclusions
Anomalous intravascular casts (AICs) exhibit a distinct and reproducible proteomic architecture incompatible with known thrombus biology. The combination of fibrinogen chain imbalance and severe plasminogen deficiency defines a clot phenotype that is structurally abnormal and functionally resistant to degradation. Importantly, these proteomic findings are concordant with prior morphological, histological, and elemental analyses, which independently indicate a dense, fibrin-bearing matrix with non-physiological composition and persistence.
Together, the convergence of structural, biochemical, and molecular evidence supports the interpretation of AICs as a discrete and atypical clot entity forming during life rather than a variant of ordinary post-mortem coagulum.
Further mechanistic investigation is warranted to determine the biochemical pathways underlying this transformation of fibrin architecture. Independent replication using complementary analytical approaches will be essential to establish the generality of this proteomic phenotype and to determine its relevance across a broader spectrum of thrombotic and clot-associated pathologies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
B.R. conceived the study, coordinated data acquisition, performed analysis, and drafted the manuscript. M.S. contributed to study design, interpretation of results, and manuscript revision. All authors reviewed and approved the final manuscript.
Funding
This work received significant funding from the NZDSOS, and a number of private donors who wished to remain anonymous, to facilitate the cost of laboratory analysis. None of these donors had any role in study design, data collection, analysis, interpretation, or manuscript preparation.
Institutional Review Board Statement
All materials analysed in this study were anonymised postmortem biological specimens obtained from waste streams generated during routine mortuary and laboratory procedures. No living human subjects were involved, no identifiable personal data were accessed, and no intervention or procedure was performed for research purposes. Under applicable local ethics regulations, research involving anonymised discarded postmortem materials does not constitute human subjects research and does not require formal ethics committee approval.
Data Availability Statement
The data supporting the findings of this study are not publicly available due to confidentiality agreements and anonymisation requirements associated with participating laboratories and suppliers of the test material.
Acknowledgments
The authors wish to acknowledge the contributions and support of the following individuals and organizations. Technical and analytical support: The authors thank the international laboratories that assisted with sample preparation, elemental analysis, and related laboratory procedures. These laboratories requested anonymity due to the nature of the work, and their request has been respected. Clinical, mortuary, or sample access support: The authors acknowledge the embalmers and undertakers from multiple countries who facilitated access to specimens, clinical context, and case material, and who cooperated during sample collection. Their wish to remain anonymous has been respected. Scholarly input and discussion: The authors thank Professor Robyn Cossford, Dr. Gerry Brady, Dr. Rob Maunsell and Mr. Nic Broomfield for constructive discussions, critical feedback, and methodological insights that informed the development of this work. Funding and material support: This work was supported in part by the NZDSOS, which had no role in study design, data collection, analysis, interpretation, or manuscript preparation. Ethical and logistical support: The authors acknowledge the cooperation of all institutions and individuals involved in the ethical handling and transfer of specimens. Additional support: The authors are grateful to all contributors who supported this work but did not meet the criteria for authorship.
Conflicts of Interest
The authors declare no competing interests.
| 1 |
describes the relationships between the quantities of reactants and products before, during, and following chemical reactions. Stoichiometry is based on the law of conservation of mass; the total mass of reactants must equal the total mass of products, so the relationship between reactants and products must form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).