1. Introduction
As global health care paradigms increasingly transition from episodic disease treatment to preventive medicine and long-term health management, personalized medicine has emerged as a central trajectory in contemporary biomedicine. Within this shift, technologies that enable continuous, real-time tracking of physiological status are particularly consequential. Wearable devices have consequently expanded within a rapidly growing commercial market, supported by portability and user-centered operation, and now serve as a practical nexus between individual health surveillance and clinical decision-making. Among wearable modalities, electrochemical biosensors have garnered substantial interest because they combine high analytical sensitivity and specificity with rapid response. Relative to conventional benchtop laboratory instrumentation, these systems also offer low cost, compact form factors, and simplified workflows, thereby enabling continuous real-time analysis beyond traditional laboratory settings. Progress in multitechnology biosensing platforms, together with scalable advanced manufacturing, has further advanced the translation of the “lab-on-a-chip” concept into wearable formats capable of capturing health-relevant chemical signals at the molecular level. Despite these favorable prospects, the performance ceiling of wearable health-monitoring devices is often defined by the efficiency and selectivity of biomolecular recognition; inadequate specificity can induce cross-interference in complex matrices, degrade quantitative reliability, and reduce confidence in actionable readouts. Accordingly, continued improvement is expected to hinge on more rigorous coupling of recognition chemistry with electrode/interface engineering, including strategies that preserve bioreceptor function while suppressing nonspecific adsorption under dynamic, on-body conditions. In this regard, rapid advances in nanomaterials have enabled effective integration of nanomaterial-enabled electrochemical transduction with wearable electrodes, strengthening interfacial performance and providing a basis for more precise detection in complex biological fluids.
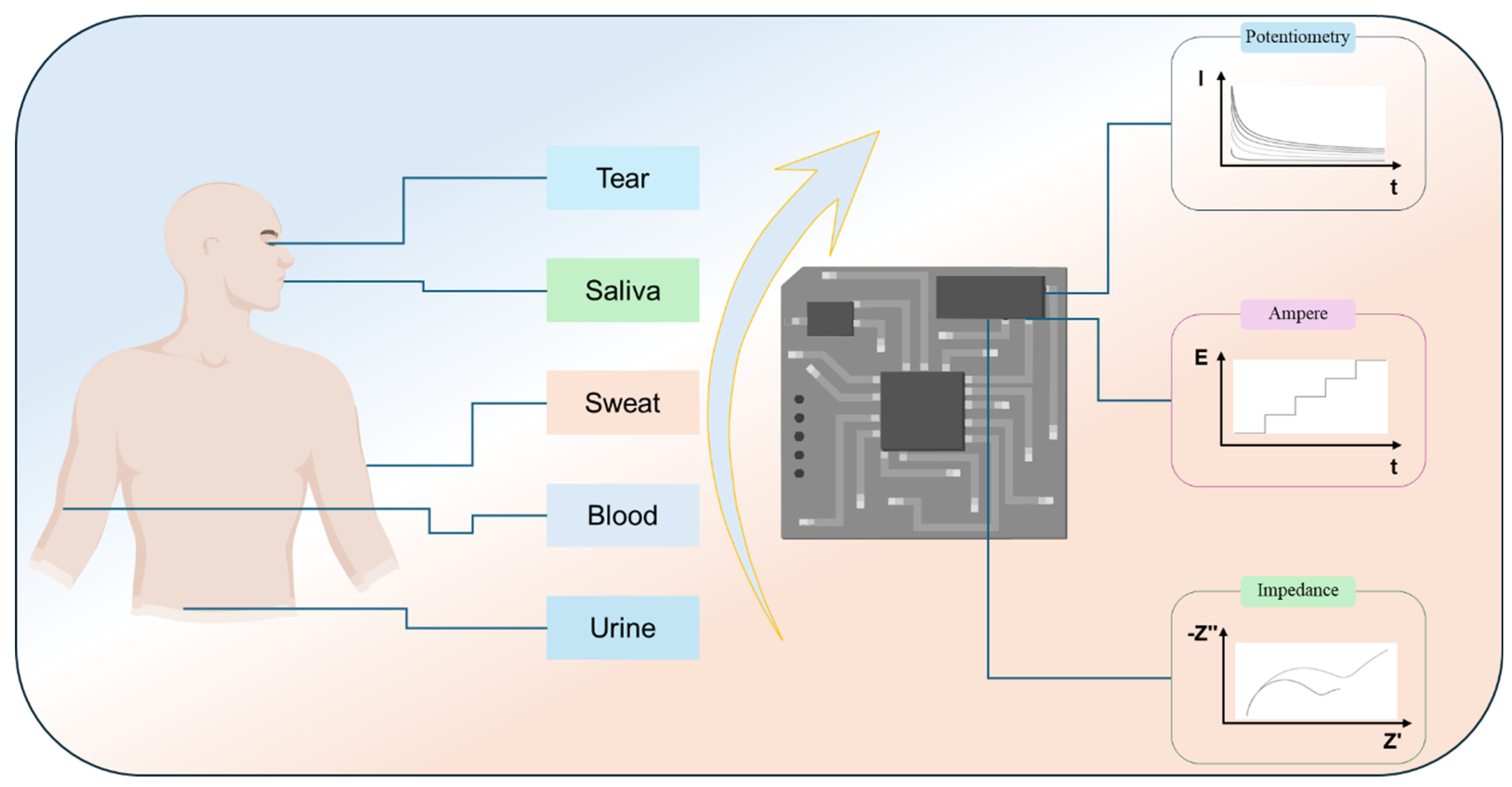
Given this context, this review systematically surveys recent advances in wearable electrochemical biosensors (as shown in
Figure 1). It first outlines biointerface design principles and core electrochemical modalities, including potentiometry, amperometry, and impedance, alongside representative wearable applications in sweat, saliva, and other biological fluids. The discussion then critically examines integrated multitechnology wearable biosensor platforms, emphasizing functional complementarities, practical trade-offs, and development priorities that may accelerate comprehensive health monitoring.
2. Wearable Electrochemical Biosensor Interface
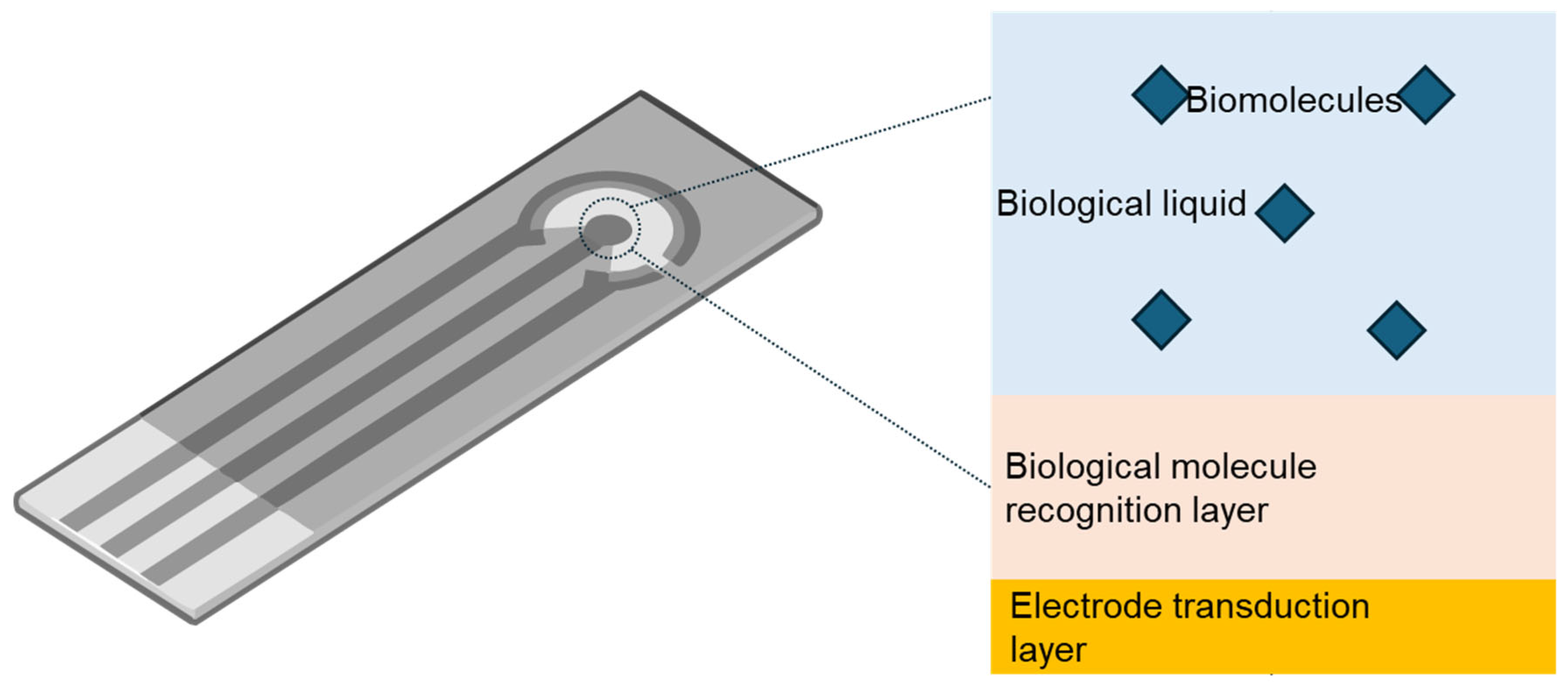
The biointerface engineering of wearable electrochemical biosensors enables highly integrated, miniaturized sensing systems (as shown in
Figure 2) whose core architecture generally comprises four functional elements: target biomolecules, the biological-fluid matrix, the biomolecular recognition interface, and the electrode transduction layer. In this framework, biological fluids such as sweat, tears, saliva, and urine—noninvasive liquid-biopsy media rich in physiological and pathological information—can reduce reliance on frequent invasive blood sampling and provide an in situ environment for interrogating diverse analytes, including ions, small-molecule metabolites (such as vitamin C, glucose), nucleic acids, proteins, and viral pathogens (such as coronaviruses). This capability is central to enabling all-day personalized health surveillance and medical assessment. However, because these matrices are chemically complex, inadequate control of interfacial selectivity and fouling can elevate background responses, distort calibration, and ultimately erode signal-to-noise ratios, thereby constraining quantitative reliability and clinical interpretability. The biomolecular recognition layer therefore functions as the critical front-end determinant of specificity, incorporating biorecognition components—such as nucleic acids, enzymes, antibodies, aptamers, or molecularly imprinted polymers—that selectively pair or bind with analyte molecules to sharpen molecular discrimination. The downstream electrode transduction layer then converts these interfacial recognition events into quantifiable electrical outputs, typically using nanomaterials with high conductivity and electrochemical activity and/or redox probes to enhance charge-transfer efficiency and signal gain. Finally, integrated back-end electronics capture, amplify, and process the signals, followed by wireless transmission and storage, thereby establishing a closed-loop analytical workflow that links biochemical interactions to digital information.
3. Types of Wearable Electrochemical Biological Sensors
The operating principle of wearable electrochemical biosensors is the efficient transduction of specific recognition events at a biointerface into quantifiable electrical outputs through appropriately engineered electrode architectures. In a basic two-electrode arrangement, the working electrode (WE) hosts the electrochemical process and, together with a reference electrode (RE) that defines a potential baseline, completes the circuit. Under an applied excitation voltage, however, the measured response reflects not only the intrinsic impedance of the electrode–electrolyte interface but also the solution resistance arising from ionic migration through the electrolyte. By Ohm’s law, the associated voltage loss (“IR drop”) is superimposed on the commanded potential, shifting the effective potential experienced at the WE. This deviation—further exacerbated by interfacial kinetics and environmental perturbations—constitutes a major source of measurement inaccuracy because it distorts the reaction-driving force and undermines quantitative comparability across conditions. To mitigate these limitations, contemporary electrochemical sensing commonly employs a three-electrode configuration by introducing a counter electrode (CE) to carry current. In this geometry, the dominant current path is established between the WE and CE, while the RE remains nearly current-free and close to equilibrium, enabling more faithful potential control at the WE. Owing to its improved suppression of IR-drop-induced potential errors, the three-electrode format has become the prevailing configuration for high-performance wearable biosensors. On this hardware basis, electrochemical methodologies such as potentiometry, amperometry, and electrochemical impedance spectroscopy are broadly deployed to interrogate interfacial kinetics and to quantify biomarkers in biological fluids, including sweat and tear fluid. This section provides a structured overview of these approaches.
3.1. Potentiometry
Potentiometric sensing is grounded in thermodynamic equilibrium: under zero current or nearly zero current conditions, the Nernst Equation relates the interfacial potential difference logarithmically to the activity of the target analyte [
1]. This logarithmic dependence affords a broad linear dynamic response range, making potentiometry well suited for ion analysis. In flexible and wearable implementations, potentiometric devices are commonly constructed as ion-selective electrodes (ISE) and applied to real-time evaluation of electrolyte balance in human body fluids (such as sodium ions, potassium ions, etc.) [
2,
3]. In emerging device designs, two-dimensional nanomaterials, including graphene and transition metal chalcogenides, are increasingly used as “ion–electron” transduction layers because of favorable charge-transport characteristics and tunable surface chemistry. These materials can establish high double-layer capacitance at the buried interface and, through hydrophobicity, suppress formation of a “water layer” between the solid-contact layer and the current collector. By attenuating a principal microscopic origin of potential drift, such interfacial engineering can markedly improve signal stability during prolonged wear [
4]. Moreover, the optimized contact structure promotes efficient conversion of ion-recognition events into robust electronic readouts and, when combined with functionalization strategies such as proton-sensitive polymers, supports promising clinical translation for wearable pH monitoring and related applications [
5].
3.2. Ampere Method
Amperometric sensing follows Faraday’s law by applying a fixed potential at the working electrode to drive redox processes and quantifying the resulting steady-state current as a measure of target biomolecule flux [
6]. Because the Faradaic current is linearly proportional to the bulk concentration of electroactive species, amperometry offers a direct and operationally straightforward route to quantitative biomarker analysis [
7] and is widely implemented in wearable platforms incorporating biocatalytic elements. In representative enzyme-based formats (e.g., glucose monitoring), glucose oxidase (GOx) immobilized on a two-dimensional nanoscale scaffold catalyzes substrate oxidation, and the generated hydrogen peroxide (H
2O
2) is subsequently oxidized at the electrode interface to produce a measurable current signal [
8,
9,
10,
11]. A persistent limitation in practical amperometric deployment, however, is analytical selectivity. In complex biological fluids, the oxidation potential required for the target often overlaps with the potentials of endogenous interferents such as ascorbic acid and uric acid, which can yield spurious responses and compromise diagnostic reliability. This deficiency directly degrades specificity and signal-to-noise ratio, limiting confident interpretation of on-body measurements. Future improvements should therefore prioritize electrode-surface nanoengineering to enable low-voltage operation by decreasing the overpotential of the desired reaction and/or incorporate semipermeable membrane systems with molecular sieving capability to attenuate interferent access and enhance performance in physiologically complex media.
3.3. Impedance Method
Electrochemical impedance spectroscopy (EIS) is a nondestructive frequency-domain technique that probes dielectric properties and charge-transfer kinetics at electrode interfaces through small-amplitude sinusoidal voltage perturbations [
12]. In contrast to methods that depend on net redox current, EIS can report binding-induced interfacial changes by tracking perturbations in impedance parameters. Results are commonly represented as Nyquist plots or Bode plots. Adsorption or binding of specific biomolecules on the electrode surface can reorganize the local dielectric environment and charge distribution, producing measurable shifts in charge transfer resistance (Rct) and double layer capacitance (Cdl). A central design tension in impedance-based biosensors is balancing electrode conductivity with interfacial responsiveness. To enhance sensitivity to low-abundance analytes, current efforts emphasize barrier layers or responsive membranes with precisely defined nanostructures that amplify resistance and/or capacitance modulation arising from recognition events without unduly penalizing electron-transport efficiency, thereby supporting high-sensitivity, label-free detection of low-concentration biomarkers.
4. Applications of Wearable Electrochemical Biosensors for the Detection of Biomolecules in Biological Liquids
Wearable electrochemical biosensors constitute a rapidly evolving platform for health care and personalized medicine by enabling continuous, decentralized assays that are noninvasive or minimally invasive. Such longitudinal measurements generate information-rich time-series datasets that can enhance physiological interpretation and clinical decision-making. Recent device generations increasingly support biochemical analysis of both small and macromolecular targets across diverse biofluids, including sweat, breath, saliva, tears, and interstitial fluid. However, translation from laboratory prototypes to clinically deployed tools remains contingent on a broader enabling ecosystem rather than sensor performance alone. In particular, wearable platforms must achieve acceptance across heterogeneous user populations, demonstrate indication-specific clinical utility, satisfy reimbursement requirements, and align with public health program needs. Deficiencies in any of these domains can impede adoption despite strong analytical performance; therefore, future progress will likely depend on coordinated advances in device validation, usability, clinical workflow integration, and evidence generation that links measured biomarkers to actionable outcomes.
4.1. Tears
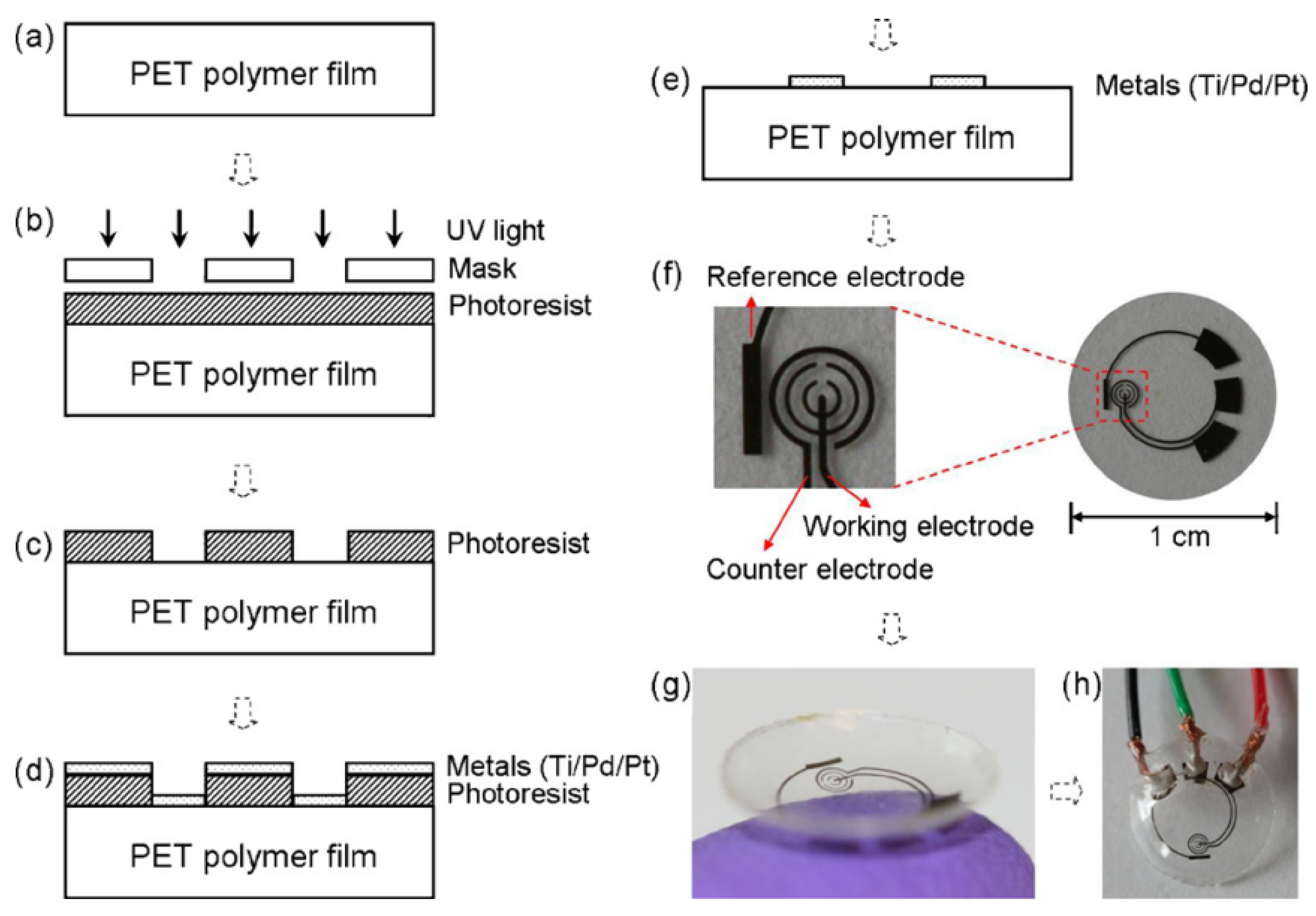
Tear fluid at the ocular surface contains abundant physiological information and, via diffusion across the blood–tear barrier, can reflect systemic biomarker levels in blood. Accordingly, tears are widely viewed as an attractive alternative matrix to venipuncture-based sampling, offering a route to reduce pain and infection risk while enabling noninvasive disease assessment. Although blood glucose monitoring still primarily relies on invasive fingertip sampling and typically does not provide continuous readouts, existing studies have established a significant correlation between tear glucose and blood glucose concentration, supporting the physiological rationale for tear-based diabetes management. Among candidate sampling formats, smart contact lenses provide intimate, stable access to the tear film and thus represent a compelling architecture for tear analytics. Early contact-lens sensing efforts relied largely on optical readouts but were constrained by substantial measurement error and challenges in meeting clinical accuracy benchmarks; by comparison, electrochemical sensors offer high sensitivity and strong miniaturization compatibility, making them better suited for continuous, high-precision monitoring of tear glucose. In this context, Yao et al. [
13] developed an electrochemical smart contact-lens sensor (as shown in
Figure 3). The highest performance was obtained when the sensor was sequentially treated with GOD (glucose oxidase), titanium dioxide, and Nafion
®, yielding a detection sensitivity of up to 240 nA/mM and a response time shortened to approximately 20 seconds. Beyond favorable analytical characteristics, feasibility of continuous operation over 24 hours was demonstrated, providing a useful technical foundation for advancing noninvasive glucose monitoring toward clinical translation.
4.2. Saliva
Relative to blood analysis that requires skin puncture or interstitial-fluid measurements that commonly depend on microneedle arrays, saliva sampling is distinctly advantageous because it is fully noninvasive. This feature reduces both physical discomfort and psychological burden, eliminates infection risks associated with needle punctures, and can improve adherence to long-term, high-frequency testing in populations such as diabetic patients, older adults, and children. Saliva contains numerous molecular species originating from blood via passive diffusion, active transport, or ultrafiltration; coupled with continuous secretion and straightforward collection, these attributes position saliva as a practical matrix for at-home health management and remote medical monitoring. Integration of electrochemical sensors into oral-worn formats (e.g., mouth guards, dentures, dental tattoos, or pacifiers) enables in situ, real-time, continuous biomarker measurement. Such continuous readouts provide analytical value that discrete sampling cannot readily deliver, particularly for capturing transient physiological dynamics, including postprandial blood glucose fluctuations, shifts in lactate threshold during exercise, and acute stress responses.
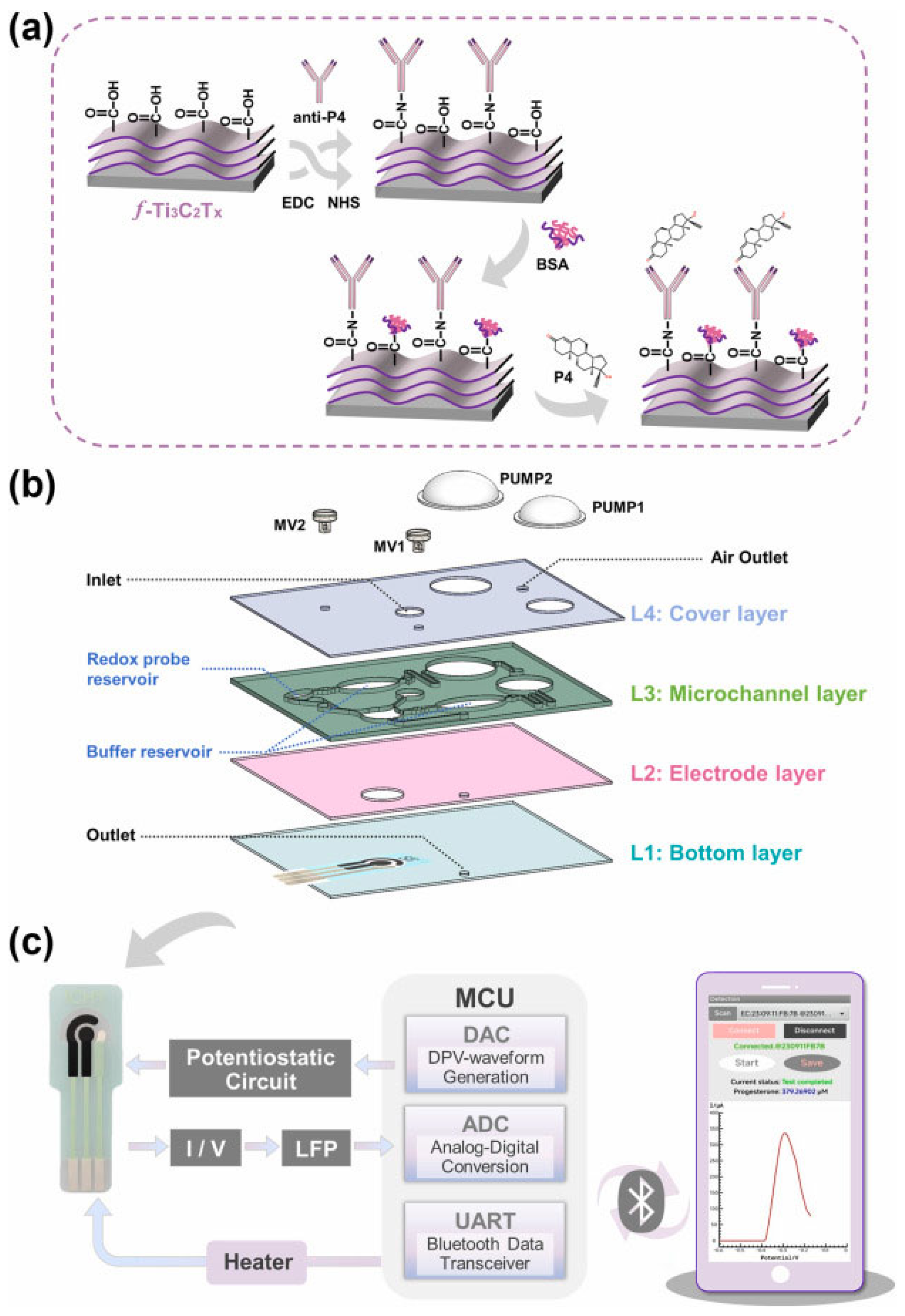
Within this emerging landscape, Yu et al. [
14] reported a microelectrochemical detection platform termed “ProgenCare” (as shown in
Figure 4) for quantitative determination of progesterone (P4) in saliva. The system integrates microfluidic handling and electrochemical sensing, using a functionalized MXene (f-Ti3C2Tx) electrode bearing carboxyl groups coupled with specific antibodies to support rapid, high-sensitivity, and high-selectivity P4 detection in standard samples. For fluidic regulation, the chip incorporates control pumps, micro valves, and Tesla valves to enable precise and selective reagent release. Differential pulse voltammetry (DPV) is executed via an embedded circuit, and data are transmitted wirelessly through Bluetooth to a smartphone interface for real-time interaction and processing. Reported measurements show strong agreement with professional laboratory instrumentation and indicate substantial clinical promise for analyzing saliva samples collected from pregnant women across different stages of pregnancy.
4.3. Sweat
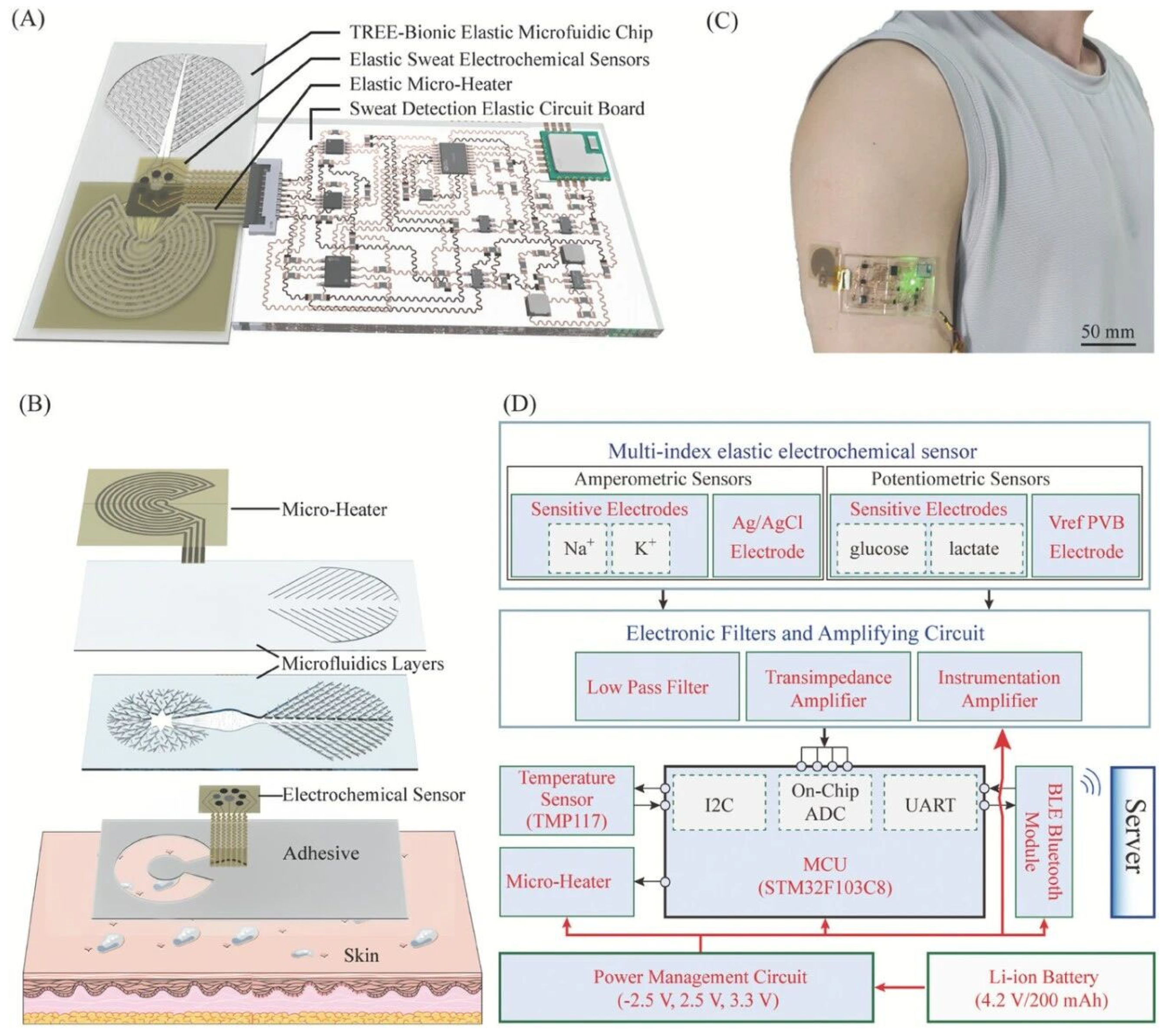
As a highly promising noninvasive biofluid, sweat occupies a central role in biomarker analysis. Sweat contains diverse chemical constituents that track physiological status: potassium ions (K+) and sodium ions (Na+) report electrolyte homeostasis and provide insight into systemic metabolic balance; lactic acid, a product of anaerobic respiration, serves as a key readout of physiological stress and the transition from aerobic to anaerobic metabolic thresholds; and the strong correlation between sweat glucose concentration and blood glucose level offers a pathophysiological rationale for noninvasive glucose assessment. Owing to its convenient, noninvasive accessibility, sweat is particularly well suited for real-time, in situ monitoring of human function during daily activities and dynamic conditions such as intense exercise. Correspondingly, analytical approaches targeting these biomarkers have expanded across clinical and nonclinical settings, including longitudinal tracking and therapeutic evaluation in chronic disorders such as diabetes and kidney disease, assessment of energy expenditure and competitive performance in sports physiology, and pharmacokinetic monitoring during drug-development stages. In high-risk operational contexts, including aerospace, continuous physiological surveillance for pilots and astronauts also carries strategic importance. To meet the demands of real-time, long-duration, and high-reliability measurement, flexible wearables have therefore become the dominant platform for sweat sensing. A persistent barrier, however, is fluidic management: unresolved challenges in sweat collection, microfluidic transport, and waste-liquid removal can prevent the sensing interface from consistently contacting freshly secreted sweat, thereby introducing lag, dilution or carryover artifacts, and compromised temporal fidelity. Addressing this limitation, Niu et al. [
15] developed a fully elastic wearable electrochemical sweat-sensing system (as shown in
Figure 5) that integrates biomimetic microfluidics, multiparameter sensing arrays, thermal management modules, and flexible circuitry. The microfluidic chip incorporates a tree-like biomimetic topology that improves fluid-dynamic performance, increasing fresh-sweat collection efficiency while accelerating removal of aged sweat, which supports real-time capture of dynamic biochemical fluctuations. The platform integrates high-precision electrochemical sensors for sodium, potassium, lactic acid, and glucose, offering excellent sensitivity and selectivity. At the systems level, the electronic components are implemented on a highly stretchable elastic circuit board that conforms to complex skin contours and motion-induced folding, improving wear comfort while enabling synchronized acquisition, on-board signal processing, and wireless transmission of multichannel physiological data; collectively, these design elements provide a useful paradigm for the form factor engineering of next-generation personalized health-monitoring devices.
4.4. Urine
Urine, the terminal output of human metabolism, carries dense physiological and biochemical information and therefore represents a compelling biofluid target for wearable biochemical sensing. Its chemically diverse composition encompasses multiple clinically relevant biomarkers, including glucose, proteins, creatinine, uric acid, electrolyte ions, hormone metabolites, and inflammatory factors. Concentration fluctuations in these species can report on metabolic homeostasis, renal filtration performance, endocrine regulation, and early pathophysiological signatures associated with systemic disease. Relative to conventional blood testing, which is invasive and typically episodic, urine collection is inherently noninvasive, convenient, and amenable to repeated or continuous sampling, positioning it for longitudinal home monitoring of chronic disorders (such as diabetes and kidney disease) and population-specific health screening. A persistent limitation, however, is the high dimensionality of the urine matrix: abundant interferents and variable background constituents can degrade molecular selectivity, elevate noise, and bias quantitative readouts, thereby compromising analytical accuracy and hindering robust remote decision-making. Accordingly, improved antifouling and interference-mitigation strategies at the recognition and transduction interfaces, together with calibration approaches that explicitly account for matrix variability, remain central directions for advancing urine-based wearables.
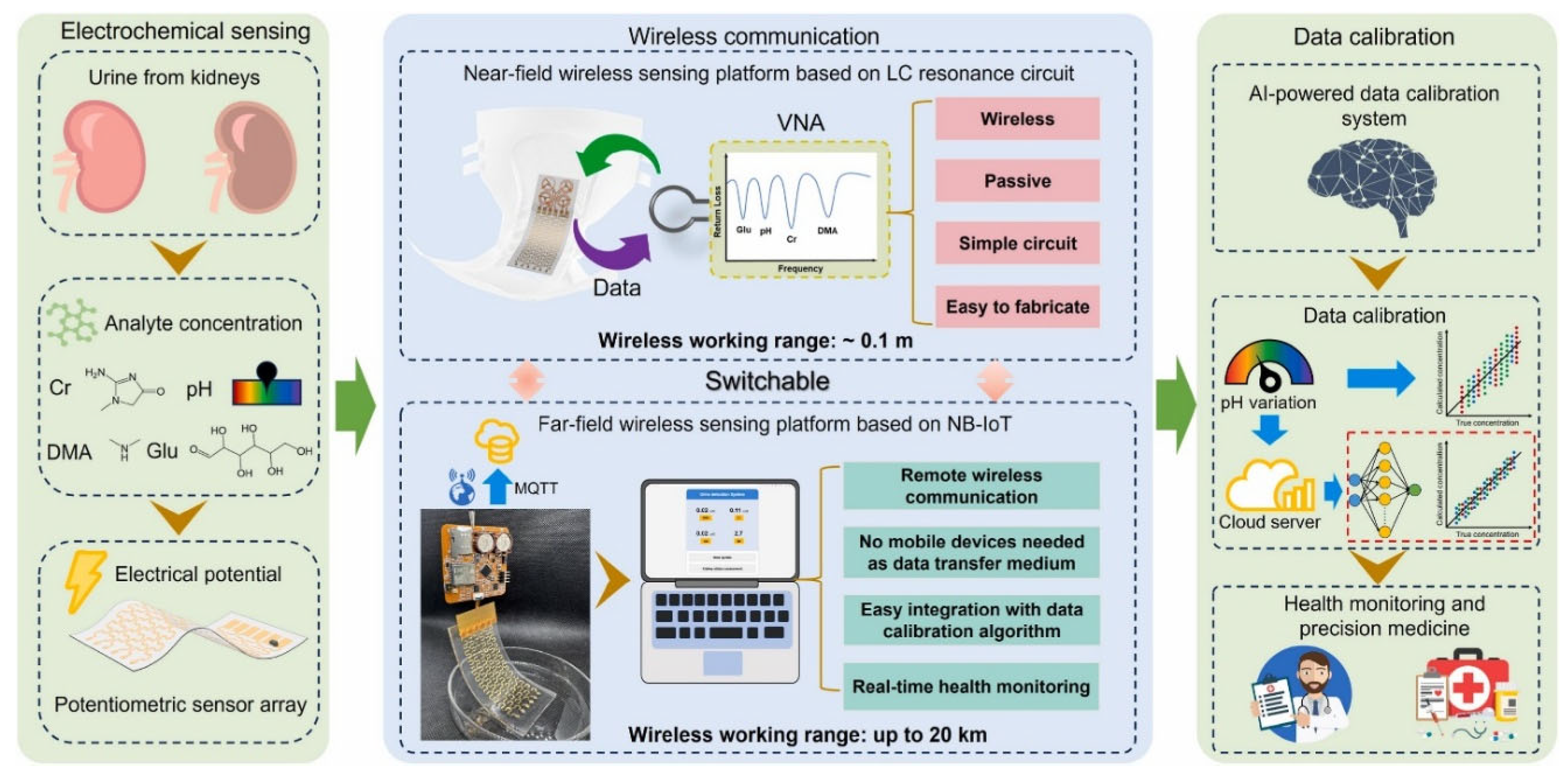
To address complex-matrix interference and enable remote operation, Dong et al. [
16] reported an AI-assisted kilometer-level wireless wearable urine biochemical sensing platform (as shown in
Figure 6). The platform integrates molecularly imprinted polymer (MIP)-modified ion-selective electrodes (ISE), enabling simultaneous measurement of four indicators—dimethylamine, creatinine, glucose, and pH value—while strengthening recognition specificity. At the system level, a “LC near-field passive + NB-IoT far-field” dual-mode wireless communication architecture is implemented to permit battery-free passive sensing under near-field conditions and to extend transmission beyond typical Bluetooth/WiFi ranges, achieving data links up to 20 kilometers. Because urine composition can vary substantially and induce signal drift, a multi-layer perceptron (MLP) AI calibration algorithm is incorporated, reducing the cross-sensitivity error associated with pH value changes from 15% to below 2%. The resulting system is designed for integration into smart diapers, with particular relevance for the elderly, infants, and disabled patients, and it establishes an end-to-end workflow—“in situ real-time sensing - long-distance wireless transmission - cloud intelligent calibration”—that supports the broader goal of scalable remote kidney disease screening and diabetes management.
5. Conclusions
Wearable electrochemical sensors have witnessed significant development in recent years (
Table 1). The wearable electrochemical biosensors surveyed herein offer instructive concepts for device construction and exhibit several compelling attributes; however, substantial barriers remain before broad deployment and commercialization can be realized. First, fabrication routes that depend on nanolithography and advanced functional nanomaterials commonly require costly high-precision instrumentation and specialized expertise. This reliance diminishes economic viability at scale and complicates industrial mass production of highly conductive, mechanically compliant electrodes. Second, robust accommodation of interindividual anatomical variability persists as a central engineering constraint. Critical unresolved questions include how to engineer flexible conductive electrodes that achieve intimate, durable conformal contact across diverse skin topographies while maintaining wearer comfort and preserving signal fidelity, as well as how to design eye-mounted sensors that reliably match variations in ocular size and curvature among users. Third, the human–device interface remains insufficiently streamlined: intricate external circuit architectures impose a steep usability threshold for practitioners without an engineering background and for nonexpert end-users. This usability deficiency materially restricts translation by increasing the likelihood of operational error, limiting reproducibility outside laboratory settings, and impeding adoption in point-of-care contexts; accordingly, future improvements should prioritize integrated system design that reduces circuit complexity while maintaining analytical performance. In parallel, the development of flexible electrodes with high biocompatibility and extremely low side effects remains pivotal for sustaining high sensitivity, long-term operational stability, and real-time monitoring of key biomarkers. Looking ahead, overcoming these bottlenecks, together with the incorporation of artificial intelligence technology capable of efficiently processing massive data, is expected to enable next-generation wearable electrochemical sensors for precise, personalized, real-time diagnosis and eventual commercialization. Overall, this article reviews emerging development strategies for wearable electrochemical biosensors in point-of-care testing applications, with the objective of accelerating progress toward real-time disease diagnosis and personalized medicine.
References
- Zhang, N.; Liu, Z.; Yu, X.; Yang, J.; Qiu, W.; Bi, S.; Jiang, T.; Yang, D. A Biocompatible Wearable Potentiometric Sensing Chip for in Situ Wound Monitoring. ACS Sens. 2025, acssensors.5c03506. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cai, Z.; Zhang, Y.; Tan, R.; Wang, L.; He, H.; He, Y.; Chang, G. SnO2 Electrolyte-Gated Thin Film Transistor for Highly Sensitive pH Detection of Beverages. Food Chem. 2025, 480, 143868. [Google Scholar] [CrossRef]
- Du, X.; Wu, G.; Dou, X.; Ding, Z.; Xie, J. Alizarin Complexone Modified UiO-66-NH2 as Dual-Mode Colorimetric and Fluorescence pH Sensor for Monitoring Perishable Food Freshness. Food Chem. 2024, 445, 138700. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, F.; Liu, G.; Lin, H.; Bao, Y.; Han, D.; Wang, W.; Ma, Y.; Zhang, B.; Niu, L. Superhydrophobic Functionalized Ti3 C2Tx MXene-Based Skin-Attachable and Wearable Electrochemical pH Sensor for Real-Time Sweat Detection. Anal. Chem. 2022, 94, 7319–7328. [Google Scholar] [CrossRef]
- Song, Y.; Min, J.; Yu, Y.; Wang, H.; Yang, Y.; Zhang, H.; Gao, W. Wireless Battery-Free Wearable Sweat Sensor Powered by Human Motion. Sci. Adv. 2020, 6, eaay9842. [Google Scholar] [CrossRef]
- Sankar, K.; Kuzmanović, U.; Schaus, S.E.; Galagan, J.E.; Grinstaff, M.W. Strategy, Design, and Fabrication of Electrochemical Biosensors: A Tutorial. ACS Sens. 2024, 9, 2254–2274. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, X.; Liu, T.; Zhang, J.; Liu, X.; Zhu, M.; Jiang, D.; Chu, Z.; Jin, W. Self-Calibrating Wearable Sweat Sensor for Precise Hyperuricemia Monitoring via Hollow-Architecture Bimetallic Oxides. Adv. Funct. Mater. 2025, e23008. [Google Scholar] [CrossRef]
- Jiang, L.; Yao, L.; Xie, H.; Liu, C.; Jin, W. An Aggregation Interpenetrating Induced Poly(Dopamine) Conductive Hydrogel Grid for Efficient Glucose Detection. Biosens. Bioelectron. 2025, 290, 117951. [Google Scholar] [CrossRef]
- Sun, X.; Wang, P.; Tang, L.; Li, N.; Lou, Y.-R.; Zhang, Y.; Li, P. Multifunctional Hydrogel Containing Oxygen Vacancy-Rich WOx for Synergistic Photocatalytic O2 Production and Photothermal Therapy Promoting Bacteria-Infected Diabetic Wound Healing. Adv. Funct. Mater. 2024, 34, 2411117. [Google Scholar] [CrossRef]
- Lopes, V.; Abreu, T.; Abrantes, M.; Nemala, S.S.; De Boni, F.; Prato, M.; Alpuim, P.; Capasso, A. Graphene-Based Glucose Sensors with an Attomolar Limit of Detection. J. Am. Chem. Soc. 2025, 147, 13059–13070. [Google Scholar] [CrossRef]
- Li, R.; Fan, H.; Chen, Y.; Yin, S.; Liu, G.L.; Li, Y.; Huang, L. MXene-Graphene Oxide Heterostructured Films for Enhanced Metasurface Plasmonic Biosensing in Continuous Glucose Monitoring. Adv. Sci. 2025, 12, 2410376. [Google Scholar] [CrossRef]
- Hu, W.; Peng, Y.; Wei, Y.; Yang, Y. Application of Electrochemical Impedance Spectroscopy to Degradation and Aging Research of Lithium-Ion Batteries. J. Phys. Chem. C 2023, 127, 4465–4495. [Google Scholar] [CrossRef]
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A Contact Lens with Embedded Sensor for Monitoring Tear Glucose Level. Biosens. Bioelectron. 2011, 26, 3290–3296. [Google Scholar] [CrossRef]
- Yu, R.; Fang, M.; Xu, H.; Meng, F.; Tang, N.; Liu, X.; Feng, S.; Wang, K.; Guo, J. ProgenCare: A Noninvasive Salivary Progesterone Monitoring Platform Based on MXene-Modified Microfluidic Electrochemical Immunosensor. Biosens. Bioelectron. 2026, 293, 118161. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Lin, S.; Chen, D.; Wang, Z.; Cao, C.; Gao, A.; Cui, S.; Liu, Y.; Hong, Y.; Zhi, X.; et al. A Fully Elastic Wearable Electrochemical Sweat Detection System of Tree-Bionic Microfluidic Structure for Real-Time Monitoring. Small 2024, 20, 2306769. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; An, W.; Zhang, Y.; Kang, Z.; Gao, B.; Lv, J.; Jiang, Y.; Niu, C.; Mao, Y.; Zhang, D. An Artificial Intelligence-Assisted, Kilometer-Scale Wireless and Wearable Biochemical Sensing Platform for Monitoring of Key Biomarkers in Urine. Biosens. Bioelectron. 2025, 288, 117844. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, Y.; Li, Y.; Wen, Z.; Peng, X.; He, Y.; Hou, Y.; Fan, J.; Zang, G.; Zhang, Y. A Wearable Non-Enzymatic Sensor for Continuous Monitoring of Glucose in Human Sweat. Talanta 2024, 278, 126499. [Google Scholar] [CrossRef]
- Chen, L.; Chen, F.; Liu, G.; Lin, H.; Bao, Y.; Han, D.; Wang, W.; Ma, Y.; Zhang, B.; Niu, L. Superhydrophobic Functionalized Ti3 C2Tx MXene-Based Skin-Attachable and Wearable Electrochemical pH Sensor for Real-Time Sweat Detection. Anal. Chem. 2022, 94, 7319–7328. [Google Scholar] [CrossRef]
- Liu, K.; Luo, B.; Zhang, L.; Hou, P.; Pan, D.; Liu, T.; Zhao, C.; Li, A. Flexible and Wearable Sensor for in Situ Monitoring of Gallic Acid in Plant Leaves. Food Chem. 2024, 460, 140740. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, G.; Chen, Y.; Gao, Y.; Hou, C.; Liao, K.; Ma, B.; Liu, H. Wearable Microfluidic Electrochemical Sensor Integrated with Iontophoresis for Non-Invasive Sweat Ketone Monitoring. Sens. Actuators. B 2024, 421, 136518. [Google Scholar] [CrossRef]
- Mi, Z.; Xia, Y.; Dong, H.; Shen, Y.; Feng, Z.; Hong, Y.; Zhu, H.; Yin, B.; Ji, Z.; Xu, Q.; et al. Microfluidic Wearable Electrochemical Sensor Based on MOF-Derived Hexagonal Rod-Shaped Porous Carbon for Sweat Metabolite and Electrolyte Analysis. Anal. Chem. 2024, 96, 16676–16685. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Broza, Y.Y.; Li, W.; Wang, Y.; Wu, S.; Liu, Z.; Wang, J.; Gui, S.; Wang, L.; Zhang, Z.; et al. Multiplexed Nanomaterial-Based Sensor Array for Detection of COVID-19 in Exhaled Breath. ACS Nano 2020, 14, 12125–12132. [Google Scholar] [CrossRef]
- Ogbeide, O.; Bae, G.; Yu, W.; Morrin, E.; Song, Y.; Song, W.; Li, Y.; Su, B.; An, K.; Hasan, T. Inkjet-printed rGO/Binary Metal Oxide Sensor for Predictive Gas Sensing in a Mixed Environment. Adv. Funct. Mater. 2022, 32, 2113348. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Lu, S.; Geng, X.; Guan, Y. Selective Detection of H2 by Pt-MCF/ZSA Bilayer Sensor Prepared in Situ via EHD Jet Printing. Sens. Actuators. B 2024, 418, 136324. [Google Scholar] [CrossRef]
- Krivačić, S.; Boček, Ž.; Zubak, M.; Kojić, V.; Kassal, P. Flexible Ammonium Ion-Selective Electrode Based on Inkjet-Printed Graphene Solid Contact. Talanta 2024, 279, 126614. [Google Scholar] [CrossRef]
- Patel, S.; Shrivas, K.; Sinha, D.; Monisha; Kumar Patle, T.; Yadav, S.; Thakur, S.S.; Deb, M.K.; Pervez, S. Smartphone-Integrated Printed-Paper Sensor Designed for on-Site Determination of Dimethoate Pesticide in Food Samples. Food Chem. 2022, 383, 132449. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A Laser-Engraved Wearable Sensor for Sensitive Detection of Uric Acid and Tyrosine in Sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).