Submitted:
11 January 2026
Posted:
27 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Conventional and Emerging Cardiovascular Risk Determinants
2.1. Traditional Risk Factor: The Chronic Driver of Vascular Injuries
2.2. Emerging and Non-Classical Risk Factor:
3. Lipid Peroxidation in Vascular Inflammation
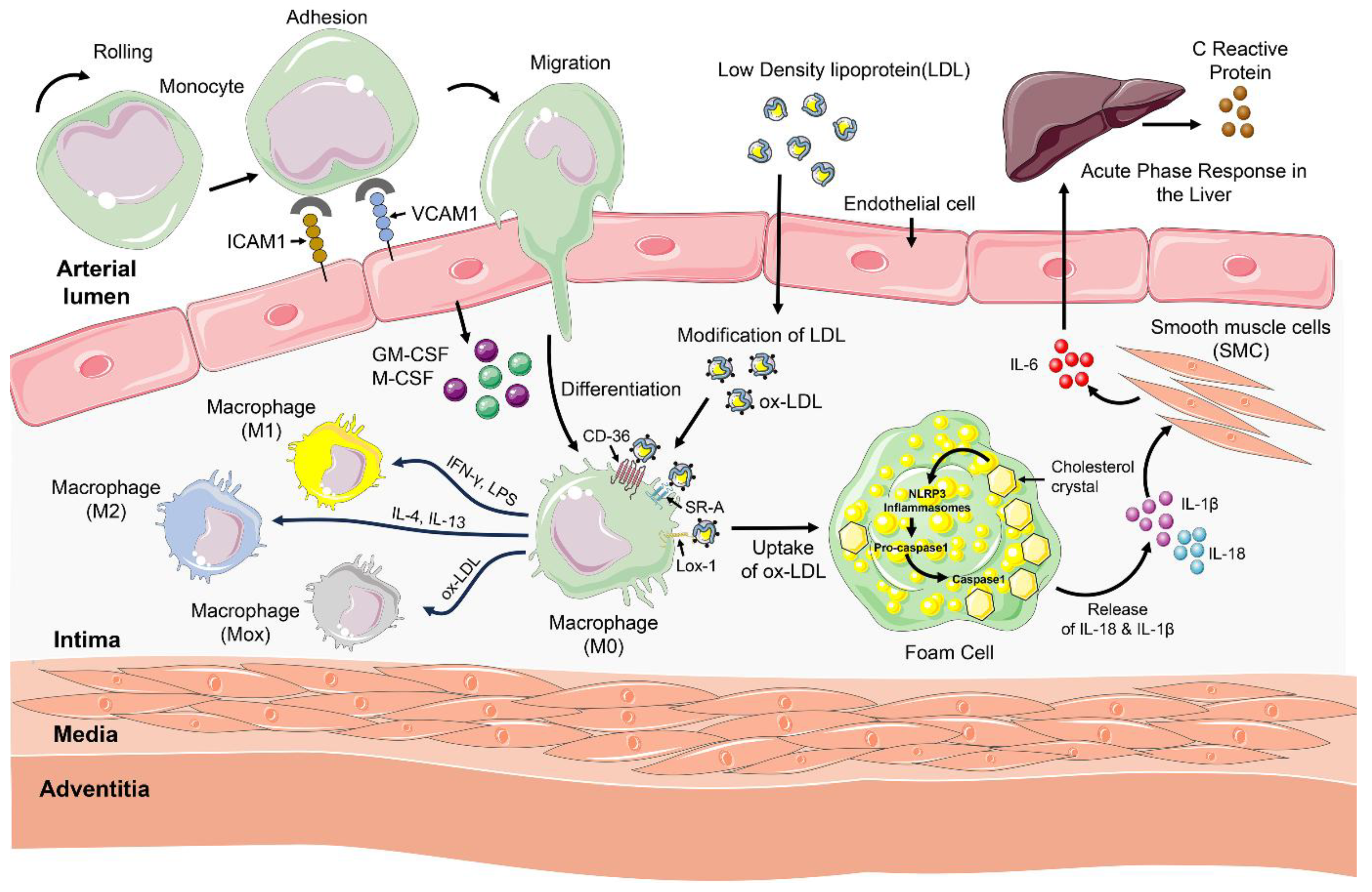
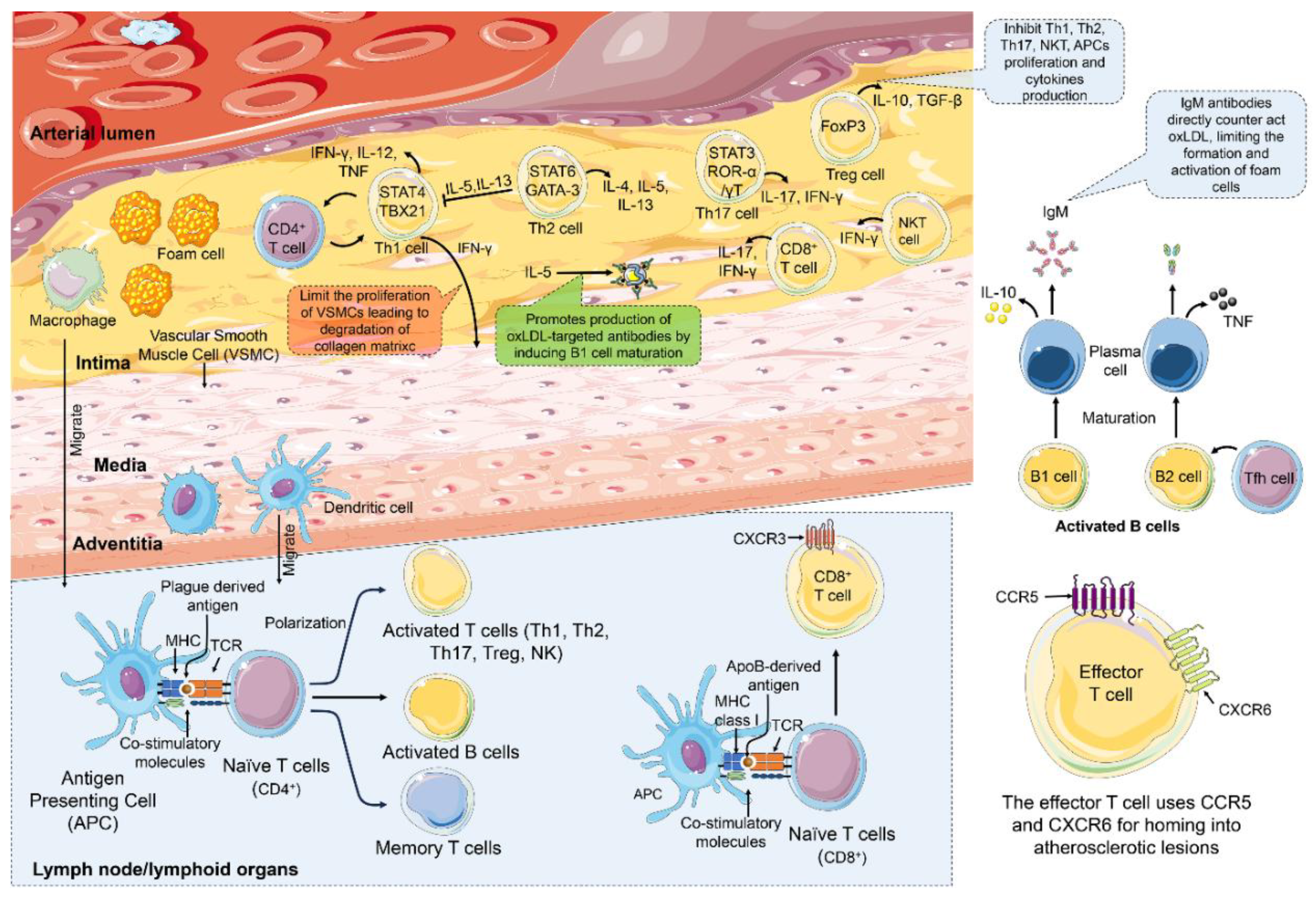
4. Immune Cells and Immune Response in Atherosclerosis
4.1. Innate Immunity in Atherosclerosis
4.2. Adaptive Immunity in Atherosclerosis
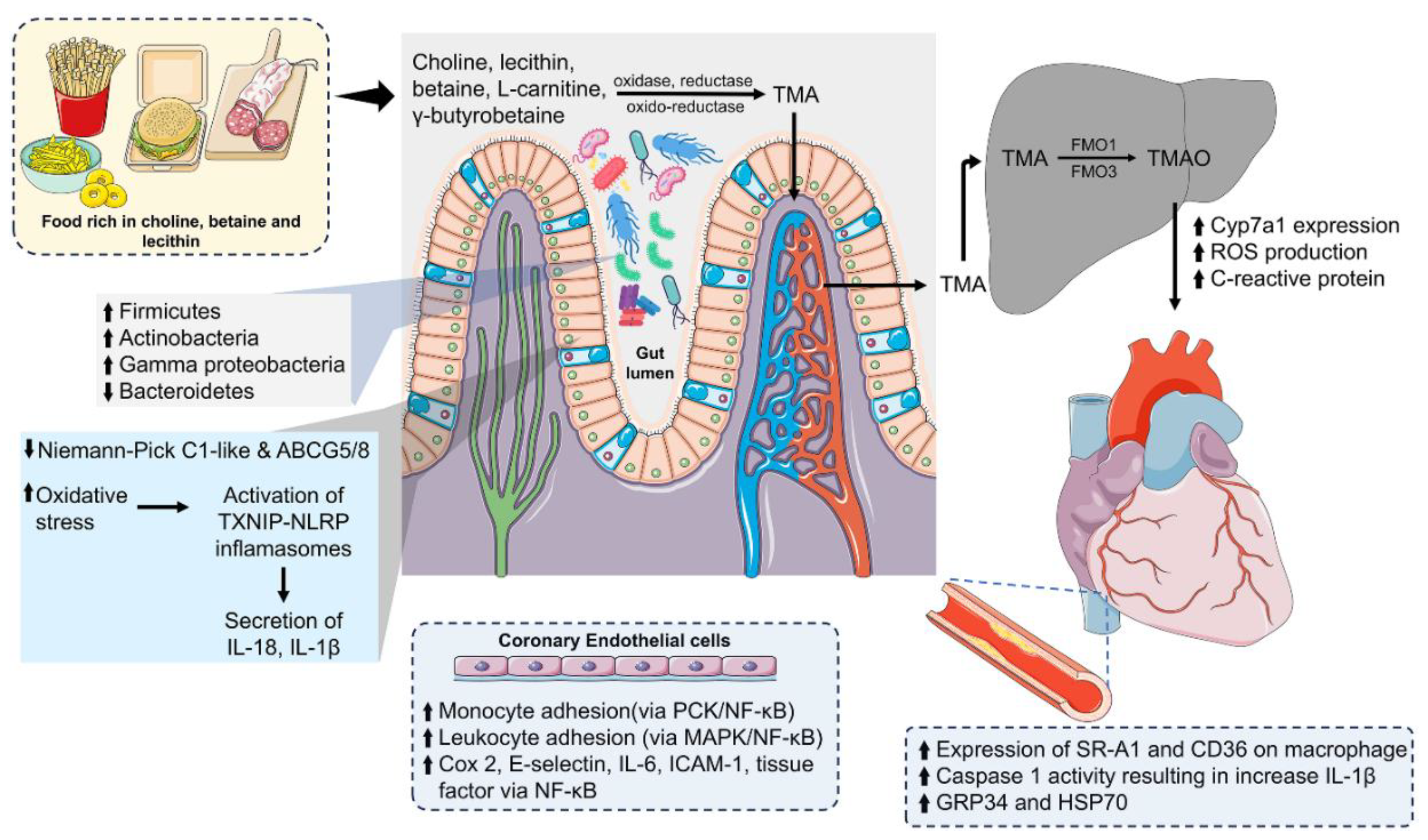
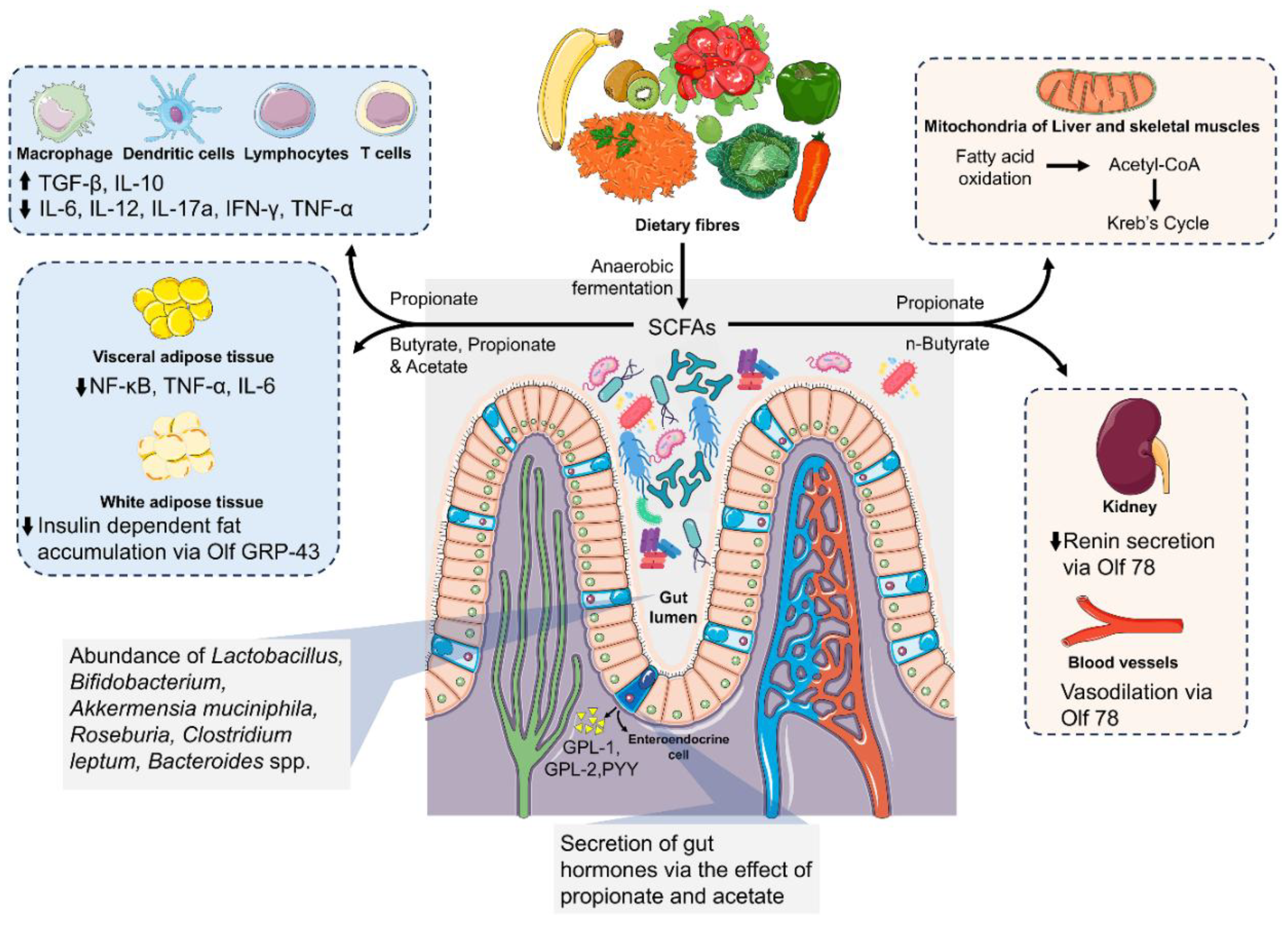
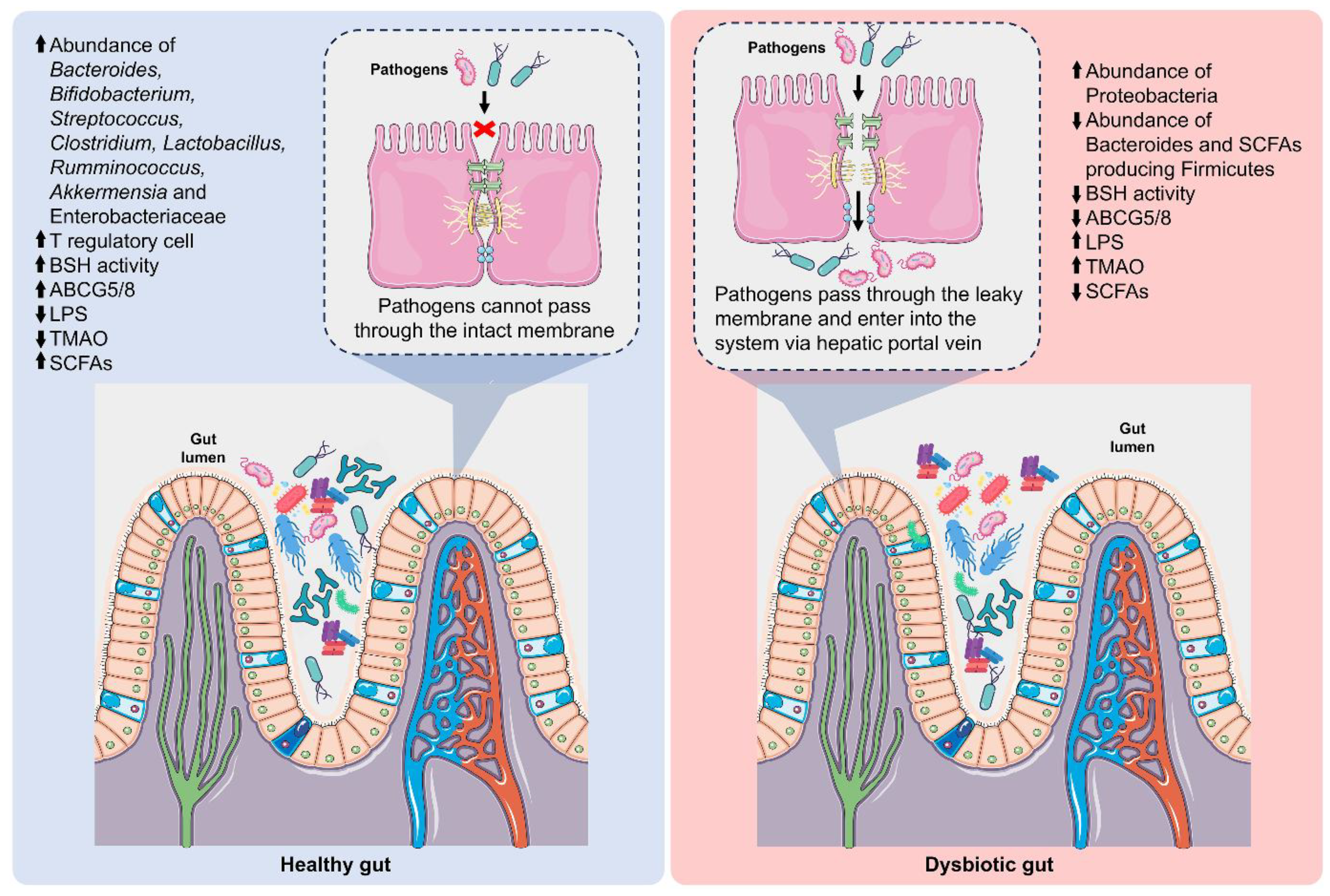
5. Gut-Derived Metabolites and Atherosclerosis
5.1. Trimethylamine N-Oxide and Its Role in Cardiovascular Disease
5.2. Short-Chain Fatty Acids and Their Role in Metabolism
5.3. Secondary Bile Acids: Metabolism, Signaling, and Immune Modulation
5.4. Metabolic Endotoxins and Their Implications in Cardiometabolic Diseases
6. Therapeutic Strategies Targeting Gut Microbiota-Derived Metabolites for Atherosclerosis
6.1. Diet and Lifestyle:
6.2. Natural Product-Based Therapies Targeting Gut Microbiota
6.3. Probiotics
6.4. Pharmacological Modulation of the Gut Microbiome in Cardiovascular Disease
6.5. Fecal Microbiome Transplantation
7. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Competing Interests
Abbreviations
| CVDs | cardiovascular diseases |
| WHO | World Health Organization |
| DALYs | Disability Adjusted Life Years |
| NCD | non-communicable disease |
| IHD | ischemic heart disease |
| SCD | sudden cardiac death |
| SMC | smooth muscle cell |
| TMAO | trimethylamine-n-oxide |
| SCFA | short-chain fatty acid |
| LPS | lipopolysaccharides |
| ASCVD | Atherosclerotic cardiovascular disease |
| LDL | Low-density lipoprotein |
| oxLDL | Oxidised Low-density lipoprotein |
| AGEs | advanced glycation-end product |
| AHA | American Heart Association |
| GI | gastrointestinal |
| MWAS | metagenome-wide association |
| T-RFLP | terminal restriction fragment length polymorphism |
| CAD | coronary artery disease |
| LCV | Lymphocystis Virus |
| TTV | Torque teno virus |
| VLDL | very low-density lipoprotein |
| HDL | high-density lipoprotein |
| Lp(a) | lipoprotein (a) |
| RCT | Reverse Cholesterol Transport |
| eNOS | endothelial nitric oxide synthase |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| PPARγ | peroxisome proliferator activator receptor γ |
| MCP-1 | monocyte chemoattractant protein-1 |
| COX-2 | cyclooxygenase-2 |
| NF-κB | nuclear factor κB |
| LOX-1 | lectin-like oxidised LDL receptor 1 |
| TGF-β1 | transforming growth factor-β1 |
| VSMCs | vascular smooth muscle cells |
| MPO | myeloperoxidase |
| ALEs | advanced lipoxidation end product |
| MDA | malondialdehyde |
| 4HNE | 4-hydroxynonenal |
| DDAH | dimethyl aminohydrolase |
| MMP | matrix metalloproteinase |
| MAPK | mitogen-activated protein kinase |
| JNK | c-jun N-terminal kinase |
| VCAM-1 | vascular cell adhesion molecule |
| ICAM | intracellular cell adhesion molecule |
| Th1 | T helper type 1 |
| INF-γ | interferon-γ |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| M-CSF | macrophage colony-stimulating factor |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| TLRs | Toll-like receptors |
| PAMPs | pathogen-associated molecular pattern |
| DAMPs | damage-associated molecular pattern |
| MyD88 | Myeloid Differentiation Primary Response Gene88 |
| Lp-PLA2 | Lipoprotein-associated phospholipase A2 |
| NLRP3 | NOD-like receptor 3 |
| NK | natural killer |
| NKT | natural killer T cells |
| APC | antigen-presenting cells |
| DC | dendritic cells |
| MHC | major histocompatibility complex |
| T-bet | T-box transcription factor |
| GATA3 | GATA binding protein 3 |
| RORγ | RARA-related orphan receptor γ |
| CTLs | cytotoxic T lymphocytes |
| BCR | B cell recptor |
| Tfh | T follicular helper |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| GBB | gamma butyrobetaine |
| TMADH | trimethylamine dehydrogenase |
| FMO | flavin monooxygenase |
| FXR | farnesoid X receptor |
| MACE | major adverse cardiovascular event |
| HAECs | human aortic endothelial cells |
| HCAECs | human coronary aortic endothelial cells |
| CYP7a1 | Cholesterol 7 alpha-hydrolase |
| ABCG5/8 | ATP-binding cassette G5/8 |
| NPC1L1 | Neimann-Pick C1-like 1 |
| ROS | reactive oxygen species |
| FHCs | fetal human colon cells |
| HIV | human immunodeficiency virus |
| EPCs | endothelial progenitor cells |
| CLDN1 | claudin1 |
| FFARs | free fatty acid receptors |
| HCA2 | hydroxycarboxylic acid receptor 2 |
| HDAC | histone deacetylase |
| PYY | peptide YY |
| GLP-1 | glucagon-like peptide 1 |
| SNS | sympathetic nervous system |
| ILCs | innate lymphoid cells |
| AhR | aryl hydrocarbon receptor |
| HIF-1α | hypoxia-inducible factor 1α |
| HSDH | hydroxysteroid dehydrogenase |
| CA | cholic acid |
| CDCA | chenodeoxycholic acid |
| LCA | lithocholic acid |
| DCA | deoxycholic acid |
| UDCA | ursodeoxycholic acid |
| IEC | intestinal epithelial cells |
| cAMP | cyclic adenosine monophosphate |
| BMDMs | bone marrow-derived macrophages |
| LBP | lipopolysaccharide-binding protein |
| BPI | bactericidal/permeability-increasing protein |
| CHF | chronic heart failure |
| SOLVD | Studies of Left Ventricular Dysfunction |
| CORDIOPREV | Coronary Diet Intervention with Olive Oil and Cardiovascular Prevention |
| PREDIMED | Prevención con dieta Mediterránea |
| ITF | inulin-type fructans |
| ASBT | apical sodium-dependent bile acid transporter |
| IMC | Iodomethylcholine |
| FMC | Fluoromethylcholine |
| FMT | fecal microbiome transplantation |
References
- Xavier, D.; Pais, P.; Devereaux, P.J.; Xie, C.; Prabhakaran, D.; Reddy, K.S.; Gupta, R.; Joshi, P.; Kerkar, P.; Thanikachalam, S.; et al. Treatment and Outcomes of Acute Coronary Syndromes in India (CREATE): A Prospective Analysis of Registry Data. Lancet 2008, 371, 1435–1442. [Google Scholar] [CrossRef]
- Sivadasanpillai, H.; Leeder, S.; M, H.; Jeemon, P.; Dorairaj, P. A Race against Time: The Challenge of Cardiovascular Diseases in Developing Economies; 2015; ISBN 978-81-930819-0-7. [Google Scholar]
- Z, R. ACE Insertion/Deletion (I/D) Polymorphism and Diabetic Nephropathy. Journal of nephropathology 2012, 1. [Google Scholar] [CrossRef]
- Azar, P.; Bochaton-Piallat, M.-L. Deciphering Metabolic Reprogramming in Atherosclerosis through a Multi-Omics Approach. Cardiovasc Res 2025, 121, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr Atheroscler Rep 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Dar, T.; Radfar, A.; Abohashem, S.; Pitman, R.K.; Tawakol, A.; Osborne, M.T. Psychosocial Stress and Cardiovascular Disease. Curr Treat Options Cardiovasc Med 2019, 21, 23. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circulation research 2017, 120, 1183. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep 2006, 7, 688–693. [Google Scholar] [CrossRef]
- F, T.; C, M.; S, D.; Ga, L.; S, B.; A, M.; D.P., F; van S., D; M, V. The Infant Gut Microbiome as a Microbial Organ Influencing Host Well-Being. Italian journal of pediatrics 2020, 46. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Zhang, T.; Xie, X.; Wu, Q. Gut Microbiota: A Novel Strategy Affecting Atherosclerosis. Microbiology Spectrum 2025, 13, e00482-24. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat Commun 2017, 8, 845. [Google Scholar] [CrossRef]
- Emoto, T.; Yamashita, T.; Sasaki, N.; Hirota, Y.; Hayashi, T.; So, A.; Kasahara, K.; Yodoi, K.; Matsumoto, T.; Mizoguchi, T.; et al. Analysis of Gut Microbiota in Coronary Artery Disease Patients: A Possible Link between Gut Microbiota and Coronary Artery Disease. J Atheroscler Thromb 2016, 23, 908–921. [Google Scholar] [CrossRef]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia Intestinalis and Diet Modulate Atherogenesis in a Murine Model. Nat Microbiol 2018, 3, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- O, K.; A, S.; J, F.; F, F.; J, S.; V, T.; Cj, B.; R, K.; B, F.; Re, L.; et al. Human Oral, Gut, and Plaque Microbiota in Patients with Atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America 2011, 108 Suppl 1. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, H.; Kang, M.; Kim, J.E.; Choi, Y.-H.; Min, Y.W.; Min, B.-H.; Lee, J.H.; Son, H.J.; Rhee, P.-L.; et al. Helicobacter Pylori Is Associated with Dyslipidemia but Not with Other Risk Factors of Cardiovascular Disease. Sci Rep 2016, 6, 38015. [Google Scholar] [CrossRef] [PubMed]
- J, L.; S, L.; Pm, V.; Cw, W.; A, X. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133. [Google Scholar] [CrossRef]
- Ott, S.J.; El Mokhtari, N.E.; Musfeldt, M.; Hellmig, S.; Freitag, S.; Rehman, A.; Kühbacher, T.; Nikolaus, S.; Namsolleck, P.; Blaut, M.; et al. Detection of Diverse Bacterial Signatures in Atherosclerotic Lesions of Patients with Coronary Heart Disease. Circulation 2006, 113, 929–937. [Google Scholar] [CrossRef]
- Dinakaran, V.; Rathinavel, A.; Pushpanathan, M.; Sivakumar, R.; Gunasekaran, P.; Rajendhran, J. Elevated Levels of Circulating DNA in Cardiovascular Disease Patients: Metagenomic Profiling of Microbiome in the Circulation. PLoS One 2014, 9, e105221. [Google Scholar] [CrossRef]
- Pothineni, N.V.K.; Subramany, S.; Kuriakose, K.; Shirazi, L.F.; Romeo, F.; Shah, P.K.; Mehta, J.L. Infections, Atherosclerosis, and Coronary Heart Disease. Eur Heart J 2017, 38, 3195–3201. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Zambon, J.J.; Trevisan, M.; Zeid, M.; Genco, R.J. Identification of Periodontal Pathogens in Atheromatous Plaques. J Periodontol 2000, 71, 1554–1560. [Google Scholar] [CrossRef]
- Kozarov, E.V.; Dorn, B.R.; Shelburne, C.E.; Dunn, W.A.; Progulske-Fox, A. Human Atherosclerotic Plaque Contains Viable Invasive Actinobacillus Actinomycetemcomitans and Porphyromonas Gingivalis. Arterioscler Thromb Vasc Biol 2005, 25, e17-18. [Google Scholar] [CrossRef]
- Padilla, C.; Lobos, O.; Hubert, E.; González, C.; Matus, S.; Pereira, M.; Hasbun, S.; Descouvieres, C. Periodontal Pathogens in Atheromatous Plaques Isolated from Patients with Chronic Periodontitis. J Periodontal Res 2006, 41, 350–353. [Google Scholar] [CrossRef]
- F, F.; V, T.; G, B.; F, B. Oral Microbiota in Patients with Atherosclerosis. Atherosclerosis 2015, 243. [Google Scholar] [CrossRef]
- Shibata, T.; Shimizu, K.; Hirano, K.; Nakashima, F.; Kikuchi, R.; Matsushita, T.; Uchida, K. Adductome-Based Identification of Biomarkers for Lipid Peroxidation. J Biol Chem 2017, 292, 8223–8235. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized Low-Density Lipoprotein. Methods Mol Biol 2010, 610, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Lj, van T.; R, S.; Pl, van L.; Mg, N.; La, J.; Af, S. Oxidized LDL Enhances Pro-Inflammatory Responses of Alternatively Activated M2 Macrophages: A Crucial Role for Krüppel-like Factor 2. Atherosclerosis 2011, 214. [Google Scholar] [CrossRef]
- Kim, C.E.; Lee, S.J.; Seo, K.W.; Park, H.M.; Yun, J.W.; Bae, J.U.; Bae, S.S.; Kim, C.D. Acrolein Increases 5-Lipoxygenase Expression in Murine Macrophages through Activation of ERK Pathway. Toxicol Appl Pharmacol 2010, 245, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular Mechanisms of Acrolein Toxicity: Relevance to Human Disease. Toxicol Sci 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; van Zanten, J.J.C.S.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc Med J 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Yoshimoto, R.; Fujita, Y.; Kakino, A.; Iwamoto, S.; Takaya, T.; Sawamura, T. The Discovery of LOX-1, Its Ligands and Clinical Significance. Cardiovasc Drugs Ther 2011, 25, 379–391. [Google Scholar] [CrossRef]
- Murphy, A.J.; Akhtari, M.; Tolani, S.; Pagler, T.; Bijl, N.; Kuo, C.-L.; Wang, M.; Sanson, M.; Abramowicz, S.; Welch, C.; et al. ApoE Regulates Hematopoietic Stem Cell Proliferation, Monocytosis, and Monocyte Accumulation in Atherosclerotic Lesions in Mice. J Clin Invest 2011, 121, 4138–4149. [Google Scholar] [CrossRef]
- Riwanto, M.; Rohrer, L.; von Eckardstein, A.; Landmesser, U. Dysfunctional HDL: From Structure-Function-Relationships to Biomarkers. Handbook of Experimental Pharmacology 2015, 224, 337–366. [Google Scholar] [CrossRef]
- Sattler, K.; Lehmann, I.; Gräler, M.; Bröcker-Preuss, M.; Erbel, R.; Heusch, G.; Levkau, B. HDL-Bound Sphingosine 1-Phosphate (S1P) Predicts the Severity of Coronary Artery Atherosclerosis. Cellular Physiology and Biochemistry 2014, 34, 172–184. [Google Scholar] [CrossRef]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid Peroxidation: Mechanisms, Inhibition, and Biological Effects. Biochemical and Biophysical Research Communications 2005, 338, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Fritz, K.S.; Petersen, D.R. An Overview of the Chemistry and Biology of Reactive Aldehydes. Free Radic Biol Med 2013, 59, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Leonarduzzi, G.; Gamba, P.; Gargiulo, S.; Biasi, F.; Poli, G. Inflammation-Related Gene Expression by Lipid Oxidation-Derived Products in the Progression of Atherosclerosis. Free Radic Biol Med 2012, 52, 19–34. [Google Scholar] [CrossRef]
- Sp, F.; Lj, D.; Je, G.; N, P.; L, Z.; Kb, G.-C.; Aj, C. Mechanism of 4-HNE Mediated Inhibition of hDDAH-1: Implications in No Regulation. Biochemistry 2008, 47. [Google Scholar] [CrossRef] [PubMed]
- N, S.; Jl, F.; Y, L.; Pm, S.; S, R.; R, N. Proinflammatory Effects of Advanced Lipoxidation End Products in Monocytes. Diabetes 2008, 57. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Fong, K.Y.; Wright, D.W. Impact of 4-Hydroxynonenal on Matrix Metalloproteinase-9 Regulation in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Cell Biochem Funct 2015, 33, 59–66. [Google Scholar] [CrossRef]
- Lee, S.J.; Seo, K.W.; Yun, M.R.; Bae, S.S.; Lee, W.S.; Hong, K.W.; Kim, C.D. 4-Hydroxynonenal Enhances MMP-2 Production in Vascular Smooth Muscle Cells via Mitochondrial ROS-Mediated Activation of the Akt/NF-kappaB Signaling Pathways. Free Radic Biol Med 2008, 45, 1487–1492. [Google Scholar] [CrossRef]
- S, X.; Ac, L.; Ai, G. Common Pathogenic Features of Atherosclerosis and Calcific Aortic Stenosis: Role of Transforming Growth Factor-Beta. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology 2010, 19. [Google Scholar] [CrossRef]
- Vatsyayan, R.; Kothari, H.; Pendurthi, U.R.; Rao, L.V.M. 4-Hydroxy-2-Nonenal Enhances Tissue Factor Activity in Human Monocytic Cells via P38 Mitogen-Activated Protein Kinase Activation-Dependent Phosphatidylserine Exposure. Arterioscler Thromb Vasc Biol 2013, 33, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Tj, L.; Jt, L.; Sk, M.; Ch, K.; Jw, P.; Tk, K. Age-Related Differential Growth Rate and Response to 4-Hydroxynonenal in Mouse Aortic Smooth Muscle Cells. International journal of molecular medicine 2006, 17. [Google Scholar]
- Zarkovic, K.; Larroque-Cardoso, P.; Pucelle, M.; Salvayre, R.; Waeg, G.; Nègre-Salvayre, A.; Zarkovic, N. Elastin Aging and Lipid Oxidation Products in Human Aorta. Redox Biol 2014, 4, 109–117. [Google Scholar] [CrossRef]
- Subbotin, V.M. Neovascularization of Coronary Tunica Intima (DIT) Is the Cause of Coronary Atherosclerosis. Lipoproteins Invade Coronary Intima via Neovascularization from Adventitial Vasa Vasorum, but Not from the Arterial Lumen: A Hypothesis. Theor Biol Med Model 2012, 9, 11. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, C.E.; Yun, M.R.; Seo, K.W.; Park, H.M.; Yun, J.W.; Shin, H.K.; Bae, S.S.; Kim, C.D. 4-Hydroxynonenal Enhances MMP-9 Production in Murine Macrophages via 5-Lipoxygenase-Mediated Activation of ERK and P38 MAPK. Toxicol Appl Pharmacol 2010, 242, 191–198. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F., Jr. New Insights on Oxidative Stress in the Artery Wall. Journal of Thrombosis and Haemostasis 2005, 3, 1825–1834. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent Developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Nakashima, Y.; Raines, E.W.; Plump, A.S.; Breslow, J.L.; Ross, R. Upregulation of VCAM-1 and ICAM-1 at Atherosclerosis-Prone Sites on the Endothelium in the ApoE-Deficient Mouse. Arterioscler Thromb Vasc Biol 1998, 18, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and Challenges in Translating the Biology of Atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Am, L.; Gk, H. Innate Immune Signals in Atherosclerosis. Clinical immunology (Orlando, Fla.) 2010, 134. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- de Beer, F.C.; Hind, C.R.; Fox, K.M.; Allan, R.M.; Maseri, A.; Pepys, M.B. Measurement of Serum C-Reactive Protein Concentration in Myocardial Ischaemia and Infarction. Br Heart J 1982, 47, 239–243. [Google Scholar] [CrossRef]
- Tennent, G.A.; Hutchinson, W.L.; Kahan, M.C.; Hirschfield, G.M.; Gallimore, J.R.; Lewin, J.; Sabin, C.A.; Dhillon, A.P.; Pepys, M.B. Transgenic Human CRP Is Not Pro-Atherogenic, pro-Atherothrombotic or pro-Inflammatory in apoE-/- Mice. Atherosclerosis 2008, 196, 248–255. [Google Scholar] [CrossRef]
- Hoefer, I.E.; Steffens, S.; Ala-Korpela, M.; Bäck, M.; Badimon, L.; Bochaton-Piallat, M.-L.; Boulanger, C.M.; Caligiuri, G.; Dimmeler, S.; Egido, J.; et al. Novel Methodologies for Biomarker Discovery in Atherosclerosis. Eur Heart J 2015, 36, 2635–2642. [Google Scholar] [CrossRef]
- Robbins, C.S.; Hilgendorf, I.; Weber, G.F.; Theurl, I.; Iwamoto, Y.; Figueiredo, J.-L.; Gorbatov, R.; Sukhova, G.K.; Gerhardt, L.M.S.; Smyth, D.; et al. Local Proliferation Dominates Lesional Macrophage Accumulation in Atherosclerosis. Nat Med 2013, 19, 1166–1172. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J Immunol 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Baron, M.; Bouhlel, M.A.; Vanhoutte, J.; Copin, C.; Sebti, Y.; Derudas, B.; Mayi, T.; Bories, G.; Tailleux, A.; et al. Human Atherosclerotic Plaque Alternative Macrophages Display Low Cholesterol Handling but High Phagocytosis Because of Distinct Activities of the PPARγ and LXRα Pathways. Circ Res 2011, 108, 985–995. [Google Scholar] [CrossRef]
- Kadl, A.; Meher, A.K.; Sharma, P.R.; Lee, M.Y.; Doran, A.C.; Johnstone, S.R.; Elliott, M.R.; Gruber, F.; Han, J.; Chen, W.; et al. Identification of a Novel Macrophage Phenotype That Develops in Response to Atherogenic Phospholipids via Nrf2. Circ Res 2010, 107, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.X.; Shapiro, M.; Trogan, E.; Fisher, E.A. Transdifferentiation of Mouse Aortic Smooth Muscle Cells to a Macrophage-like State after Cholesterol Loading. Proc Natl Acad Sci U S A 2003, 100, 13531–13536. [Google Scholar] [CrossRef] [PubMed]
- Feil, S.; Fehrenbacher, B.; Lukowski, R.; Essmann, F.; Schulze-Osthoff, K.; Schaller, M.; Feil, R. Transdifferentiation of Vascular Smooth Muscle Cells to Macrophage-like Cells during Atherogenesis. Circ Res 2014, 115, 662–667. [Google Scholar] [CrossRef]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.C.; Greene, E.S.; Straub, A.C.; et al. KLF4-Dependent Phenotypic Modulation of Smooth Muscle Cells Has a Key Role in Atherosclerotic Plaque Pathogenesis. Nat Med 2015, 21, 628–637. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of Intimal Smooth Muscle Cells to Cholesterol Accumulation and Macrophage-like Cells in Human Atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef]
- Edfeldt, K.; Swedenborg, J.; Hansson, G.K.; Yan, Z. Expression of Toll-like Receptors in Human Atherosclerotic Lesions: A Possible Pathway for Plaque Activation. Circulation 2002, 105, 1158–1161. [Google Scholar] [CrossRef]
- West, X.Z.; Malinin, N.L.; Merkulova, A.A.; Tischenko, M.; Kerr, B.A.; Borden, E.C.; Podrez, E.A.; Salomon, R.G.; Byzova, T.V. Oxidative Stress Induces Angiogenesis by Activating TLR2 with Novel Endogenous Ligands. Nature 2010, 467, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Björkbacka, H.; Kunjathoor, V.V.; Moore, K.J.; Koehn, S.; Ordija, C.M.; Lee, M.A.; Means, T.; Halmen, K.; Luster, A.D.; Golenbock, D.T.; et al. Reduced Atherosclerosis in MyD88-Null Mice Links Elevated Serum Cholesterol Levels to Activation of Innate Immunity Signaling Pathways. Nat Med 2004, 10, 416–421. [Google Scholar] [CrossRef] [PubMed]
- H.-C., E; G, C.; H, P.; K, O.; P, K. Phospholipase A(2) in Vascular Disease. Circulation research 2001, 89. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci 2019, 20, 3328. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.H.; Genest, J.; Gotto, A.M.; Kastelein, J.J.P.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N Engl J Med 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Maier, W.; Altwegg, L.A.; Corti, R.; Gay, S.; Hersberger, M.; Maly, F.E.; Sütsch, G.; Roffi, M.; Neidhart, M.; Eberli, F.R.; et al. Inflammatory Markers at the Site of Ruptured Plaque in Acute Myocardial Infarction: Locally Increased Interleukin-6 and Serum Amyloid A but Decreased C-Reactive Protein. Circulation 2005, 111, 1355–1361. [Google Scholar] [CrossRef]
- M, D.; Rt, M.; van Z., M; C, W.; O, S. Hyperlipidemia-Triggered Neutrophilia Promotes Early Atherosclerosis. Circulation 2010, 122. [Google Scholar] [CrossRef]
- Tupin, E.; Nicoletti, A.; Elhage, R.; Rudling, M.; Ljunggren, H.-G.; Hansson, G.K.; Berne, G.P. CD1d-Dependent Activation of NKT Cells Aggravates Atherosclerosis. J Exp Med 2004, 199, 417–422. [Google Scholar] [CrossRef]
- Whitman, S.C.; Rateri, D.L.; Szilvassy, S.J.; Yokoyama, W.; Daugherty, A. Depletion of Natural Killer Cell Function Decreases Atherosclerosis in Low-Density Lipoprotein Receptor Null Mice. Arteriosclerosis, Thrombosis, and Vascular Biology 2004, 24, 1049–1054. [Google Scholar] [CrossRef]
- Gh, van P.; Ej, van W.; Ad, H.; de V., P; Tj, van B.; J, K. Effect of Natural Killer T Cell Activation on the Initiation of Atherosclerosis. Thrombosis and haemostasis 2009, 102. [Google Scholar] [CrossRef]
- Hy, C.; R, W.; Cc, H. Gammadelta (Γδ) T Lymphocytes Do Not Impact the Development of Early Atherosclerosis. Atherosclerosis 2014, 234. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Lord, R.S.A. Mapping of Vascular Dendritic Cells in Atherosclerotic Arteries Suggests Their Involvement in Local Immune-Inflammatory Reactions. Cardiovasc Res 1998, 37, 799–810. [Google Scholar] [CrossRef]
- Saigusa, R.; Winkels, H.; Ley, K. T Cell Subsets and Functions in Atherosclerosis. Nat Rev Cardiol 2020, 17, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Decisions about Dendritic Cells: Past, Present, and Future. Annu Rev Immunol 2012, 30, 1–22. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ Res 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Kim, H.I.; Park, J.; Guo, J.; Huang, W. The Role of Immune Cells in Different Stages of Atherosclerosis. Int J Med Sci 2024, 21, 1129–1143. [Google Scholar] [CrossRef]
- Li, J.; McArdle, S.; Gholami, A.; Kimura, T.; Wolf, D.; Gerhardt, T.; Miller, J.; Weber, C.; Ley, K. CCR5+T-bet+FoxP3+ Effector CD4 T Cells Drive Atherosclerosis. Circ Res 2016, 118, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Harry, B.L.; Ludwig, A.; Liehn, E.A.; Sanders, J.M.; Bruce, A.; Weber, C.; Ley, K. CXCR6 Promotes Atherosclerosis by Supporting T-Cell Homing, Interferon-Gamma Production, and Macrophage Accumulation in the Aortic Wall. Circulation 2007, 116, 1801–1811. [Google Scholar] [CrossRef]
- Brinkman, C.C.; Peske, J.D.; Engelhard, V.H. Peripheral Tissue Homing Receptor Control of Naïve, Effector, and Memory CD8 T Cell Localization in Lymphoid and Non-Lymphoid Tissues. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A Novel Transcription Factor, T-Bet, Directs Th1 Lineage Commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J Immunol 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Edamitsu, S.; Matsukawa, A.; Ohkawara, S.; Takagi, K.; Nariuchi, H.; Yoshinaga, M. Role of TNF Alpha, IL-1, and IL-1ra in the Mediation of Leukocyte Infiltration and Increased Vascular Permeability in Rabbits with LPS-Induced Pleurisy. Clin Immunol Immunopathol 1995, 75, 68–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Bian, F.; Wu, P.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zheng, T.; et al. TNF-α Promotes Early Atherosclerosis by Increasing Transcytosis of LDL across Endothelial Cells: Crosstalk between NF-κB and PPAR-γ. J Mol Cell Cardiol 2014, 72, 85–94. [Google Scholar] [CrossRef]
- Nakayama, T.; Hirahara, K.; Onodera, A.; Endo, Y.; Hosokawa, H.; Shinoda, K.; Tumes, D.J.; Okamoto, Y. Th2 Cells in Health and Disease. Annual Review of Immunology 2017, 35, 53–84. [Google Scholar] [CrossRef]
- Marks, B.R.; Nowyhed, H.N.; Choi, J.-Y.; Poholek, A.C.; Odegard, J.M.; Flavell, R.A.; Craft, J. Thymic Self-Reactivity Selects Natural Interleukin 17-Producing T Cells That Can Regulate Peripheral Inflammation. Nat Immunol 2009, 10, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Snijckers, R.P.M.; Foks, A.C. Adaptive Immunity and Atherosclerosis: Aging at Its Crossroads. Front. Immunol. 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.; Tipping, P.G. The Role of Interleukin-4 and Interleukin-12 in the Progression of Atherosclerosis in Apolipoprotein E-Deficient Mice. Am J Pathol 2003, 163, 1117–1125. [Google Scholar] [CrossRef]
- Fernández-Gallego, N.; Castillo-González, R.; Méndez-Barbero, N.; López-Sanz, C.; Obeso, D.; Villaseñor, A.; Escribese, M.M.; López-Melgar, B.; Salamanca, J.; Benedicto-Buendía, A.; et al. The Impact of Type 2 Immunity and Allergic Diseases in Atherosclerosis. Allergy 2022, 77, 3249–3266. [Google Scholar] [CrossRef]
- Smith, E.; Prasad, K.-M.R.; Butcher, M.; Dobrian, A.; Kolls, J.K.; Ley, K.; Galkina, E. Blockade of Interleukin-17A Results in Reduced Atherosclerosis in Apolipoprotein E-Deficient Mice. Circulation 2010, 121, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, C.E.; Mele, F.; Aschenbrenner, D.; Jarrossay, D.; Ronchi, F.; Gattorno, M.; Monticelli, S.; Lanzavecchia, A.; Sallusto, F. Pathogen-Induced Human TH17 Cells Produce IFN-γ or IL-10 and Are Regulated by IL-1β. Nature 2012, 484, 514–518. [Google Scholar] [CrossRef]
- Foks, A.C.; Lichtman, A.H.; Kuiper, J. Treating Atherosclerosis with Regulatory T Cells. Arterioscler Thromb Vasc Biol 2015, 35, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Freuchet, A.; Roy, P.; Armstrong, S.S.; Oliaeimotlagh, M.; Kumar, S.; Orecchioni, M.; Ali, A.J.; Khan, A.; Makings, J.; Lyu, Q.; et al. Identification of Human exTreg Cells as CD16+CD56+ Cytotoxic CD4+ T Cells. Nat Immunol 2023, 24, 1748–1761. [Google Scholar] [CrossRef]
- Gaddis, D.E.; Padgett, L.E.; Wu, R.; McSkimming, C.; Romines, V.; Taylor, A.M.; McNamara, C.A.; Kronenberg, M.; Crotty, S.; Thomas, M.J.; et al. Apolipoprotein AI Prevents Regulatory to Follicular Helper T Cell Switching during Atherosclerosis. Nat Commun 2018, 9, 1095. [Google Scholar] [CrossRef]
- Kyaw, T.; Winship, A.; Tay, C.; Kanellakis, P.; Hosseini, H.; Cao, A.; Li, P.; Tipping, P.; Bobik, A.; Toh, B.-H. Cytotoxic and Proinflammatory CD8+ T Lymphocytes Promote Development of Vulnerable Atherosclerotic Plaques in apoE-Deficient Mice. Circulation 2013, 127, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Sage, A.P.; Tsiantoulas, D.; Binder, C.J.; Mallat, Z. The Role of B Cells in Atherosclerosis. Nat Rev Cardiol 2019, 16, 180–196. [Google Scholar] [CrossRef]
- Kyaw, T.; Tipping, P.; Bobik, A.; Toh, B.-H. Protective Role of Natural IgM-Producing B1a Cells in Atherosclerosis. Trends Cardiovasc Med 2012, 22, 48–53. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ Res 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Zheng, X.; Xie, G.; Zhao, A.; Zhao, L.; Yao, C.; Chiu, N.H.L.; Zhou, Z.; Bao, Y.; Jia, W.; Nicholson, J.K.; et al. The Footprints of Gut Microbial-Mammalian Co-Metabolism. J Proteome Res 2011, 10, 5512–5522. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Chen, Y.; Smith, T.C.; Karna, S.L.R.; Seshu, J. Short-Chain Fatty Acids Alter Metabolic and Virulence Attributes of Borrelia Burgdorferi. Infect Immun 2018, 86, e00217-18. [Google Scholar] [CrossRef] [PubMed]
- Y, M.; Ja, G.; C.-T., L; Bc, O.; E, J.; Ej, S.; W, L.; Sl, P.; Ar, H.; Is, O. Association between Dietary Fiber and Serum C-Reactive Protein. The American journal of clinical nutrition 2006, 83. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab 2017, 26, 110–130. [Google Scholar] [CrossRef]
- Uchimura, Y.; Fuhrer, T.; Li, H.; Lawson, M.A.; Zimmermann, M.; Yilmaz, B.; Zindel, J.; Ronchi, F.; Sorribas, M.; Hapfelmeier, S.; et al. Antibodies Set Boundaries Limiting Microbial Metabolite Penetration and the Resultant Mammalian Host Response. Immunity 2018, 49, 545–559.e5. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the Trimethylamine-Producing Bacteria of the Human Gut Microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef]
- Craciun, S.; Balskus, E.P. Microbial Conversion of Choline to Trimethylamine Requires a Glycyl Radical Enzyme. Proc Natl Acad Sci U S A 2012, 109, 21307–21312. [Google Scholar] [CrossRef]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. γ-Butyrobetaine Is a Proatherogenic Intermediate in Gut Microbial Metabolism of L-Carnitine to TMAO. Cell Metab 2014, 20, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu Rev Nutr 2017, 37, 157–181. [Google Scholar] [CrossRef]
- G, F.; V.-S., S; J, R. Microbiology Meets Big Data: The Case of Gut Microbiota-Derived Trimethylamine. Annual review of microbiology 2015, 69. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab Dispos 2016, 44, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- C.-G., J; A, G.; V, S.; N, P.; B, B.; A, S. The Complex Metabolism of Trimethylamine in Humans: Endogenous and Exogenous Sources. Expert reviews in molecular medicine 2016, 18. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Trøseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjørndal, B.; Halvorsen, B.; et al. Microbiota-Dependent Metabolite Trimethylamine-N-Oxide Is Associated with Disease Severity and Survival of Patients with Chronic Heart Failure. J Intern Med 2015, 277, 717–726. [Google Scholar] [CrossRef]
- K, C.; X, Z.; M, F.; D, L.; H, Z. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Frontiers in physiology 2017, 8. [Google Scholar] [CrossRef]
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-Oxide in Atherogenesis: Impairing Endothelial Self-Repair Capacity and Enhancing Monocyte Adhesion. Biosci Rep 2017, 37, BSR20160244. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J Am Heart Assoc 2016, 5, e002767. [Google Scholar] [CrossRef]
- X, C.; X, Q.; Y, L.; C, Y.; X, Y. Trimethylamine N-Oxide Promotes Tissue Factor Expression and Activity in Vascular Endothelial Cells: A New Link between Trimethylamine N-Oxide and Atherosclerotic Thrombosis. Thrombosis research 2019, 177. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Dzutsev, A.; Aghayev, T.; McCulloch, J.A.; Thovarai, V.; Badger, J.H.; Vats, R.; Sundd, P.; Tang, H.-Y.; et al. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity 2018, 49, 943–957.e9. [Google Scholar] [CrossRef]
- Buonocore, S.; Ahern, P.P.; Uhlig, H.H.; Ivanov, I.I.; Littman, D.R.; Maloy, K.J.; Powrie, F. Innate Lymphoid Cells Drive Interleukin-23-Dependent Innate Intestinal Pathology. Nature 2010, 464, 1371–1375. [Google Scholar] [CrossRef]
- Peshkova, I.O.; Schaefer, G.; Koltsova, E.K. Atherosclerosis and Aortic Aneurysm - Is Inflammation a Common Denominator? FEBS J 2016, 283, 1636–1652. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaide, M.A.I.; Singh, R.; Datta, P.; Rewers-Felkins, K.A.; Salguero, M.V.; Al-Obaidi, I.; Kottapalli, K.R.; Vasylyeva, T.L. Gut Microbiota-Dependent Trimethylamine-N-Oxide and Serum Biomarkers in Patients with T2DM and Advanced CKD. J Clin Med 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Feskens, E.J.M.; Boer, J.M.A.; Müller, M. The Potential Influence of Genetic Variants in Genes along Bile Acid and Bile Metabolic Pathway on Blood Cholesterol Levels in the Population. Atherosclerosis 2010, 210, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Ra, K.; Z, W.; Bs, L.; Ja, B.; E, O.; Bt, S.; Eb, B.; X, F.; Y, W.; L, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nature medicine 2013, 19. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-Lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- J, G.; C, Y.; B, W.; X, Z.; T, H.; Y, G.; J, L. Trimethylamine N-Oxide Promotes Atherosclerosis via CD36-Dependent MAPK/JNK Pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2018, 97. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.; Li, X.; Mehta, J.L.; Wang, X. Role of NLRP3 Inflammasome in the Pathogenesis of Cardiovascular Diseases. Basic Res Cardiol 2017, 113, 5. [Google Scholar] [CrossRef]
- X, S.; X, J.; Y, M.; Y, L.; L, Z.; Y, H.; Y, C. Trimethylamine N-Oxide Induces Inflammation and Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells via Activating ROS-TXNIP-NLRP3 Inflammasome. Biochemical and biophysical research communications 2016, 481. [Google Scholar] [CrossRef]
- Yue, C.; Yang, X.; Li, J.; Chen, X.; Zhao, X.; Chen, Y.; Wen, Y. Trimethylamine N-Oxide Prime NLRP3 Inflammasome via Inhibiting ATG16L1-Induced Autophagy in Colonic Epithelial Cells. Biochem Biophys Res Commun 2017, 490, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Kränkel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arterioscler Thromb Vasc Biol 2018, 38, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Clish, C.B.; Hua, S.; Scott, J.M.; Hanna, D.B.; Burk, R.D.; Haberlen, S.A.; Shah, S.J.; Margolick, J.B.; Sears, C.L.; et al. Gut Microbial-Related Choline Metabolite Trimethylamine-N-Oxide Is Associated With Progression of Carotid Artery Atherosclerosis in HIV Infection. J Infect Dis 2018, 218, 1474–1479. [Google Scholar] [CrossRef]
- Haissman, J.M.; Haugaard, A.K.; Ostrowski, S.R.; Berge, R.K.; Hov, J.R.; Trøseid, M.; Nielsen, S.D. Microbiota-Dependent Metabolite and Cardiovascular Disease Marker Trimethylamine-N-Oxide (TMAO) Is Associated with Monocyte Activation but Not Platelet Function in Untreated HIV Infection. BMC Infect Dis 2017, 17, 445. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-Oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J Nutr 2016, 146, 283–289. [Google Scholar] [CrossRef]
- Chou, R.-H.; Chen, C.-Y.; Chen, I.-C.; Huang, H.-L.; Lu, Y.-W.; Kuo, C.-S.; Chang, C.-C.; Huang, P.-H.; Chen, J.-W.; Lin, S.-J. Trimethylamine N-Oxide, Circulating Endothelial Progenitor Cells, and Endothelial Function in Patients with Stable Angina. Sci Rep 2019, 9, 4249. [Google Scholar] [CrossRef]
- Burke-Gaffney, A.; Brooks, A.V.S.; Bogle, R.G. Regulation of Chemokine Expression in Atherosclerosis. Vascul Pharmacol 2002, 38, 283–292. [Google Scholar] [CrossRef]
- B, D.; V.O., L; B, V.; K, V. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nature reviews. Gastroenterology & hepatology 2019, 16. [Google Scholar] [CrossRef]
- Jh, C.; Ew, P.; Wj, B.; Cp, N.; Gt, M. Short Chain Fatty Acids in Human Large Intestine, Portal, Hepatic and Venous Blood. Gut 1987, 28. [Google Scholar] [CrossRef] [PubMed]
- Soldavini, J.; Kaunitz, J.D. Pathobiology and Potential Therapeutic Value of Intestinal Short-Chain Fatty Acids in Gut Inflammation and Obesity. Dig Dis Sci 2013, 58, 2756–2766. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J Lipid Res 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Colon Microbiota Fermentation of Dietary Prebiotics towards Short-Chain Fatty Acids and Their Roles as Anti-Inflammatory and Antitumour Agents: A Review. Journal of Functional Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J Atheroscler Thromb 2017, 24, 660–672. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- D, B.; Ab, T.; G, M.; Ce, M. The Pharmacology and Function of Receptors for Short-Chain Fatty Acids. Molecular pharmacology 2016, 89. [Google Scholar] [CrossRef]
- Pluznick, J.L. Gut Microbiota in Renal Physiology: Focus on Short-Chain Fatty Acids and Their Receptors. Kidney Int 2016, 90, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation - Protective or Causative? Front Immunol 2016, 7, 28. [Google Scholar] [CrossRef]
- L, A.; M, N.; O, B.; A, P.; L, A.; L, D.; T, M.; X, Z.; I, E.; L, M. Short Chain Fatty Acids (SCFA) Reprogram Gene Expression in Human Malignant Epithelial and Lymphoid Cells. PloS one 2016, 11. [Google Scholar] [CrossRef]
- G, T.; H, H.; Ys, L.; He, P.; Am, H.; E, D.; J, C.; J, G.; F, R.; Fm, G. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61. [Google Scholar] [CrossRef]
- Caylak, E. Anorexigenic Peptides in Health and Disease. In eLS; John Wiley & Sons, Ltd, 2012; ISBN 978-0-470-01590-2. [Google Scholar]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Evidence for the Gut Microbiota Short-Chain Fatty Acids as Key Pathophysiological Molecules Improving Diabetes. Mediators Inflamm 2014, 2014, 162021. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Bs, S.; A, S.; T, M.; Fe, R.; F, B.; Jk, M.; Re, H.; Sc, W.; J, C.; M, Y.; et al. Effects of the Gut Microbiota on Host Adiposity Are Modulated by the Short-Chain Fatty-Acid Binding G Protein-Coupled Receptor, Gpr41. Proceedings of the National Academy of Sciences of the United States of America 2008, 105. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The Gut Microbiota Suppresses Insulin-Mediated Fat Accumulation via the Short-Chain Fatty Acid Receptor GPR43. Nat Commun 2013, 4, 1829. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-Chain Fatty Acids and Ketones Directly Regulate Sympathetic Nervous System via G Protein-Coupled Receptor 41 (GPR41). Proceedings of the National Academy of Sciences 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Jl, P.; Rj, P.; H, G.; Z, P.; A, S.; J, H.; I, B.; Lx, W.; F, R.; T, W.; et al. Olfactory Receptor Responding to Gut Microbiota-Derived Signals Plays a Role in Renin Secretion and Blood Pressure Regulation. Proceedings of the National Academy of Sciences of the United States of America 2013, 110. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat Commun 2015, 6, 6734. [Google Scholar] [CrossRef]
- W, Y.; T, Y.; X, H.; Aj, B.; L, X.; Y, L.; J, S.; F, P.; J, Z.; W, Z.; et al. Intestinal Microbiota-Derived Short-Chain Fatty Acids Regulation of Immune Cell IL-22 Production and Gut Immunity. Nature communications 2020, 11. [Google Scholar] [CrossRef]
- Me, K.; R, S.; Aj, N.; Ac, O.; Lj, M.; L, B.; Cr, M.; Ch, W. G Protein-Coupled Receptor 43 Modulates Neutrophil Recruitment during Acute Inflammation. PloS one 2016, 11. [Google Scholar] [CrossRef]
- Singh, N.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Lambert, N.A.; Boettger, T.; Offermanns, S.; Ganapathy, V. Blockade of Dendritic Cell Development by Bacterial Fermentation Products Butyrate and Propionate through a Transporter (Slc5a8)-Dependent Inhibition of Histone Deacetylases. J Biol Chem 2010, 285, 27601–27608. [Google Scholar] [CrossRef] [PubMed]
- Alenghat, T.; Artis, D. Epigenomic Regulation of Host-Microbiota Interactions. Trends Immunol 2014, 35, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The Microbiome and Innate Immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of Inflammation by Microbiota Interactions with the Host. Nat Immunol 2017, 18, 851–860. [Google Scholar] [CrossRef]
- Kaisar, M.M.M.; Pelgrom, L.R.; van der Ham, A.J.; Yazdanbakhsh, M.; Everts, B. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells via Both Histone Deacetylase Inhibition and G Protein-Coupled Receptor 109A Signaling. Front Immunol 2017, 8, 1429. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut Microbiota Metabolism of Dietary Fiber Influences Allergic Airway Disease and Hematopoiesis. Nat Med 2014, 20, 159–166. [Google Scholar] [CrossRef]
- N, A.; C, C.; X, F.; S, D.; van der V., J; deRoos, P; H, L.; Jr, C.; K, P.; Pj, C.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504. [Google Scholar] [CrossRef]
- T, A.; G, G.; T, G.; T, M.; L, W.; Jl, R.; Ww, H. Histone/Protein Deacetylase Inhibitors Increase Suppressive Functions of Human FOXP3+ Tregs. Clinical immunology (Orlando, Fla.) 2010, 136. [Google Scholar] [CrossRef]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B Cell-Intrinsic Epigenetic Modulation of Antibody Responses by Dietary Fiber-Derived Short-Chain Fatty Acids. Nat Commun 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Busnelli, M.; Manzini, S.; Chiesa, G. The Gut Microbiota Affects Host Pathophysiology as an Endocrine Organ: A Focus on Cardiovascular Disease. Nutrients 2019, 12, 79. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.M.; Marchesi, J.R. Functional and Comparative Metagenomic Analysis of Bile Salt Hydrolase Activity in the Human Gut Microbiome. Proc Natl Acad Sci U S A 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chiu, C.; Hung, S.; Huang, W.-C.; Lee, Y.-P.; Liu, J.-Y.; Huang, Y.-T.; Chen, T.; Chuang, H. Gnotobiotic Mice Inoculated with Firmicutes, but Not Bacteroidetes, Deteriorate Nonalcoholic Fatty Liver Disease Severity by Modulating Hepatic Lipid Metabolism. Nutrition research 2019, 69, 20–29. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Bajaj, J.S. The Human Gut Sterolbiome: Bile Acid-Microbiome Endocrine Aspects and Therapeutics. Acta Pharm Sin B 2015, 5, 99–105. [Google Scholar] [CrossRef]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision Microbiome Reconstitution Restores Bile Acid Mediated Resistance to Clostridium Difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef] [PubMed]
- N, M.; L, R.; B, S.; A, M.; S, D. Intestinal Bacteria Interplay With Bile and Cholesterol Metabolism: Implications on Host Physiology. Frontiers in physiology 2019, 10. [Google Scholar] [CrossRef]
- Branchereau, M.; Burcelin, R.; Heymes, C. The Gut Microbiome and Heart Failure: A Better Gut for a Better Heart. Rev Endocr Metab Disord 2019, 20, 407–414. [Google Scholar] [CrossRef]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of Bile Acids and Bile Acid Receptors in Metabolic Regulation. Physiol Rev 2009, 89, 147–191. [Google Scholar] [CrossRef]
- Hanafi, N.I.; Mohamed, A.S.; Sheikh Abdul Kadir, S.H.; Othman, M.H.D. Overview of Bile Acids Signaling and Perspective on the Signal of Ursodeoxycholic Acid, the Most Hydrophilic Bile Acid, in the Heart. Biomolecules 2018, 8, 159. [Google Scholar] [CrossRef]
- Dossa, A.Y.; Escobar, O.; Golden, J.; Frey, M.R.; Ford, H.R.; Gayer, C.P. Bile Acids Regulate Intestinal Cell Proliferation by Modulating EGFR and FXR Signaling. Am J Physiol Gastrointest Liver Physiol 2016, 310, G81–92. [Google Scholar] [CrossRef]
- H, Z.; S, U.; B, R.; D, L.; M, B. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. International journal of molecular sciences 2019, 20. [Google Scholar] [CrossRef]
- Parker, H.E.; Wallis, K.; le Roux, C.W.; Wong, K.Y.; Reimann, F.; Gribble, F.M. Molecular Mechanisms Underlying Bile Acid-Stimulated Glucagon-like Peptide-1 Secretion. Br J Pharmacol 2012, 165, 414–423. [Google Scholar] [CrossRef]
- Wammers, M.; Schupp, A.-K.; Bode, J.G.; Ehlting, C.; Wolf, S.; Deenen, R.; Köhrer, K.; Häussinger, D.; Graf, D. Reprogramming of Pro-Inflammatory Human Macrophages to an Anti-Inflammatory Phenotype by Bile Acids. Sci Rep 2018, 8, 255. [Google Scholar] [CrossRef]
- Guo, C.; Xie, S.; Chi, Z.; Zhang, J.; Liu, Y.; Zhang, L.; Zheng, M.; Zhang, X.; Xia, D.; Ke, Y.; et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity 2016, 45, 802–816. [Google Scholar] [CrossRef]
- Pols, T.W.H.; Puchner, T.; Korkmaz, H.I.; Vos, M.; Soeters, M.R.; de Vries, C.J.M. Lithocholic Acid Controls Adaptive Immune Responses by Inhibition of Th1 Activation through the Vitamin D Receptor. PLoS One 2017, 12, e0176715. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu Rev Biochem 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Pj, T.; Re, L.; M, H.; Cm, F.-L.; R, K.; Ji, G. The Human Microbiome Project. Nature 2007, 449. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb Ecol Health Dis 2015, 26, 26191. [Google Scholar] [CrossRef]
- Hb, S.; Ke, W.-W.; M, C.; L, T.; Ca, P. Lipopolysaccharide Induces Oxidative Cardiac Mitochondrial Damage and Biogenesis. Cardiovascular research 2004, 64. [Google Scholar] [CrossRef]
- Ll, S.; Gm, D.; Nl, W. Potential Role of Endotoxin as a Proinflammatory Mediator of Atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 2004, 24. [Google Scholar] [CrossRef]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a Receptor for Complexes of Lipopolysaccharide (LPS) and LPS Binding Protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 Signal Transduction Pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- M.B., F; Jg, V.; N, R. Toll-like Receptor Signaling Pathways in Cardiovascular Diseases: Challenges and Opportunities. International reviews of immunology 2012, 31. [Google Scholar] [CrossRef]
- Blomkalns, A.L.; Stoll, L.L.; Shaheen, W.; Romig-Martin, S.A.; Dickson, E.W.; Weintraub, N.L.; Denning, G.M. Low Level Bacterial Endotoxin Activates Two Distinct Signaling Pathways in Human Peripheral Blood Mononuclear Cells. J Inflamm (Lond) 2011, 8, 4. [Google Scholar] [CrossRef]
- Tan, Y.; Kagan, J.C. A Cross-Disciplinary Perspective on the Innate Immune Responses to Bacterial Lipopolysaccharide. Mol Cell 2014, 54, 212–223. [Google Scholar] [CrossRef]
- Jankowska, E.A.; von Haehling, S.; Czarny, A.; Zaczynska, E.; Kus, A.; Anker, S.D.; Banasiak, W.; Ponikowski, P. Activation of the NF-kappaB System in Peripheral Blood Leukocytes from Patients with Chronic Heart Failure. Eur J Heart Fail 2005, 7, 984–990. [Google Scholar] [CrossRef]
- Földes, G.; von Haehling, S.; Okonko, D.O.; Jankowska, E.A.; Poole-Wilson, P.A.; Anker, S.D. Fluvastatin Reduces Increased Blood Monocyte Toll-like Receptor 4 Expression in Whole Blood from Patients with Chronic Heart Failure. Int J Cardiol 2008, 124, 80–85. [Google Scholar] [CrossRef]
- Kuwahata, S.; Fujita, S.; Orihara, K.; Hamasaki, S.; Oba, R.; Hirai, H.; Nagata, K.; Ishida, S.; Kataoka, T.; Oketani, N.; et al. High Expression Level of Toll-like Receptor 2 on Monocytes Is an Important Risk Factor for Arteriosclerotic Disease. Atherosclerosis 2010, 209, 248–254. [Google Scholar] [CrossRef]
- Torre-Amione, G.; Kapadia, S.; Benedict, C.; Oral, H.; Young, J.B.; Mann, D.L. Proinflammatory Cytokine Levels in Patients with Depressed Left Ventricular Ejection Fraction: A Report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 1996, 27, 1201–1206. [Google Scholar] [CrossRef]
- Ohlsson, B.G.; Englund, M.C.; Karlsson, A.L.; Knutsen, E.; Erixon, C.; Skribeck, H.; Liu, Y.; Bondjers, G.; Wiklund, O. Oxidized Low Density Lipoprotein Inhibits Lipopolysaccharide-Induced Binding of Nuclear Factor-kappaB to DNA and the Subsequent Expression of Tumor Necrosis Factor-Alpha and Interleukin-1beta in Macrophages. J Clin Invest 1996, 98, 78–89. [Google Scholar] [CrossRef]
- B, J.; Ys, B.; D, P.; Cs, S.; K, G.; Bs, L.; V, M.; M, B. Increased Circulatory Levels of Lipopolysaccharide (LPS) and Zonulin Signify Novel Biomarkers of Proinflammation in Patients with Type 2 Diabetes. Molecular and cellular biochemistry 2014, 388. [Google Scholar] [CrossRef]
- Sanz, Y.; Santacruz, A.; Palma, G.D. Insights into the Roles of Gut Microbes in Obesity. Interdisciplinary Perspectives on Infectious Diseases 2008. [Google Scholar] [CrossRef]
- Am, B.; Hw, H. Triglyceride-Rich Lipoproteins as Agents of Innate Immunity. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2005, 41 Suppl 7. [Google Scholar] [CrossRef]
- Larsen, C.G.; Anderson, A.O.; Appella, E.; Oppenheim, J.J.; Matsushima, K. The Neutrophil-Activating Protein (NAP-1) Is Also Chemotactic for T Lymphocytes. Science 1989, 243, 1464–1466. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, Its Components, and Cardiovascular Disease. Am J Med 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med 2018, 378, e34. [Google Scholar] [CrossRef]
- Tørris, C.; Molin, M.; Cvancarova Småstuen, M. Fish Consumption and Its Possible Preventive Role on the Development and Prevalence of Metabolic Syndrome - a Systematic Review. Diabetol Metab Syndr 2014, 6, 112. [Google Scholar] [CrossRef]
- Jimenez-Torres, J.; Alcalá-Diaz, J.F.; Torres-Peña, J.D.; Gutierrez-Mariscal, F.M.; Leon-Acuña, A.; Gómez-Luna, P.; Fernández-Gandara, C.; Quintana-Navarro, G.M.; Fernandez-Garcia, J.C.; Perez-Martinez, P.; et al. Mediterranean Diet Reduces Atherosclerosis Progression in Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. Stroke 2021, 52, 3440–3449. [Google Scholar] [CrossRef]
- Griffin, L.E.; Djuric, Z.; Angiletta, C.J.; Mitchell, C.M.; Baugh, M.E.; Davy, K.P.; Neilson, A.P. A Mediterranean Diet Does Not Alter Plasma Trimethylamine N-Oxide Concentrations in Healthy Adults at Risk for Colon Cancer. Food Funct 2019, 10, 2138–2147. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-Oxide, Mediterranean Diet, and Nutrition in Healthy, Normal-Weight Adults: Also a Matter of Sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; Graziadio, C.; Maisto, M.; Pivari, F.; Falco, A.; Tenore, G.C.; Colao, A.; Savastano, S. Association of the Chronotype Score with Circulating Trimethylamine N-Oxide (TMAO) Concentrations. Nutrients 2021, 13, 1671. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Yamagata, K.; Hashiguchi, K.; Yamamoto, H.; Tagami, M. Dietary Apigenin Reduces Induction of LOX-1 and NLRP3 Expression, Leukocyte Adhesion, and Acetylated Low-Density Lipoprotein Uptake in Human Endothelial Cells Exposed to Trimethylamine-N-Oxide. J Cardiovasc Pharmacol 2019, 74, 558–565. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med 2018, 378, e34. [Google Scholar] [CrossRef]
- Choo, J.M.; Murphy, K.J.; Wade, A.T.; Wang, Y.; Bracci, E.L.; Davis, C.R.; Dyer, K.A.; Woodman, R.J.; Hodgson, J.M.; Rogers, G.B. Interactions between Mediterranean Diet Supplemented with Dairy Foods and the Gut Microbiota Influence Cardiovascular Health in an Australian Population. Nutrients 2023, 15, 3645. [Google Scholar] [CrossRef]
- Ma, W.; Nguyen, L.H.; Song, M.; Wang, D.D.; Franzosa, E.A.; Cao, Y.; Joshi, A.; Drew, D.A.; Mehta, R.; Ivey, K.L.; et al. Dietary Fiber Intake, the Gut Microbiome, and Chronic Systemic Inflammation in a Cohort of Adult Men. Genome Med 2021, 13, 102. [Google Scholar] [CrossRef]
- Murga-Garrido, S.M.; Hong, Q.; Cross, T.-W.L.; Hutchison, E.R.; Han, J.; Thomas, S.P.; Vivas, E.I.; Denu, J.; Ceschin, D.G.; Tang, Z.-Z.; et al. Gut Microbiome Variation Modulates the Effects of Dietary Fiber on Host Metabolism. Microbiome 2021, 9, 117. [Google Scholar] [CrossRef]
- Catry, E.; Bindels, L.B.; Tailleux, A.; Lestavel, S.; Neyrinck, A.M.; Goossens, J.-F.; Lobysheva, I.; Plovier, H.; Essaghir, A.; Demoulin, J.-B.; et al. Targeting the Gut Microbiota with Inulin-Type Fructans: Preclinical Demonstration of a Novel Approach in the Management of Endothelial Dysfunction. Gut 2018, 67, 271–283. [Google Scholar] [CrossRef]
- Liu, F.; Prabhakar, M.; Ju, J.; Long, H.; Zhou, H.-W. Effect of Inulin-Type Fructans on Blood Lipid Profile and Glucose Level: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur J Clin Nutr 2017, 71, 9–20. [Google Scholar] [CrossRef]
- Cosola, C.; De Angelis, M.; Rocchetti, M.T.; Montemurno, E.; Maranzano, V.; Dalfino, G.; Manno, C.; Zito, A.; Gesualdo, M.; Ciccone, M.M.; et al. Beta-Glucans Supplementation Associates with Reduction in P-Cresyl Sulfate Levels and Improved Endothelial Vascular Reactivity in Healthy Individuals. PLoS One 2017, 12, e0169635. [Google Scholar] [CrossRef]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary Running Exercise Alters Microbiota Composition and Increases N-Butyrate Concentration in the Rat Cecum. Biosci Biotechnol Biochem 2008, 72, 572–576. [Google Scholar] [CrossRef]
- Varghese, S.; Rao, S.; Khattak, A.; Zamir, F.; Chaari, A. Physical Exercise and the Gut Microbiome: A Bidirectional Relationship Influencing Health and Performance. Nutrients 2024, 16, 3663. [Google Scholar] [CrossRef]
- Lira, F.S.; Rosa, J.C.; Pimentel, G.D.; Souza, H.A.; Caperuto, E.C.; Carnevali, L.C.; Seelaender, M.; Damaso, A.R.; Oyama, L.M.; de Mello, M.T.; et al. Endotoxin Levels Correlate Positively with a Sedentary Lifestyle and Negatively with Highly Trained Subjects. Lipids Health Dis 2010, 9, 82. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, L.; Chen, L.; Li, L. Therapeutic Potential of Traditional Chinese Medicine in Atherosclerosis: A Review. Phytother Res 2022, 36, 4080–4100. [Google Scholar] [CrossRef]
- Jing, J.; Guo, J.; Dai, R.; Zhu, C.; Zhang, Z. Targeting Gut Microbiota and Immune Crosstalk: Potential Mechanisms of Natural Products in the Treatment of Atherosclerosis. Front Pharmacol 2023, 14, 1252907. [Google Scholar] [CrossRef]
- Liu, S.; He, F.; Zheng, T.; Wan, S.; Chen, J.; Yang, F.; Xu, X.; Pei, X. Ligustrum Robustum Alleviates Atherosclerosis by Decreasing Serum TMAO, Modulating Gut Microbiota, and Decreasing Bile Acid and Cholesterol Absorption in Mice. Mol Nutr Food Res 2021, 65, e2100014. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat Rev Gastroenterol Hepatol 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Ghanbari, F.; Hasani, S.; Aghili, Z.S.; Asgary, S. The Potential Preventive Effect of Probiotics, Prebiotics, and Synbiotics on Cardiovascular Risk Factors through Modulation of Gut Microbiota: A Review. Food Sci Nutr 2024, 12, 4569–4580. [Google Scholar] [CrossRef]
- O’Morain, V.L.; Ramji, D.P. The Potential of Probiotics in the Prevention and Treatment of Atherosclerosis. Mol Nutr Food Res 2020, 64, e1900797. [Google Scholar] [CrossRef]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus Plantarum ZDY04 Exhibits a Strain-Specific Property of Lowering TMAO via the Modulation of Gut Microbiota in Mice. Food Funct 2018, 9, 4299–4309. [Google Scholar] [CrossRef]
- Moludi, J.; Kafil, H.S.; Qaisar, S.A.; Gholizadeh, P.; Alizadeh, M.; Vayghyan, H.J. Effect of Probiotic Supplementation along with Calorie Restriction on Metabolic Endotoxemia, and Inflammation Markers in Coronary Artery Disease Patients: A Double Blind Placebo Controlled Randomized Clinical Trial. Nutr J 2021, 20, 47. [Google Scholar] [CrossRef]
- de Araújo Henriques Ferreira, G.; Magnani, M.; Cabral, L.; Brandão, L.R.; Noronha, M.F.; de Campos Cruz, J.; de Souza, E.L.; de Brito Alves, J.L. Potentially Probiotic Limosilactobacillus Fermentum Fruit-Derived Strains Alleviate Cardiometabolic Disorders and Gut Microbiota Impairment in Male Rats Fed a High-Fat Diet. Probiotics Antimicrob Proteins 2022, 14, 349–359. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus Rhamnosus GG Derived Extracellular Vesicles Modulate Gut Microbiota and Attenuate Inflammatory in DSS-Induced Colitis Mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, M.; Liu, Y.; Xu, M.; Shi, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Bifidobacterium Breve and Bifidobacterium Longum Attenuate Choline-Induced Plasma Trimethylamine N-Oxide Production by Modulating Gut Microbiota in Mice. Nutrients 2022, 14, 1222. [Google Scholar] [CrossRef]
- Yang, D.; Lyu, W.; Hu, Z.; Gao, J.; Zheng, Z.; Wang, W.; Firrman, J.; Ren, D. Probiotic Effects of Lactobacillus Fermentum ZJUIDS06 and Lactobacillus Plantarum ZY08 on Hypercholesteremic Golden Hamsters. Front Nutr 2021, 8, 705763. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Li, X.; Zhang, T.; Liang, M.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus Reuteri CCFM8631 Alleviates Hypercholesterolaemia Caused by the Paigen Atherogenic Diet by Regulating the Gut Microbiota. Nutrients 2022, 14, 1272. [Google Scholar] [CrossRef]
- Pham, Q.H.; Bui, T.V.A.; Sim, W.-S.; Lim, K.H.; Law, C.O.K.; Tan, W.; Kim, R.Y.; Chow, K.T.; Park, H.-J.; Ban, K.; et al. Daily Oral Administration of Probiotics Engineered to Constantly Secrete Short-Chain Fatty Acids Effectively Prevents Myocardial Injury from Subsequent Ischaemic Heart Disease. Cardiovasc Res 2024, 120, 1737–1751. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia Muciniphila Inversely Correlates with the Onset of Inflammation, Altered Adipose Tissue Metabolism and Metabolic Disorders during Obesity in Mice. Sci Rep 2015, 5, 16643. [Google Scholar] [CrossRef]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides Vulgatus and Bacteroides Dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Inagawa, H.; Kohchi, C.; Kazumura, K.; Tsuchiya, H.; Miwa, T.; Okazaki, K.; Soma, G.-I. Oral Administration of Pantoea Agglomerans-Derived Lipopolysaccharide Prevents Development of Atherosclerosis in High-Fat Diet-Fed apoE-Deficient Mice via Ameliorating Hyperlipidemia, pro-Inflammatory Mediators and Oxidative Responses. PLoS One 2018, 13, e0195008. [Google Scholar] [CrossRef]
- Kuka, J.; Liepinsh, E.; Makrecka-Kuka, M.; Liepins, J.; Cirule, H.; Gustina, D.; Loza, E.; Zharkova-Malkova, O.; Grinberga, S.; Pugovics, O.; et al. Suppression of Intestinal Microbiota-Dependent Production of pro-Atherogenic Trimethylamine N-Oxide by Shifting L-Carnitine Microbial Degradation. Life Sciences 2014, 117, 84–92. [Google Scholar] [CrossRef]
- Sethi, N.J.; Safi, S.; Korang, S.K.; Hróbjartsson, A.; Skoog, M.; Gluud, C.; Jakobsen, J.C. Antibiotics for Secondary Prevention of Coronary Heart Disease. Cochrane Database Syst Rev 2021, 2, CD003610. [Google Scholar] [CrossRef]
- Heianza, Y.; Zheng, Y.; Ma, W.; Rimm, E.B.; Albert, C.M.; Hu, F.B.; Rexrode, K.M.; Manson, J.E.; Qi, L. Duration and Life-Stage of Antibiotic Use and Risk of Cardiovascular Events in Women. Eur Heart J 2019, 40, 3838–3845. [Google Scholar] [CrossRef]
- Vrieze, A.; Nood, E.V.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, H.; Xiang, Q.; Shen, L.; Guo, X.; Zhai, C.; Hu, H. Targeting Trimethylamine N-Oxide: A New Therapeutic Strategy for Alleviating Atherosclerosis. Front Cardiovasc Med 2022, 9, 864600. [Google Scholar] [CrossRef]
- Lindskog Jonsson, A.; Hållenius, F.F.; Akrami, R.; Johansson, E.; Wester, P.; Arnerlöv, C.; Bäckhed, F.; Bergström, G. Bacterial Profile in Human Atherosclerotic Plaques. Atherosclerosis 2017, 263, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Fåk, F.; Tremaroli, V.; Bergström, G.; Bäckhed, F. Oral Microbiota in Patients with Atherosclerosis. Atherosclerosis 2015, 243, 573–578. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human Oral, Gut, and Plaque Microbiota in Patients with Atherosclerosis. Proceedings of the National Academy of Sciences 2011, 108, 4592–4598. [Google Scholar] [CrossRef] [PubMed]
| SL no. | Diseases | Origin of microbiota | Types of microbiota present | Inference | Reference |
|---|---|---|---|---|---|
| 1. | Coronary heart disease | Oral | Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans | Provide evidence for the presence of viable oral pathogens at the vascular sites and also at the atherosclerotic plaque. | [22] |
| 2. | Coronary Artery Disease | Gut | Phylum Bacteroidetes, Firmicutes | Decrease abundance of phylum Bacteroidetes and increase in phylum Firmicutes in patients with CAD | [13] |
| 3. | Atherosclerotic cardiovascular disease (ACVD) | Oral and Gut | Phylum Proteobacteria, Actinobacteria, Firmicutes, Cyanobacteria and Bacteroidetes. | Proteobacteria (48.3%) and Actinobacteria (40.2%), and three less dominating phyla: Firmicutes (4.0%), Cyanobacteria (3.9%) and Bacteroidetes (2.2%) in both symptomatic and asymptomatic patients |
[253] |
| 4. | Atherosclerotic cardiovascular disease (ACVD) | Oral cavity | Veillonella and Streptococcus | Bacteria from oral cavity may correlate with the disease marker of atherosclerosis. | [15] |
| 5. | Atherosclerotic cardiovascular disease (ACVD) | Gut | Enterobacteriaceae and Streptococcus spp. | Increase abundance of Enterobacteriaceae and Streptococcus spp. in ACVD patients compared to normal. | [12] |
| 6. | Atherosclerotic cardiovascular disease (ACVD) | Gut | Roseburia intestinalis | Provide protection against atherosclerosis | [14] |
| 7. | Atherosclerotic cardiovascular disease (ACVD) | Gut | Akkermansia muciniphila | Provide protection against atherosclerosis | [17] |
| 8. | Cardiovascular disease | Gut | Chlamydia pneumoniae, Staphylococcus spp., Streptococcus spp., Klebsiella pneumoniae, Proteus vulgaris, Burkholderia and Pseudomonas aeruginosa | Associated with CVD progression | [18] |
| 9. | Atherosclerotic cardiovascular disease (ACVD) | Oral and gut | Chlamydia pneumoniae and Helicobacter pylori | Associate with progression of atherosclerosis | [20] |
| 11. | Atherosclerotic cardiovascular disease (ACVD) | Oral | Anaeroglobus | Most abundant oral bacteria present in the symptomatic ACVD patient | [254] |
| 12 | Atherosclerotic cardiovascular disease (ACVD) | Oral | Chryseomonas | May contribute to the progression of Atherosclerosis | [255] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




