1. Introduction
Pediatric myopia has emerged as a global public health crisis, affecting >80% of urban Taiwanese schoolchildren with projections estimating 2.5 billion cases worldwide by 2050 [
1]. The global escalation of myopia represents a critical shift from a simple refractive error to a major public health crisis. While Vitale et al. established a baseline prevalence of 33.1% in the U.S. [
2], the situation is more dire in East Asian urban centers, where Morgan et al. reported myopia rates of 80–90% among youth, driven by intensive education and limited outdoor sunlight [
3]. This rising prevalence inevitably increases the risk of irreversible vision loss. Wong et al. highlighted that pathologic myopia affects up to 3% of the population, often leading to myopic choroidal neovascularization and legal blindness during an individual's most productive working years [
4].
Current pharmacotherapies remain suboptimal. Low-dose atropine (0.01%–0.05%) slows progression by approximately 50% in the LAMP and ATOM trials; however, side effects such as photophobia (35%) and near-vision blur (25%) lead to nearly 20% discontinuation [
5,
6,
7,
8]. The biological rationale for alternative therapies lies in the excessive remodeling of the scleral extracellular matrix, a process mediated by matrix metalloproteinases (MMPs) that facilitates axial elongation. Specifically, MMP-2 and MMP-9 are key drivers of collagen degradation in the myopic sclera [
9,
10,
11]. Emerging MMP-targeted alternatives include tetracycline derivatives like doxycycline hyclate, which inhibit MMP transcription and activity at sub-antimicrobial doses (1–10 μg/mL) [
12]. These preclinical studies have demonstrated exploratory in preventing scleral thinning and achieving >60% MMP-9 reduction in animal models [
13]. Such findings support the repurposing of doxycycline as a topical ophthalmic solution, leveraging its favorable ocular pharmacokinetics to stabilize the scleral matrix.
Given the pediatric focus, ICH M3(R2) mandates 6–9 month non-rodent studies for chronic pediatric applications [
14]; ICH S4 validates 9-month rabbit duration [
15]. This GLP-compliant study (No. 2098-005, Charles River Laboratories) in juvenile Dutch Belted rabbits—exhibiting human-like corneal permeability (limited systemic absorption / low corneal penetration typical of topical dosing) and developmental timelines [
16]—characterizes doxycycline hyclate ophthalmic solution's ocular/systemic safety and toxicokinetics during maturation, generating regulatory-grade data for pediatric IND submission.
2. Results
2.1. Clinical and Ocular Observations
No mortalities occurred during the study. Clinical observations revealed no Doxycycline-related systemic effects. Body weights in both male and female animals increased steadily over the 9-month study period, and these changes were consistent with the control group. Ophthalmic examinations showed no clinically significant or test article-related findings. Intraocular pressure (IOP) measurements remained stable and showed no clinically significant differences compared to the control group.
2.2. Clinical Pathology and Histopathology
Clinical pathology (hematology, coagulation, clinical chemistry, and urinalysis) evaluations revealed no test article-related changes in hematology, coagulation, clinical chemistry, or urinalysis parameters in either sex at any dose level. All observed fluctuations were considered sporadic, consistent with biological and/or procedural variability, and not related to Doxycycline Hyclate administration. There were no test article-related macroscopic findings at terminal or recovery necropsy. Histopathologic evaluation demonstrated no test article-related microscopic findings in ocular or systemic tissues.
2.3. Toxicokinetics
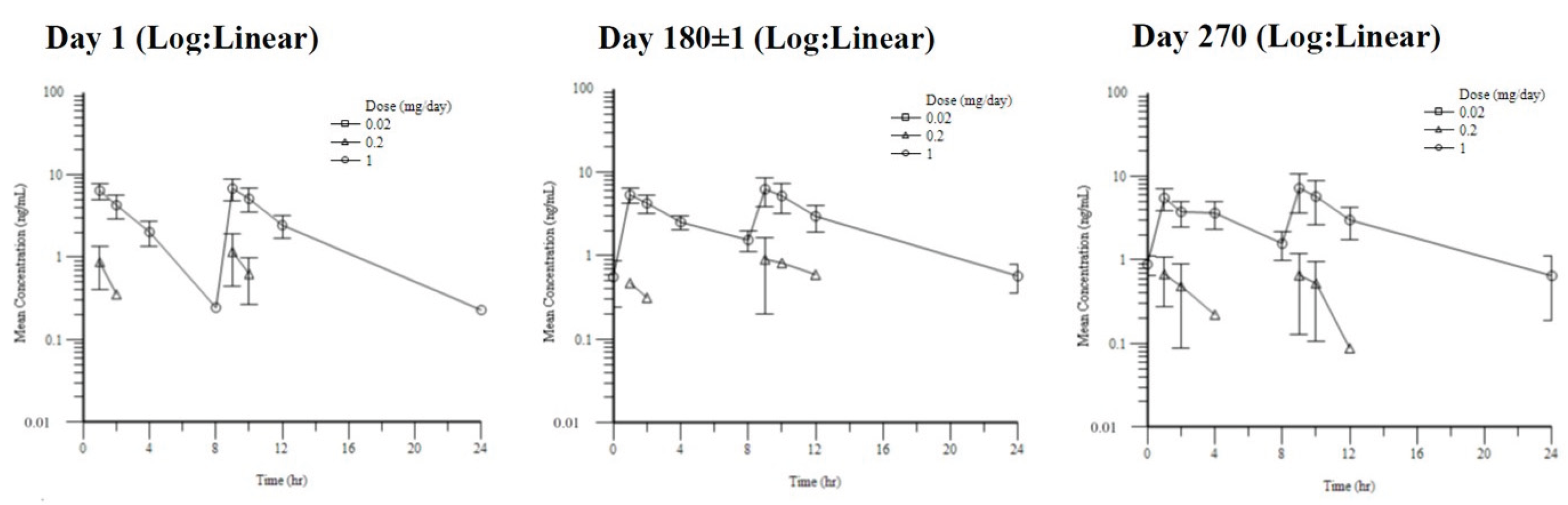
Toxicokinetic parameters demonstrated low systemic accumulation following repeated dosing, with no significant sex-related differences observed; therefore, data from male and female animals were pooled for analysis (
Table 1,
Figure 1). Dose proportionality was not formally assessed due to the low systemic exposure and limited sample size.
3. Discussion
This 9-month GLP study (No. 2098-005) establishes a favorable nonclinical safety profile following long-term topical administration, with no adverse findings at the highest tested dose (0.25 mg/eye BID (0.5% w/v)) without ocular/systemic effects [Study 2098-005]. The juvenile Dutch Belted rabbit emulates human corneal absorption and scleral ontogeny [
17], consistent with ICH M3(R2) and S4 considerations for chronic nonclinical safety assessment.
No ocular irritation was observed in the present study. Ocular tolerability remains an important consideration for existing myopia therapies. Intraocular pressure remained within the physiological range throughout the study. [
18]. Topical ophthalmic administration further mitigates the potential for systemic adverse effects traditionally associated with tetracyclines, such as dental staining. Notably, while tetracycline-class antibiotics are historically linked to permanent tooth discoloration. Previous clinical observations have suggested that doxycycline may differ from other tetracyclines with respect to dental staining risk. [
19] Systemic exposure remained substantially below antibacterial levels. [
20]. Accumulation ratios remained close to unity and were not considered indicative of systemic accumulation. Thus, toxicokinetic parameters demonstrated low accumulation following repeated administration support BID dosing.
This 9-month GLP-compliant study (Study No. 2098-005) demonstrated a favorable nonclinical safety profile for doxycycline hyclate ophthalmic solution following long-term topical administration in juvenile Dutch Belted rabbits, with no ocular or systemic adverse findings observed at the highest tested dose. The juvenile rabbit model, which exhibits ocular developmental features relevant to pediatric populations, is commonly used for the assessment of chronic ophthalmic safety.
Ocular tolerability was unremarkable throughout the study, with no treatment-related irritation or changes in intraocular pressure observed. Systemic exposure following topical administration increased with dose but remained low, with accumulation ratios close to unity and not indicative of systemic accumulation after repeated dosing. These findings are consistent with the localized route of administration and support the feasibility of chronic ophthalmic dosing.
Collectively, the ocular and systemic safety findings from this long-term juvenile animal study provide a nonclinical foundation to support further early-phase clinical evaluation of doxycycline hyclate ophthalmic solution for chronic myopia control. Given the high prevalence of pediatric myopia and the limitations associated with current therapeutic options, continued investigation of alternative strategies targeting scleral remodeling pathways, including matrix metalloproteinase modulation, is warranted.
4. Materials and Methods
4.1. Test Article and Dosing
Doxycycline hyclate ophthalmic solution (developed at National Taiwan University, Taipei, Taiwan) comprised sterile, isotonic, preservative-free aqueous vehicle (pH 7.0–7.4). GLP dose formulation analysis (HPLC; Days 1/28/90/180/270) confirmed >88% recovery, CV<9% across concentrations.
Juvenile Dutch Belted rabbits ({Haz:(DB)SPF}, Envigo RMS, USA, ~7 weeks). Dosing: 50 μL/eye BID (~8–10 h interval), inferior conjunctival sac via calibrated micropipette. Recovery: 14 days post-270 days dosing (Groups 1,4) (
Table 2).
4.2. Histopathology and Toxicokinetics
TK sampling (K₂EDTA plasma; Days 1/180/270; predose/0.5–24 h; n=3~5 /sex/group/time): LC-MS/MS (LLOQ 1 ng/mL), Phoenix WinNonlin v8.0 non-compartmental analysis.
At scheduled necropsy (Day 284 or 298), a full examination was performed. Over 40 tissues per animal were collected; eyes and optic nerves were preserved in Davidson’s fixative, while other systemic tissues were fixed in 10% neutral buffered formalin. All tissues were paraffin-embedded, sectioned, and stained with H&E. Histopathologic evaluation was conducted via a blinded, peer-reviewed process.
4.3. Statistical Analysis
Statistical Analysis
Continuous data (e.g., body weights, IOP, and clinical pathology parameters) were analyzed using one-way ANOVA followed by Dunnett’s post-hoc test (α=0.05) for multiple comparisons; Kruskal-Wallis tests were employed for non-parametrically distributed data7. Trends over time were evaluated using repeated-measures ANOVA8. Categorical data, such as the incidence of clinical or macroscopic observations, were analyzed using Fisher’s Exact Test9. All statistical analyses were performed using SAS software (v9.4).
5. Conclusions
Doxycycline hyclate ophthalmic solution was well tolerated following long-term topical administration in juvenile rabbits, with no evidence of ocular or systemic toxicity at the highest tested dose. Toxicokinetic evaluations indicated increasing systemic exposure with dose and low accumulation following repeated administration. Collectively, the GLP findings from Study No. 2098-005 support further clinical development of doxycycline hyclate for chronic ophthalmic indications.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Summary of Ophthalmic Examination Findings; Table S2 : Summary of Hematology Values.
Author Contributions
Conceptualization, I-Jong, Wang and Chi-Jr Liao ; resources, I-Jong, Wang . Yi-Chau Chen. Shi-Ju, Huang and Hsin-Ming Chen; writing—original draft preparation, Chi-Jr Liao; writing—review and editing, Chi-Jr Liao.
Funding
This research was funded by National Science and Technology Council (NSTC), grant number MOST106-3114-B002-010 and MOST107-2321-B002-069.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of National Taiwan University College of Medicine and College of Public Health (protocol code 20170242; approval date: 30 October 2017).
Data Availability Statement
Data are available on request from the corresponding author.
Acknowledgments
This research was supported by National Science and Technology Council (NSTC), Taiwan project funding (Grant No. [MOST106-3114-B002-010 and MOST107-2321-B002-069.]). The authors gratefully acknowledge professional guidance and from Biomedical Commercialization Center (BMCC), Taiwan. GLP toxicology execution was conducted at Charles River Laboratories, Mattawan, MI, USA (Study No. 2098-005). The multidisciplinary study team is thanked for their technical expertise and dedication.
Conflicts of Interest
Charles River Laboratories staff were involved in the independent execution of Study No. 2098-005. The test article was supplied by the National Taiwan University team via LifeOptimal Technology. The study was funded by NSTC Taiwan. Charles River Laboratories maintained independent oversight of data collection and reporting. The authors declare no other conflicts.
Abbreviations
The following abbreviations are used in this manuscript:
| BID |
Twice Daily |
| BMCC |
Biomedical Commercialization Center |
| CRL |
Charles River Laboratories |
| CV |
Coefficient of Variation |
| ECM |
Extracellular Matrix |
| GLP |
Good Laboratory Practice |
| H&E |
Hematoxylin and Eosin |
| HPLC |
High-Performance Liquid Chromatography |
| IACUC |
Institutional Animal Care and Use Committee |
| ICH |
International Council for Harmonisation |
| IOP |
Intraocular Pressure |
| LAMP |
Low-Concentration Atropine for Myopia Progression |
| LC-MS/MS |
Liquid Chromatography-Tandem Mass Spectrometry |
| LLOQ |
Lower Limit of Quantification |
| MMP |
Matrix Metalloproteinase |
| NSTC |
National Science and Technology Council |
| NOAEL |
No Observed Adverse Effect Level |
| NTU |
National Taiwan University |
| SAD |
Single Ascending Dose |
| TK |
Toxicokinetics |
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Ellwein, L.; Cotch, M.F.; Ferris, F.L., 3rd; Sperduto, R. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol 2008, 126, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Ferreira, A.; Hughes, R.; Carter, G.; Mitchell, P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol 2014, 157, 9–25 e12. [Google Scholar] [CrossRef] [PubMed]
- Chia, A.; Chua, W.H.; Cheung, Y.B.; Wong, W.L.; Lingham, A.; Fong, A.; Tan, D. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012, 119, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.H.; Balakrishnan, V.; Chan, Y.H.; Tong, L.; Ling, Y.; Quah, B.L.; Tan, D. Atropine for the treatment of childhood myopia. Ophthalmology 2006, 113, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Yam, J.C. Low-Concentration Atropine Eye Drops for Myopia Progression. Asia Pac J Ophthalmol (Phila) 2019, 8, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Yam, J.C.; Jiang, Y.; Tang, S.M.; Law, A.K.P.; Chan, J.J.; Wong, E.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J.; et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology 2019, 126, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ge, J. The Role of Scleral Changes in the Progression of Myopia: A Review and Future Directions. Clin Ophthalmol 2025, 19, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhou, J.B. Scleral remodeling in myopia development. Int J Ophthalmol 2022, 15, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhou, Q.; Reinach, P.S.; Yang, J.; Ma, L.; Wang, X.; Wen, Y.; Srinivasalu, N.; Qu, J.; Zhou, X. Cause and Effect Relationship between Changes in Scleral Matrix Metallopeptidase-2 Expression and Myopia Development in Mice. Am J Pathol 2018, 188, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Luo, L.; Pflugfelder, S.C.; Li, D.Q. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci 2005, 46, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Lin, I.T.; Wu, Y.C.; Wang, I.J. Stepwise candidate drug screening for myopia control by using zebrafish, mouse, and Golden Syrian Hamster myopia models. EBioMedicine 2021, 65, 103263. [Google Scholar] [CrossRef] [PubMed]
- GUIDANCE ON NONCLINICAL SAFETY STUDIES FOR THE CONDUCT OF HUMAN CLINICAL TRIALS AND MARKETING AUTHORIZATION FOR PHARMACEUTICALS M3(R2) . 2009.
- DURATION OF CHRONIC TOXICITY TESTING IN ANIMALS (RODENT AND NON RODENT TOXICITY TESTING) S4 . 1998.
- Yamagiwa, Y.; Kurata, M.; Satoh, H. Histological Features of Postnatal Development of the Eye in White Rabbits. Toxicol Pathol 2021, 49, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Schuh, J.C.L.; Holve, D.L.; Mundwiler, K.E. Corneal Dystrophy in Dutch Belted Rabbits as a Possible Model of Thiel-Behnke Subtype of Epithelial-Stromal TGFbeta-Induced Corneal Dystrophy. Toxicol Pathol 2021, 49, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.W.; de Vries, M.M.; Junoy Montolio, F.G.; Jansonius, N.M. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology 2011, 118, 1989–1994 e1982. [Google Scholar] [CrossRef] [PubMed]
- Poyhonen, H.; Nurmi, M.; Peltola, V.; Alaluusua, S.; Ruuskanen, O.; Lahdesmaki, T. Dental staining after doxycycline use in children. J Antimicrob Chemother 2017, 72, 2887–2890. [Google Scholar] [CrossRef] [PubMed]
- Saivin, S.; Houin, G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet 1988, 15, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |