1. Introduction
Since the first laparoscopic assisted colectomies reported by Jacobs [
1] and Fowler [
2] independently in 1991, laparoscopic colorectal surgery has undergone widespread adoption and significant evolution. This approach has demonstrated several well-established advantages over open surgery, including reduced postoperative pain with decreased opioid requirements, lower rates of surgical site infection, and reduced incidence of incisional hernia formation. These benefits were further enhanced with the introduction of totally laparoscopic procedures with intracorporeal anastomosis. Early reports of intracorporeal anastomosis in colorectal surgery date back to 1992, with subsequent descriptions of the technique for laparoscopic right hemicolectomy provided by Casciola [
3] in 2003 and Lechaux [
4] in 2005.
Despite these advances, an ongoing debate persists regarding the comparative merits of intracorporeal versus extracorporeal anastomosis in minimally invasive colorectal surgery, contributing to the limited adoption of totally laparoscopic techniques. Over the past decades, multiple studies have demonstrated comparable, if not superior, outcomes associated with intracorporeal when compared to extracorporeal anastomosis [
5,
6].
These investigations have primarily focused on the safety and efficacy of laparoscopic intracorporeal anastomosis, particularly in the context of malignant disease, while also assessing oncologic adequacy and radicality.
The principal concern associated with intracorporeal anastomosis remains its safety, with anastomotic leakage representing the most clinically significant manifestation of anastomotic failure. Reported rates of anastomotic leakage in laparoscopic right colectomies in international literature range from 1% to 8% [
37], although a declining trend has been observed in recent years. Several factors influence anastomotic healing and are commonly categorized as modifiable and non-modifiable preoperative factors, tumor-related factors and intraoperative risk factors [
7].
Fundamental surgical principles identify three key technical determinants of successful anastomosis: (1) meticulous surgical technique with careful prevention of hematoma formation (2) preservation of adequate blood supply to the bowel ends to prevent ischemia during dissection and (3) the creation of a tension- free anastomosis [
8,
9,
10,
11]
. When these principles are rigorously applied during the performance of intracorporeal anastomosis, the risk of anastomotic failure remains low.
Currently, most surgeons perform intracorporeal anastomosis using mechanical devices such as linear or circular staplers, selected according to the type of anastomosis. In laparoscopic right colectomies, linear staplers are the most commonly employed due to their convenience and adaptability, particularly with the advent of advanced stapling devices offering articulation angles up to 110 degrees. Although intracorporeal handsewn anastomosis is a cost-effective alternative, it requires advanced laparoscopic skills and is associated with a steep learning curve. Furthermore, handsewn anastomosis is generally more time-consuming. Consequently, due to the technical demands and training requirements, this approach is less frequently adopted and limited literature exists evaluating its outcomes in comparison with stapled intracorporeal anastomosis.
The aim of the present study is to describe the intracorporeal handsewn anastomotic technique implemented in our surgical unit following laparoscopic right colectomy and to evaluate its safety and efficacy through review of the relevant literature.
2. Surgical Technique
Optimal positioning of both the surgical instruments, surgeons, as well as the tissues handled is essential to ensure precise dissections and the creation of a tension free anastomosis. Patients are placed in the Lloyd-Davies position, with a gentle left- sided reverse Trendelenburg tilt, facilitating displacement of the abdominal viscera to the left and improving exposure of the right colon. The surgeon stands between the patient’s legs, with the first assistant positioned on the patient’s left side and the second assistant on the right.
Pneumoperitoneum is established at 12-15mmHg using a 5-mm trocar inserted in the left upper quadrant at Palmer’s point, below the left costal margin along the anterior axillary line. Following insufflation, the working trocars are placed as follows: an 11-mm in the left upper quadrant in the midline between the umbilicus and the Palmer’s point, a 12-mm trocar in the midline between the umbilicus and the left anterior superior iliac spine. An additional 5-mm trocar is inserted in the right lower quadrant in the midline between the umbilicus and the right anterior superior iliac spine for retraction assistance. This trocar configuration provides optimal triangulation for both dissection and intracorporeal suturing.
The procedure begins with assessing the peritoneal cavity in order to exclude peritoneal dissemination. In the presence of suspicious secondary lesions, biopsies are obtained. The colonic lesion is subsequently identified to determine its precise location and define the extent of resection.
Dissection is performed using a medial-to-lateral approach. The ileocecal junction is first identified, followed by exposure of the ileocolic pedicle. The mesenteric peritoneum is incised using an ultrasonic scalpel and the avascular plane between the mesocolon and the retroperitoneum is developed through a combination of blunt and sharp dissection. Dissection proceeds laterally along the embryologic plane following the Told’s fascia. Once the dissection is completed the ileocolic vessels are divided between hemolock clips at their origin just off and below the duodenum. Cranially the dissection follows the anterior mesenteric vein up to the middle colic vein where the right branches of middle colic vessels are divided between hemolock clips. The dissection then proceeds from above through the gastrocolic ligaments with the patient in reverse Trendelenburg position. Care must be taken to find the correct plane through the lesser sac and keep the mesogastrium intact. The dissection then follows the transverse colon up to the hepatic flexure and then caudally towards the cecum, while mobilizing the ascending colon from the lateral peritoneal attachments.
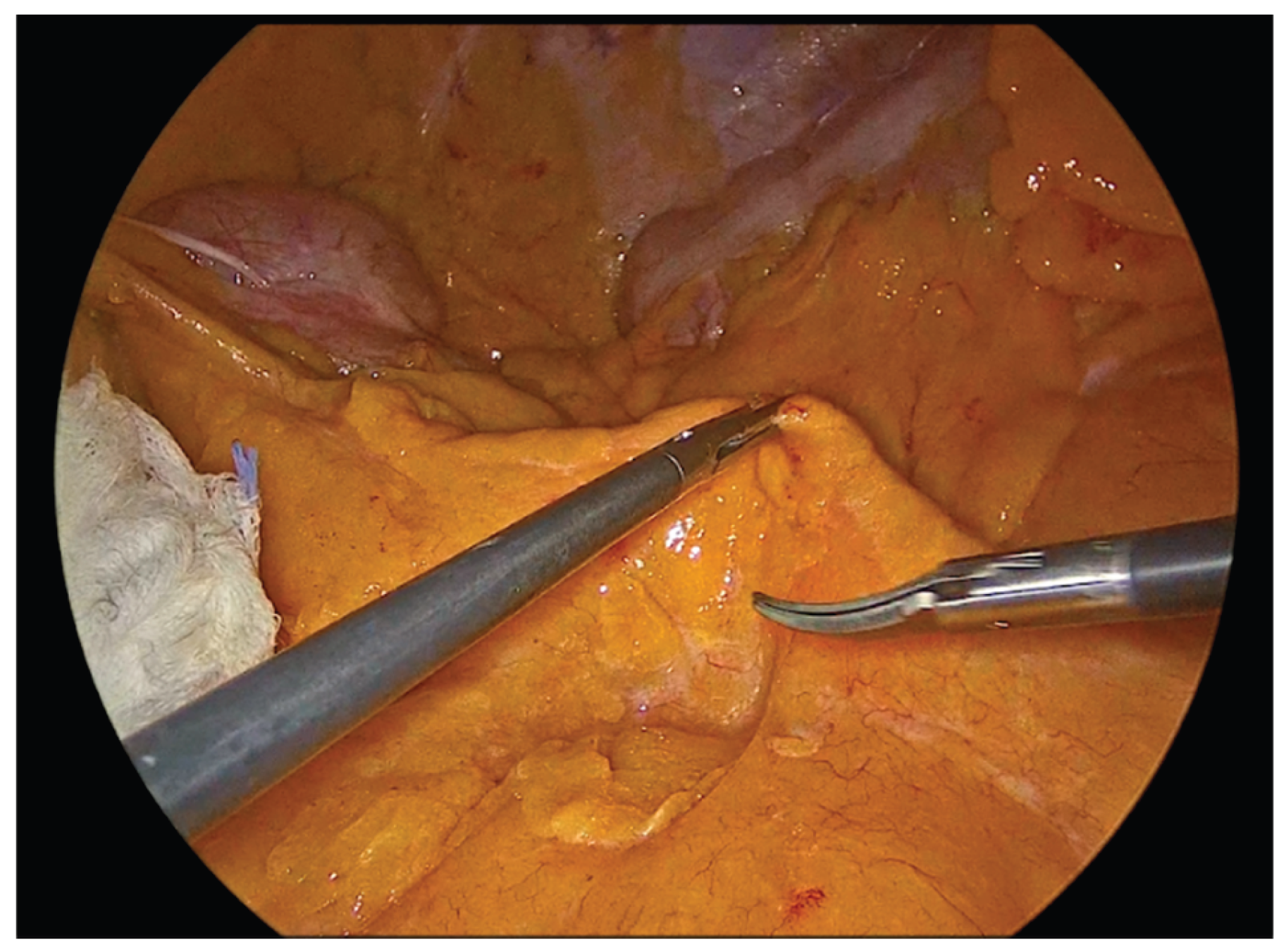
Figure 1.
Identification of the Ileocolic pedicle.
Figure 1.
Identification of the Ileocolic pedicle.
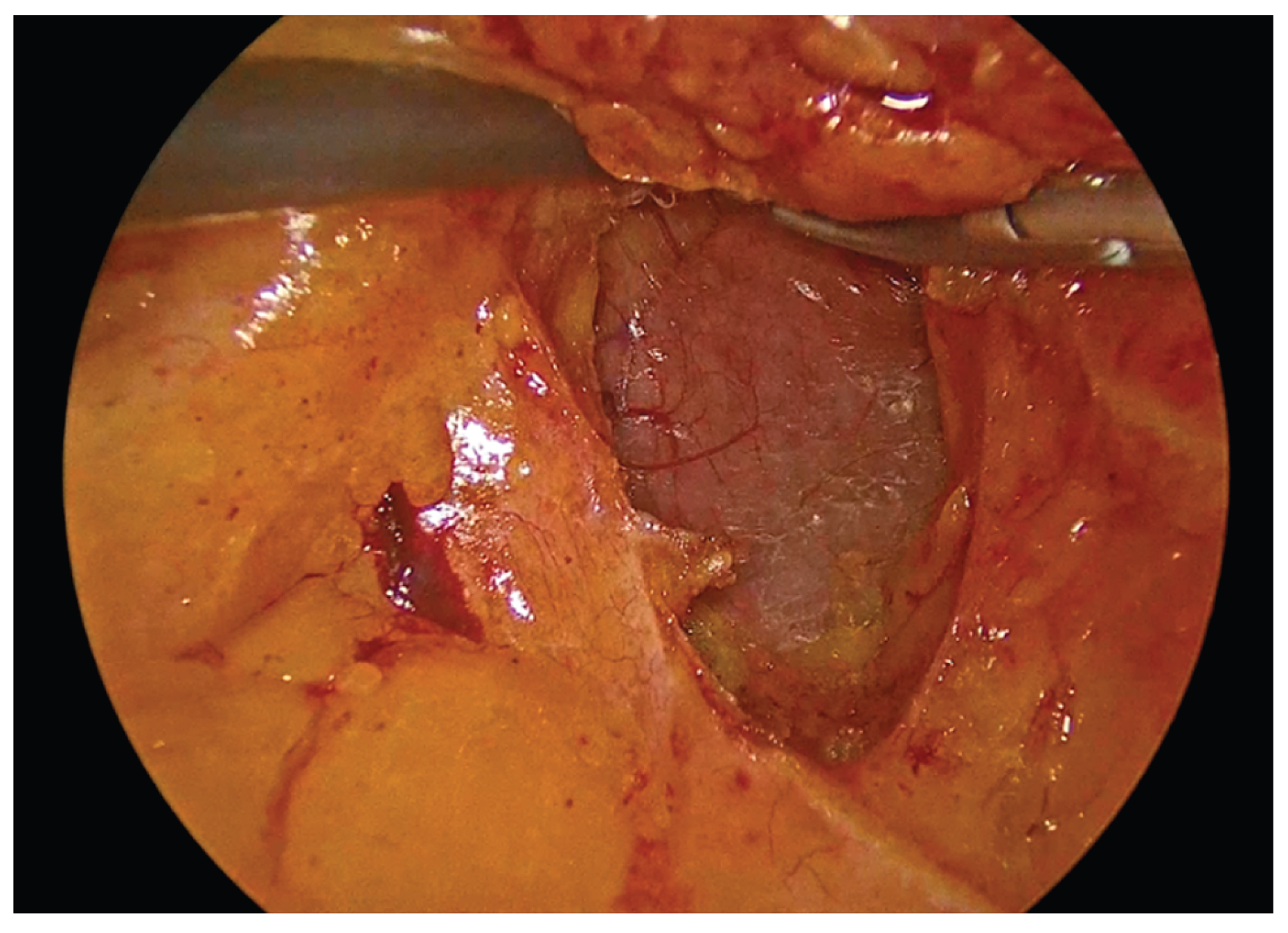
Figure 2.
Incision of the mesenteric peritoneum and development of the avascular plane to the retroperitoneum with protection of the duodenum.
Figure 2.
Incision of the mesenteric peritoneum and development of the avascular plane to the retroperitoneum with protection of the duodenum.
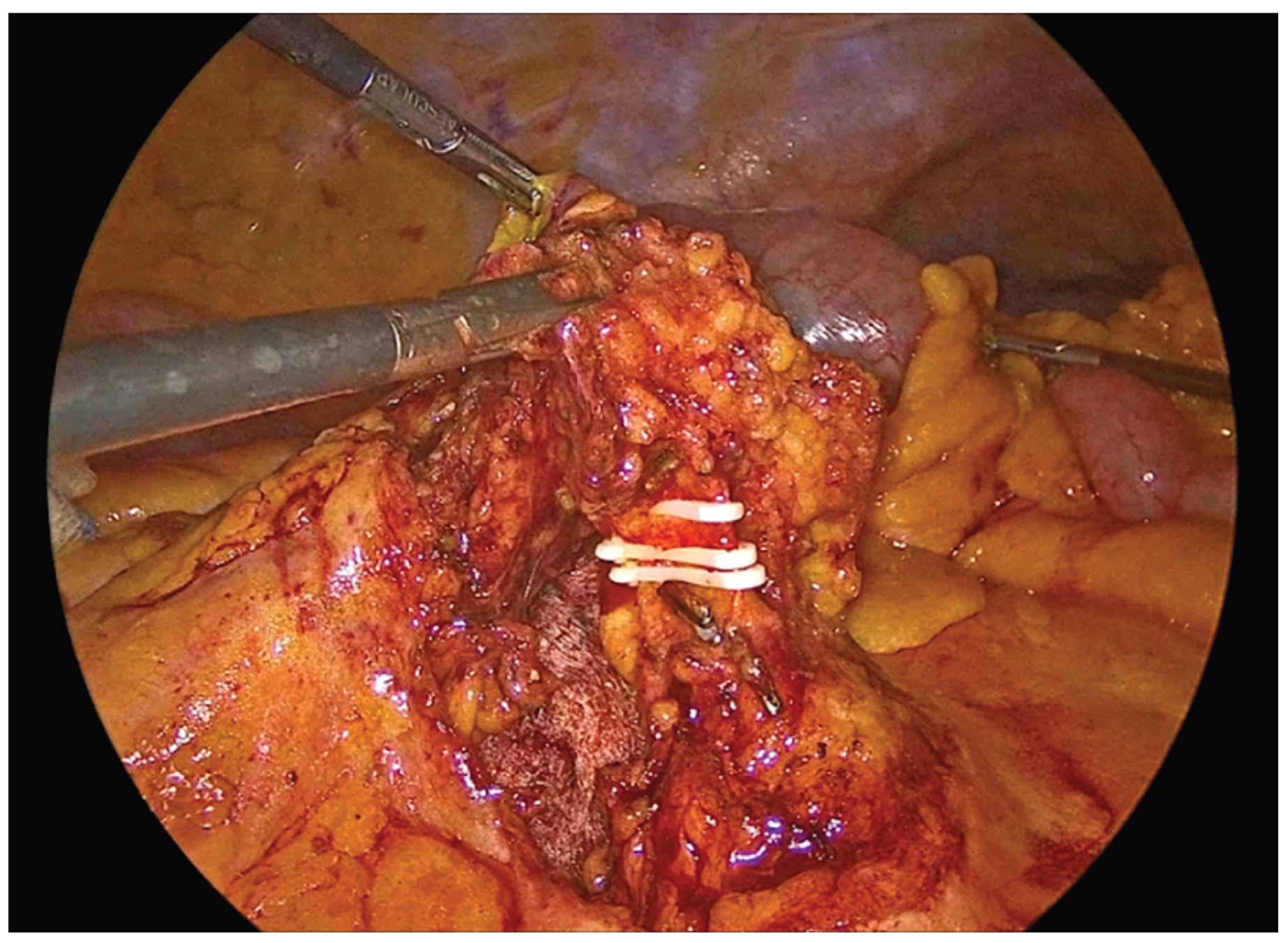
Figure 3.
Transection of the ileocolic vessels.
Figure 3.
Transection of the ileocolic vessels.
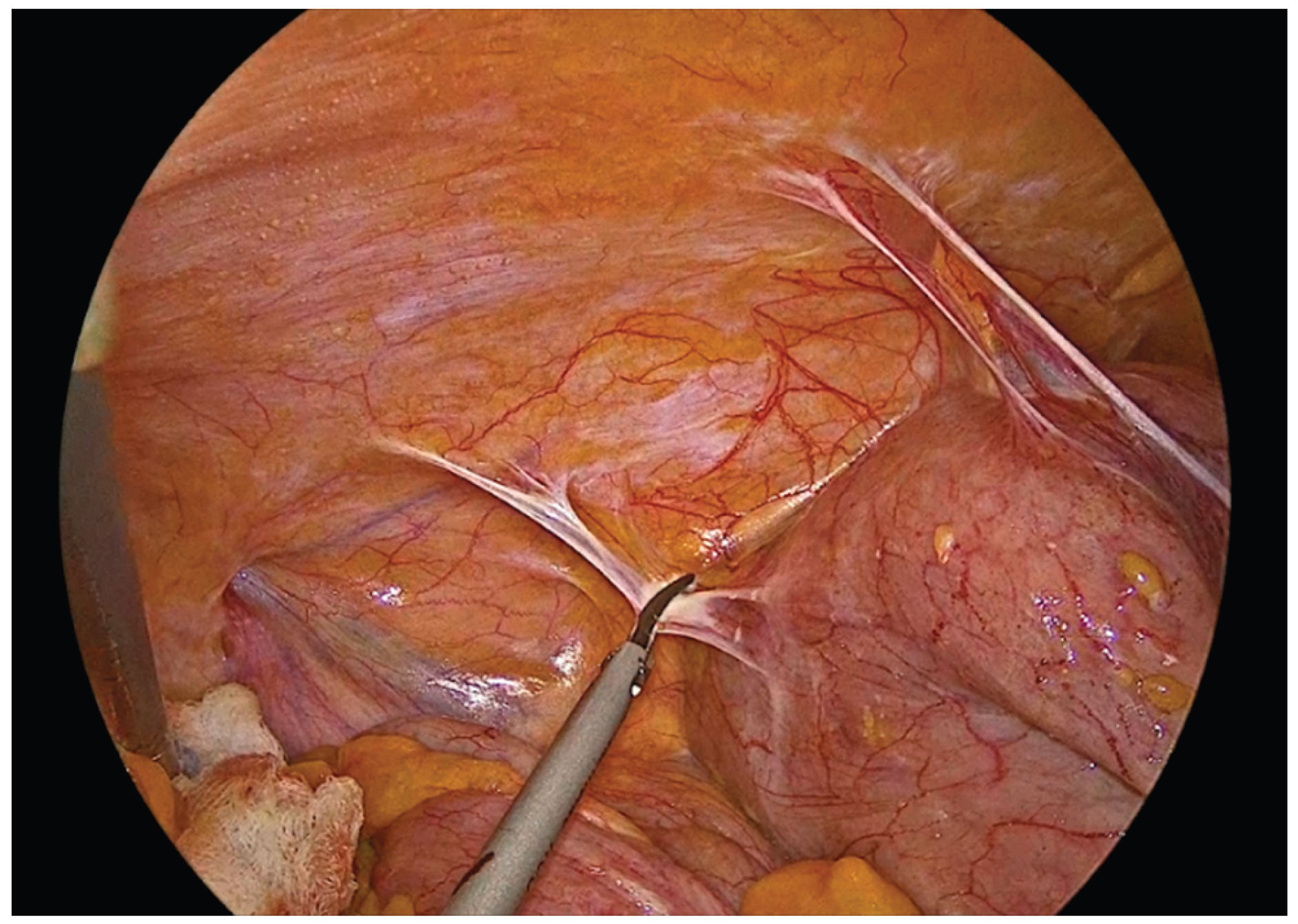
Figure 4.
Lateral mobilization of the ascending colon.
Figure 4.
Lateral mobilization of the ascending colon.
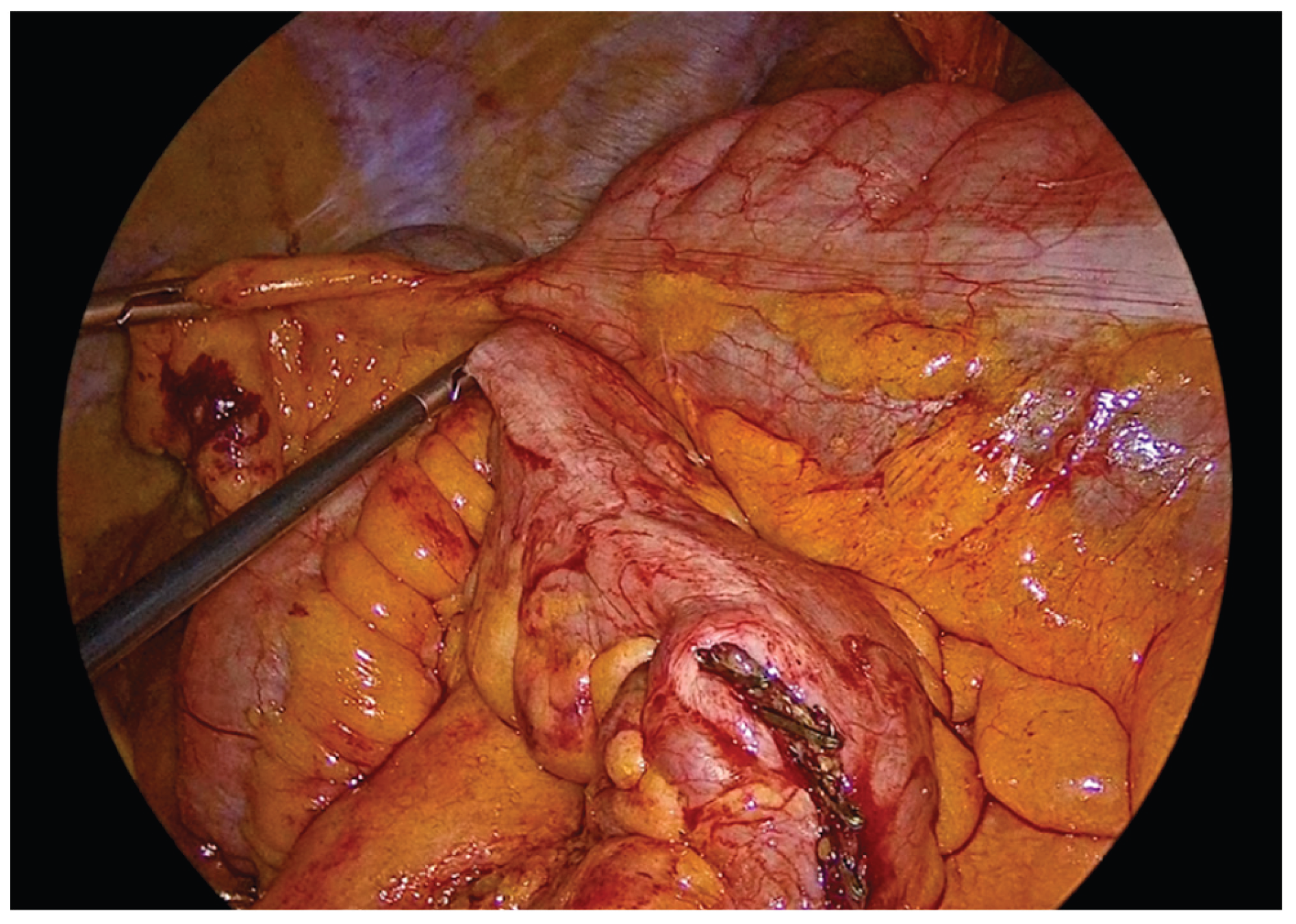
Following complete mobilization of the specimen and the transection of the mesocolon and associated vessels, the terminal ileum and transverse colon are divided using a 60mm endoscopic linear stapler. The specimen is temporarily positioned over the liver to maximize the operative field and facilitate construction of the anastomosis.
The transverse colon and terminal ileum are aligned in an isoperistaltic, side-to-side orientation, approximately 5 cm proximal to the stapled ends using barbed No.3-0 suture 15cm long. A single - layer handsewn intracorporeal anastomosis is then fashioned using a Vicryl No.3-0 suture 34cm long.
Figure 5.
Alignment and positioning of the terminal ileum and transverse colon in an isoperistaltic, side-to-side configuration.
Figure 5.
Alignment and positioning of the terminal ileum and transverse colon in an isoperistaltic, side-to-side configuration.
The anastomosis begins with a continuous seromuscular layer using a barbed 3-0 non - absorbable suture, extending for approximately 8 to 10 cm, providing reinforcement and support to the anastomosis.
Figure 6.
Side to side isoperistaltic sutured alignment of transverse colon with ileum.
Figure 6.
Side to side isoperistaltic sutured alignment of transverse colon with ileum.
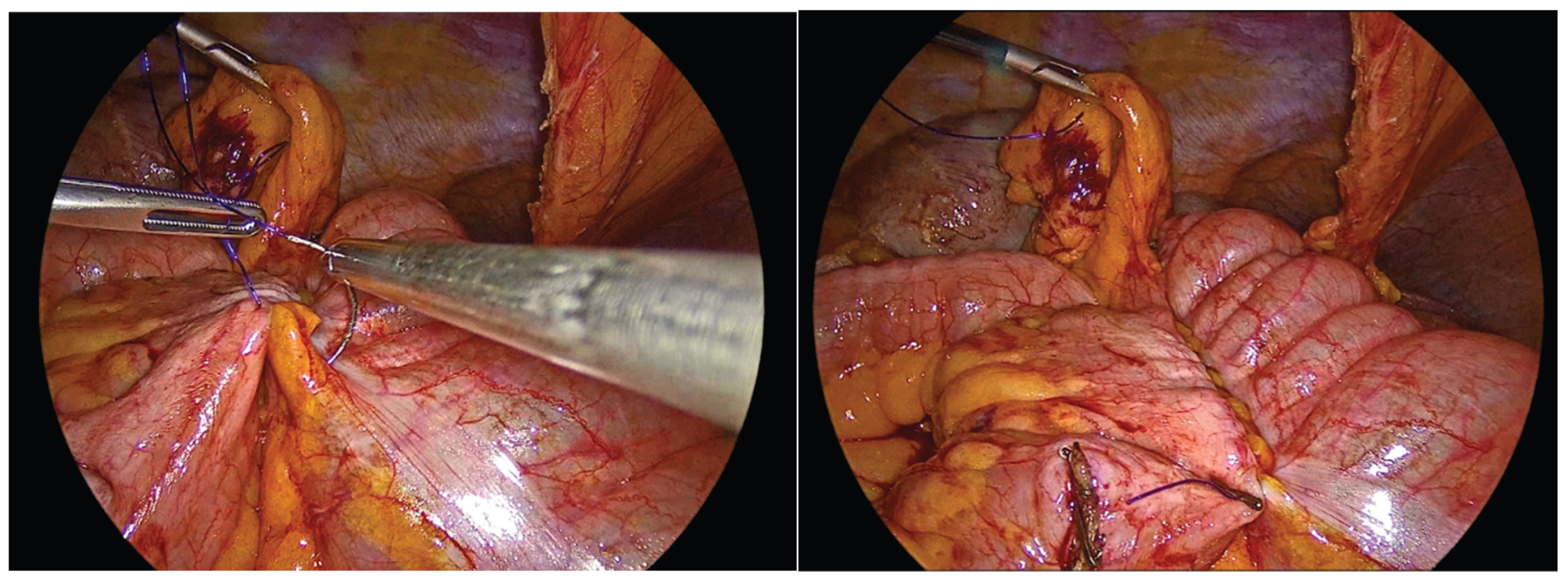
Enterotomies are subsequently created on the antimesenteric borders of both bowel segments, at approximately 1 cm from the outer suture line, with a length of 3 to 4 cm.
Figure 7.
Enterotomies of bowel ends.
Figure 7.
Enterotomies of bowel ends.
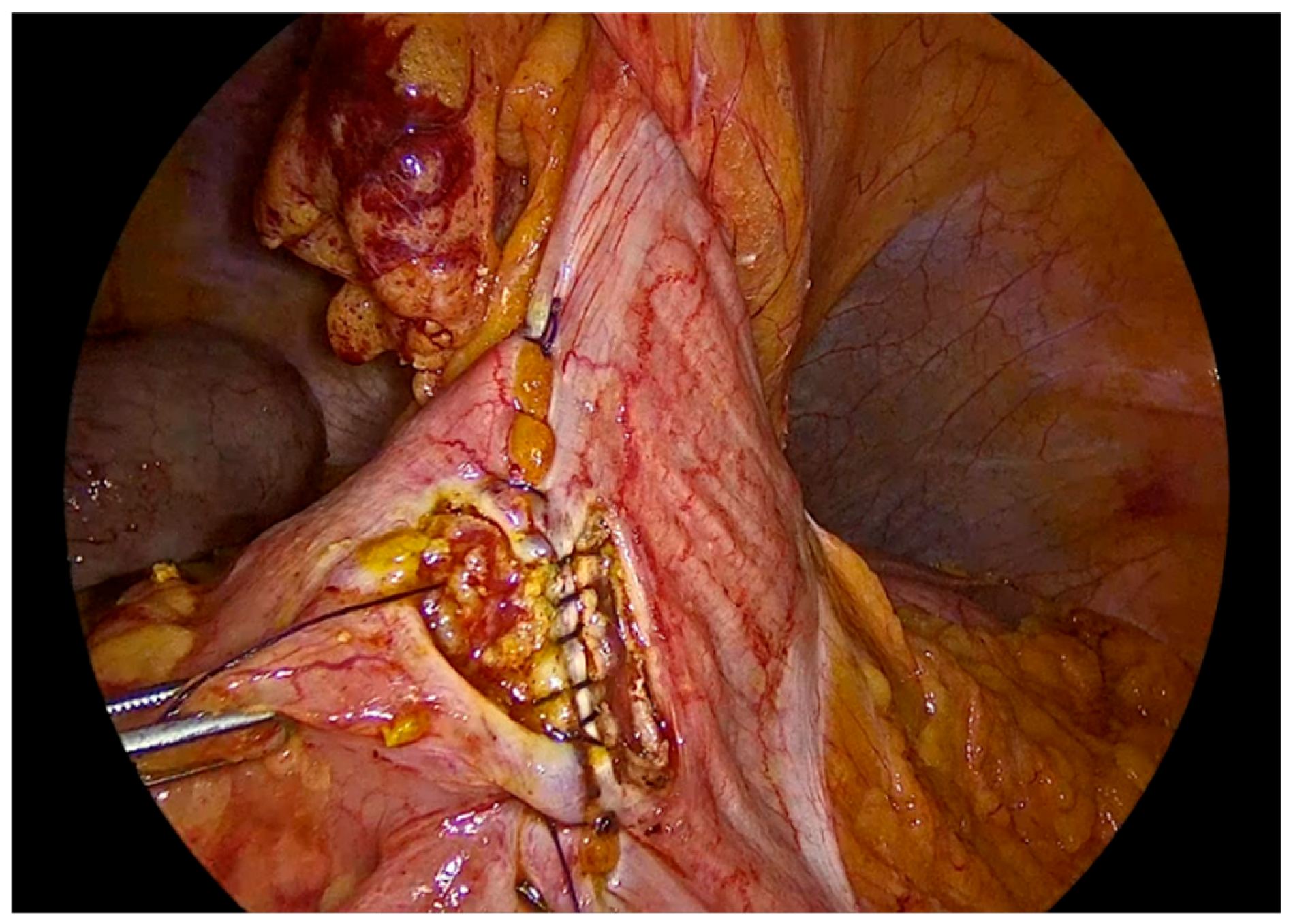
The inner layer of the anastomosis is constructed using a continuous full-thickness absorbable 3-0 Vicryl Plus suture, initiated proximally to the surgeon. Suturing proceeds from the proximal aspect toward the antimesenteric border, incorporating all the layers of the bowel lumen, with bites placed at intervals of 0,5 to 1 cm. Upon reaching the distal end of the enterotomy, the suture is continued along the opposite side, and the anterior layer of the anastomosis is completed after reaching back the proximal initial part of the anastomosis and tying the suture and to the initial knot.
Figure 8.
First suture of the anastomosis, using an absorbable 3-0 Vicryl Plus suture.
Figure 8.
First suture of the anastomosis, using an absorbable 3-0 Vicryl Plus suture.
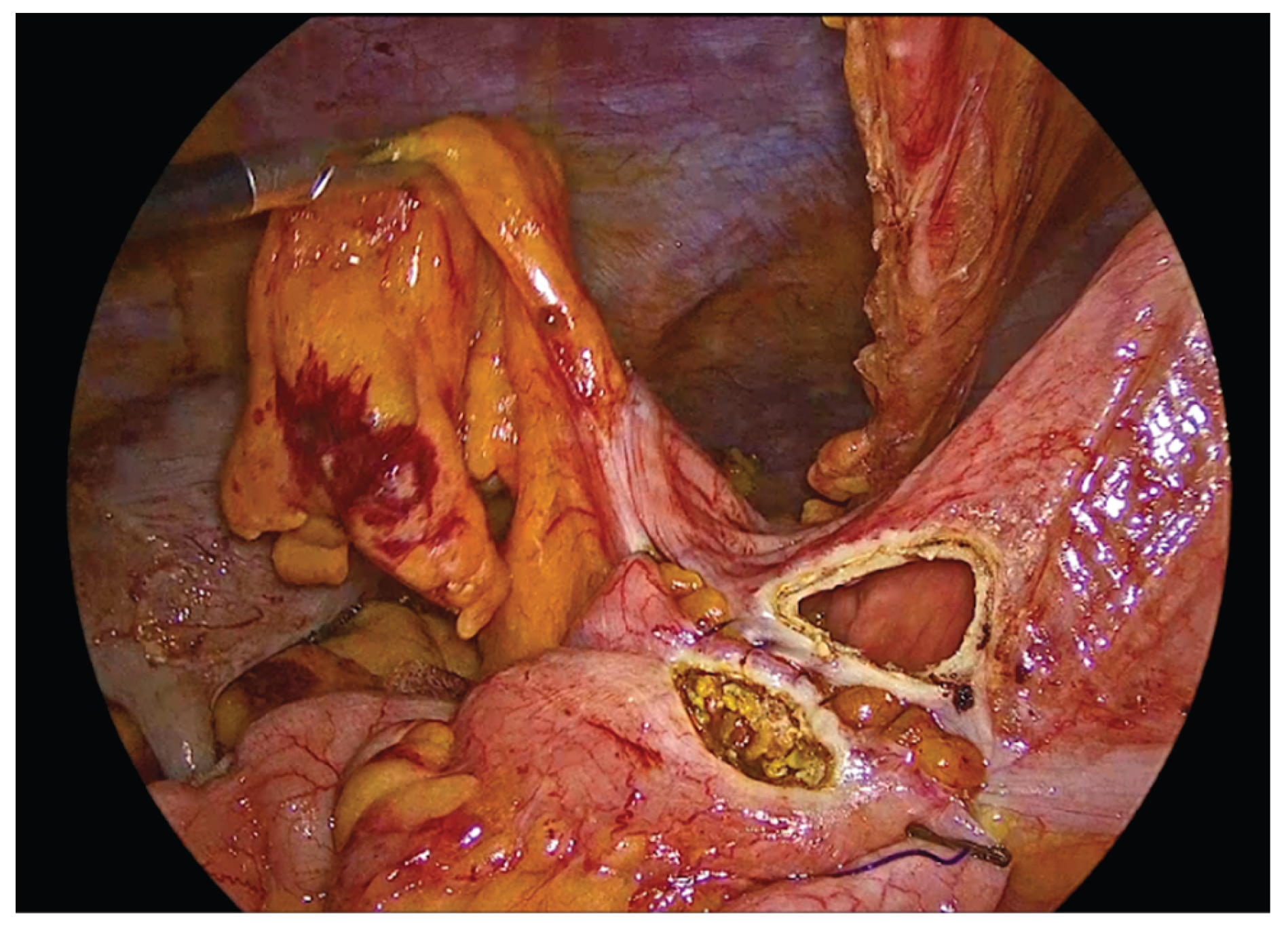
Figure 9.
The inner layer of the anastomosis.
Figure 9.
The inner layer of the anastomosis.
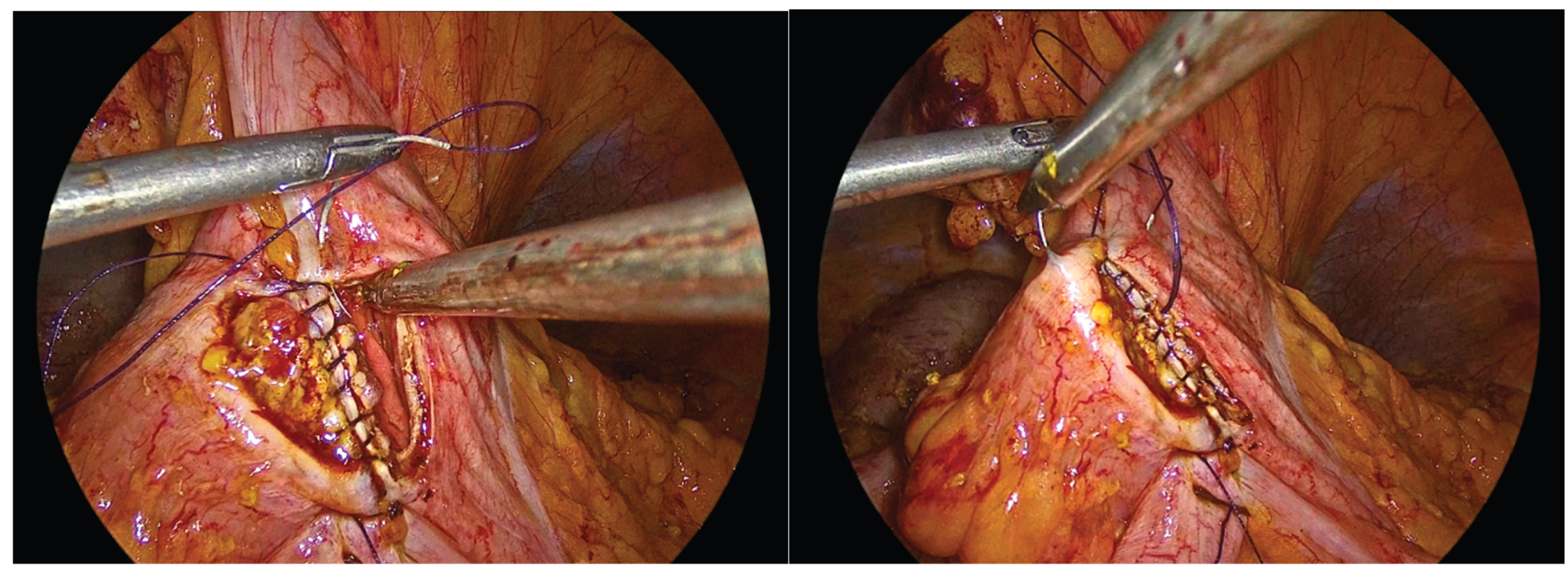
Figure 10.
Completion of the inner full-thickness layer of the anastomosis and transition to construction of the anterior layer.
Figure 10.
Completion of the inner full-thickness layer of the anastomosis and transition to construction of the anterior layer.
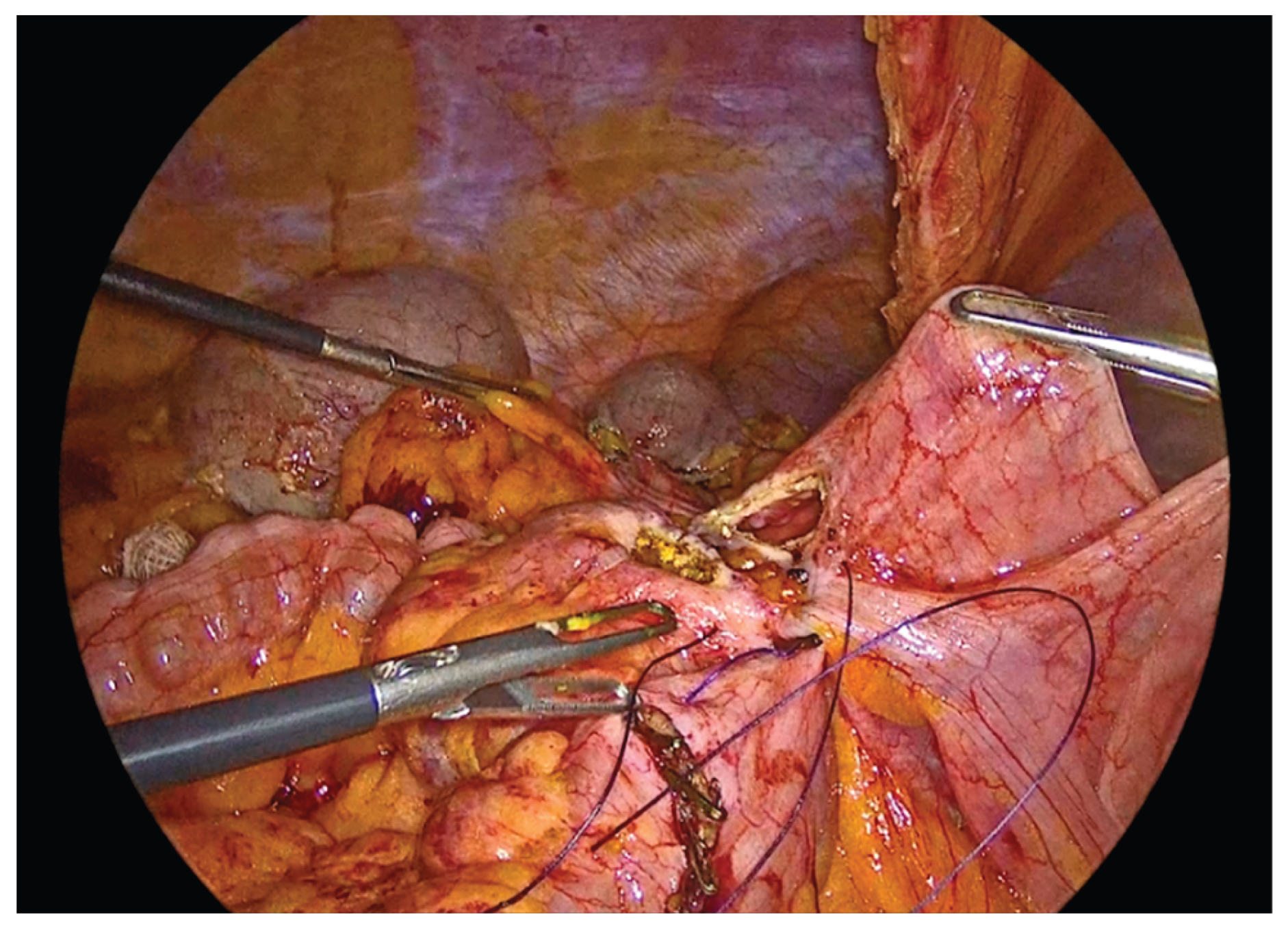
Figure 11.
Anterior outer layer of the anastomosis.
Figure 11.
Anterior outer layer of the anastomosis.
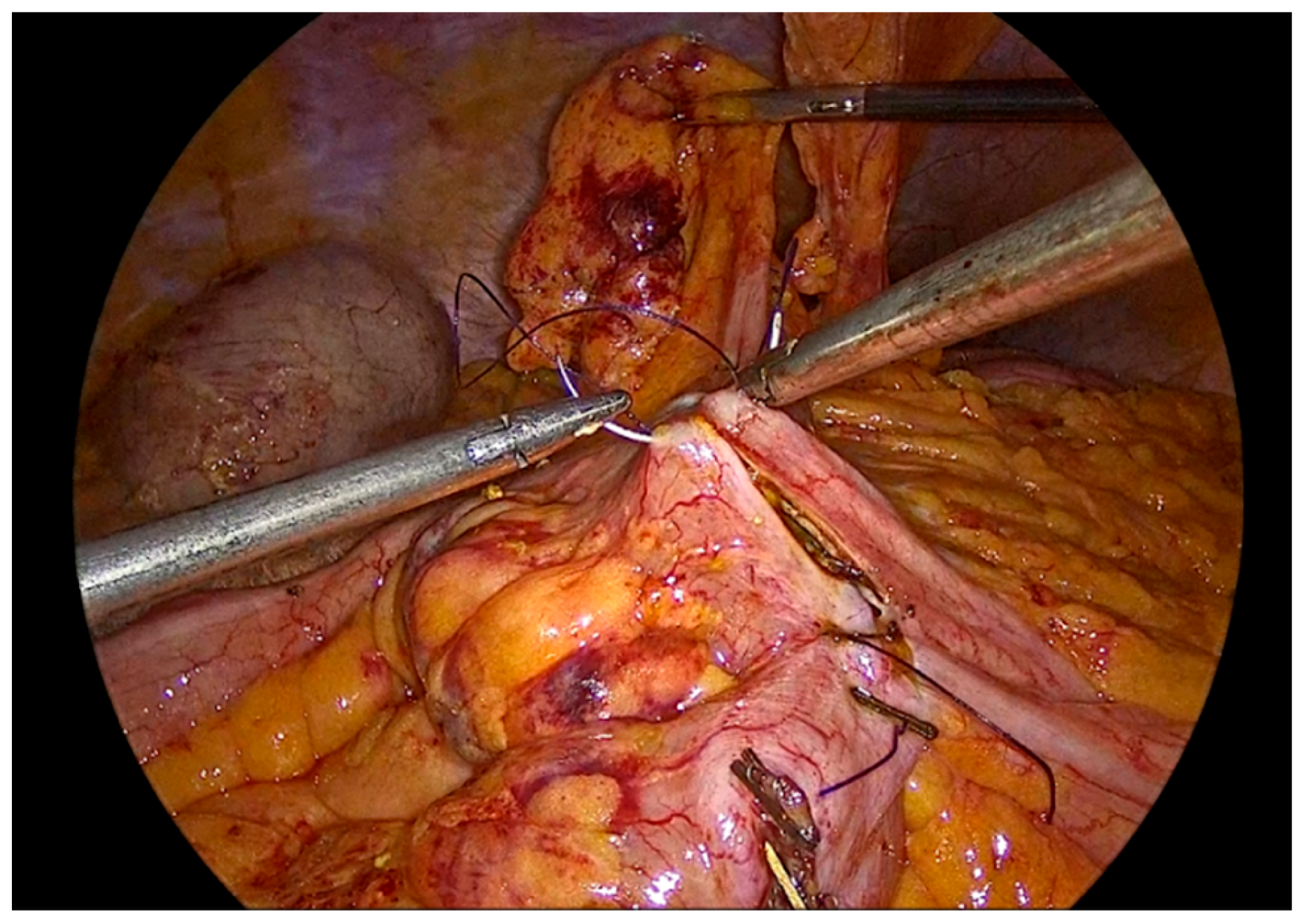
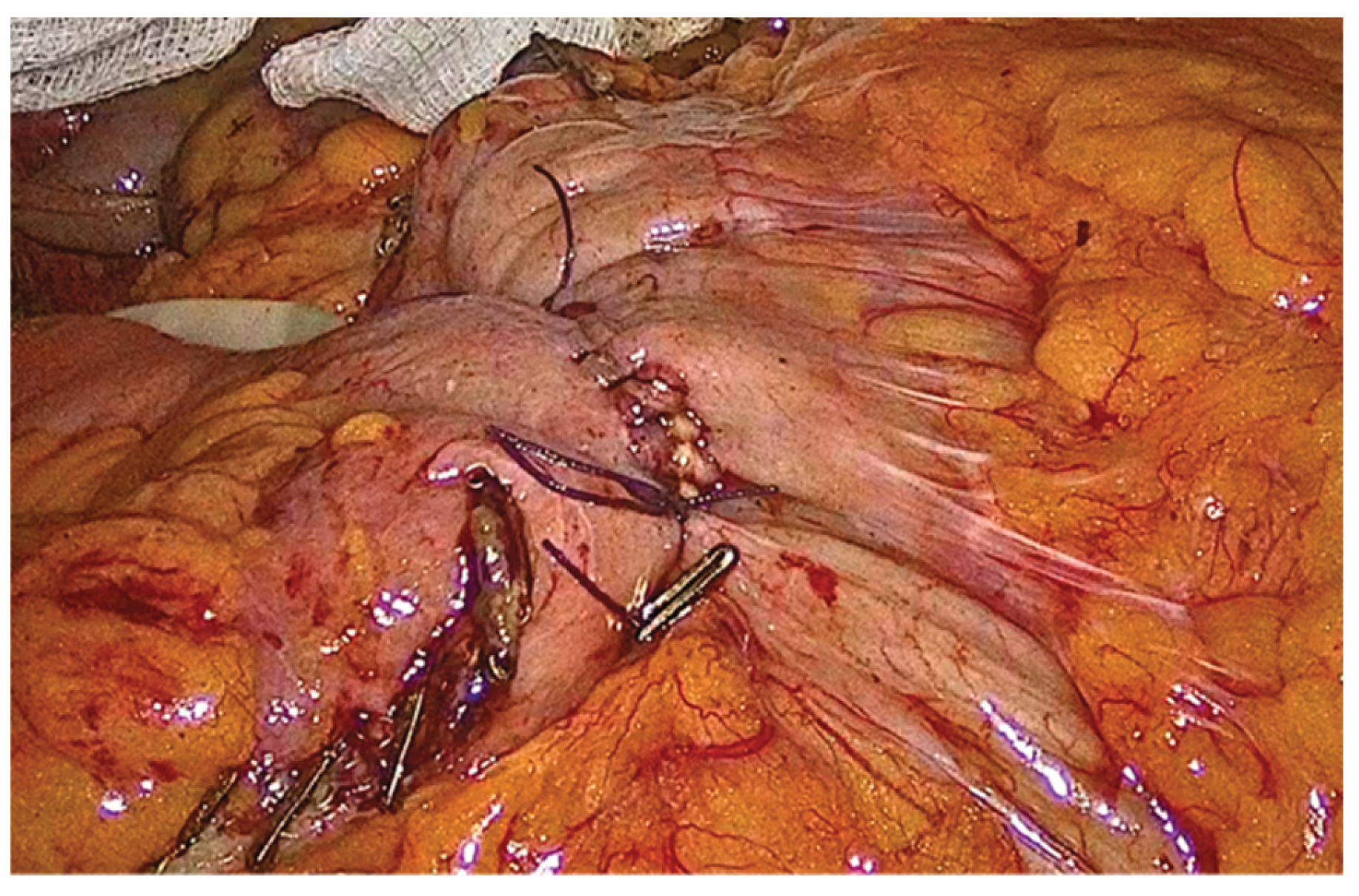
Figure 12.
The completed single-layer handsewn intracorporeal anastomosis.
Figure 12.
The completed single-layer handsewn intracorporeal anastomosis.
In order to facilitate the ease of the construction of this anastomosis, we use right-hand, left-hand, back-hand, and fore-hand techniques. We take care not to evert the mucosa of the colon or ileum, but on the contrary to invert both mucosa into the suture line. We also try to be precise with the sutures and as far as possible the anastomosis to be symmetric. Completion of the anastomosis is followed by meticulous inspection of the suture line and stapled bowel ends to ensure adequate hemostasis and the absence of hematoma formation.
Specimen extraction is performed through a Pfannenstiel incision, using a wound protector, aiming to minimize the risk of incisional hernia, infection rate and definitely pain. The procedure is concluded laparoscopically with placement of a Penrose drain posterior to the anastomosis. A final inspection of the abdominal cavity is performed to confirm hemostasis, after which pneumoperitoneum is released.
3. Results
All patients underwent standardized preoperative preparation, including mechanical bowel preparation with oral polyethylene glycol and intravenous antibiotic prophylaxis administered approximately one hour prior to surgical incision, in accordance with institutional protocols.
The mean operative time was approximately 240 minutes, which is comparable to operative durations reported for intracorporeal stapled anastomosis during the early and intermediate phases of the learning curve [
20,
25]
. Estimated intraoperative blood loss was minimal. Importantly, no bleeding from the anastomotic line or hematoma formation was observed intraoperatively, a finding of particular relevance given the higher rates of anastomotic bleeding reported in stapled techniques in both laparoscopic and conventional colorectal surgery [
23].
Postoperatively, patients commenced a liquid diet on the first postoperative day and progressed gradually according to the return of bowel function in the absence of nausea, vomiting, or abdominal distention. The time to first passage of flatus ranged from the second to the third postoperative day, consistent with the accelerated postoperative recovery commonly reported following intracorporeal anastomosis when compared with extracorporeal approaches [
16,
17,
18]. Length of hospital stay ranged from three to four days, which is comparable to, and in some cases shorter than, stays reported for intracorporeal stapled anastomosis in laparoscopic right colectomy [
25]. Postoperative pain was limited, with minimal requirement for opioid analgesics, reflecting the reduced surgical trauma associated with totally laparoscopic techniques.
This handsewn intracorporeal anastomotic technique has been routinely implemented in laparoscopic right colectomies, predominantly for malignant disease, over the past three years. A total of 68 procedures were completed laparoscopically using this method, all performed by the same surgeon, ensuring technical standardization and procedural consistency. No anastomotic leaks were observed, and no anastomosis-related postoperative complications, including stenosis or hematoma formation, were identified. These outcomes are comparable to those reported for intracorporeal stapled anastomosis in the literature and suggest that a handsewn approach does not compromise anastomotic safety when performed by experienced surgeons [
20,
25].
Oncologic outcomes were appropriate and in accordance with established oncologic principles. Adequate proximal and distal resection margins were achieved, and lymph node harvest was satisfactory in all malignant cases, indicating that the use of a handsewn intracorporeal anastomosis did not adversely affect oncologic radicality. Taken together, these findings support the feasibility, safety, and oncologic adequacy of handsewn intracorporeal anastomosis and provide a clinical framework for its comparison with stapled techniques, as discussed in the subsequent sections.
4. Discussion
The concept of the ideal anastomosis in colorectal surgery has traditionally been defined by fundamental surgical principles, namely a technique that is safe, reproducible, and technically accessible, while minimizing postoperative morbidity and complication rates. Ideally, such an anastomosis should be easily performed by surgeons with varying levels of expertise, should not require highly advanced technical skills, and should demonstrate consistently low rates of anastomotic failure [
32]. Despite significant advances in surgery, no single anastomotic technique has yet fulfilled all these criteria. Consequently, there remains no universally accepted gold standard for anastomotic construction in colorectal surgery, and the optimal approach continues to be a matter of debate.
As previously discussed, numerous studies have compared extracorporeal (EA) to intracorporeal anastomosis (IA) in colorectal surgery, particularly in the setting of laparoscopic right colectomy. The majority of available evidence supports the superiority of IA over EA in several operative and postoperative parameters. Operative outcomes such as conversion rates to open surgery, incision length, and estimated blood loss have consistently been shown to be significantly lower in IA cohorts, provided that procedures are performed by experienced surgeons with advanced laparoscopic skills. Reduced blood loss is of particular clinical relevance, as is associated with improved short-term outcomes and reduced postoperative morbidity [
12,
14].
Furthermore, the intracorporeal approach minimizes excessive bowel manipulation and mesenteric traction, which is often unavoidable during extracorporeal anastomosis. By avoiding exteriorization of the bowel through a mini laparotomy, IA reduced the risk of mesenteric stretching, vascular compromise, hemorrhage, hematoma formation, and devascularization of the bowel ends, all of which may negatively impact anastomotic integrity and healing. These advantages likely contribute to the improved anastomotic viability observed in IA groups and support is increasing adoption in minimally invasive colorectal surgery [
12,
13]
.
Another well-documented benefit of intracorporeal anastomosis is the reduction of postoperative infectious complications, particularly surgical site infections. Smaller extraction incisions, reduced tissue trauma and the lack of exposure to bowel contents, translate into lower wound infection rates and a diminished need for wound-related reoperation [
12,
14]. This advantage is particularly relevant in high-risk patient populations, including obese patients and those with multiple comorbidities, in whom wound- related complications are more frequent and associated with prolonged hospital stay and increased healthcare costs [
15].
When evaluating the role of intracorporeal anastomosis across different patient risk profiles, studies have demonstrated that totally laparoscopic colectomies provide comparable surgical outcomes in high-risk patients when compared with low-risk populations [
15]. Importantly, rates of anastomotic leakage, internal hernia formation, reoperation, and overall mortality do not differ significantly between IA and EA groups [
12,
13]
. These findings suggest that IA can be safely applied across a broad spectrum of patients when appropriate surgical expertise is available.
A noteworthy observation in literature is that completion of the learning curve for intracorporeal anastomosis does not appear to adversely affect anastomotic leakage rates. On the contrary, perioperative complication rates are often lower in IA groups compared with EA cohorts, even during early adoption phase [
12]
. This observation highlights the inherent advantages of the technique itself and suggests that once basic proficiency is achieved, intracorporeal anastomosis may offer more consistent outcomes than extracorporeal approaches.
Postoperative bowel function represents another important outcome parameter favoring intracorporeal anastomosis. Multiple studies have reported faster recovery of bowel movements, earlier tolerance of oral intake, and reduced postoperative ileus in IA patients [
16,
17,
18]
. These benefits are likely multifactorial, reflecting reduced bowel manipulation, diminished surgical stress response, and improved anastomotic geometry. In particular, IA allows easier construction of a tension-free anastomosis without the need for extensive mobilization of the transverse colon to facilitate exteriorization through a mini-laparotomy [
13,
19,
28]. Despite these advantages, widespread adoption of IA remains limited, largely due to the advanced laparoscopic skills and manual dexterity required for intracorporeal anastomosis.
To date, to our knowledge, there is a notable absence of studies comparing intracorporeal handsewn and stapled anastomosis in laparoscopic colorectal surgeries. In contrast, such comparisons have been more extensively explored in upper gastrointestinal surgery, where intracorporeal handsewn anastomosis has been routinely performed for decades [
36]
, and more recently in robotic-assisted colorectal surgery [
20,
21,
22,
25]
.
The scarcity of laparoscopic data may be partially explained by the technical advantages offered by robotic platforms, including three-dimensional visualization, articulated instruments that replicate wrist-like movements, enhanced ergonomics, and a tremor filtration. These features substantially lower the technical barriers to performing intracorporeal handsewn anastomosis and may explain the earlier adoption of this technique in robotic surgery [
25,
26]
.
Evidence from robotic studies suggests that handsewn anastomosis may be associated with increased operative time but shorter overall hospital stay [
25]
. The prolonged operative duration is likely attributable to the technical demands of handsewn suturing. However, this difference appears to diminish with increasing surgeon experience [
20]. Additionally, standardization of the individual steps involved in construction of a handsewn anastomosis has been shown to significantly reduce operative time, supporting the notion that reproducibility and efficiency improve with protocol-driven approaches [
19]
.
Bleeding from the anastomotic line represents another critical determinant of anastomotic success. Several studies have demonstrated that anastomotic bleeding occurs more frequently in stapled anastomoses and, although uncommon, can occasionally necessitate endoscopic or surgical intervention [
23]. Similar findings have been reported in conventional colorectal surgery, where stapled anastomoses have been associated with higher rates of postoperative bleeding and anastomotic strictures compared with handsewn techniques [
24,
29]. These observations suggest that handsewn anastomosis may offer superior control over tissue approximation and hemostasis, potentially translating into improved anastomotic quality.
5. Conclusions
In this study, we present a standardized technique for an isoperistaltic side-to-side handsewn intracorporeal ileocolic anastomosis following laparoscopic right colectomy. Our experience demonstrates that this technique can be safely and effectively performed in a fully intracorporeal manner in both malignant and benign conditions requiring right colectomy or ileocecal resection. When fundamental surgical principles are meticulously applied, intracorporeal handsewn anastomosis represents a feasible and safe alternative to stapled techniques.
Author Contributions
Conceptualization, G.A.; methodology, T.C.; validation, E.K., and G.A.; investigation, T.C., L.K., K.N and K.S; resources, T.C., D.N, M.T. and G.A.; data curation, M.L., D.M, A.K. and M.P.; writing—original draft preparation, T.C.; writing—review and editing, G.A. and T.C; visualization, T.C., G.A, and P.D. ; supervision, C.I., and G.A; project administration, G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IA |
Intracorporeal Anastomosis |
| EA |
Extracorporeal Anastomosis |
References
- Jacobs, M; Verdeja, JC; Goldstein, HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991, 1(3), 144–50. [Google Scholar] [PubMed]
- Fowler, D.L.; White, S.A. Laparoscopy-assisted sigmoid resection. Surgical Laparoscopy & Endoscopy 1991, 1(3), 183–188. [Google Scholar]
- Casciola, L.; et al. [Laparoscopic right hemicolectomy with intracorporeal anastomosis. Technical aspects and personal experience]. Minerva Chirurgica 2003, 58(4), 621–627. [Google Scholar]
- Lechaux, D. [Intra-corporeal anastomosis in laparoscopic right hemicolectomy]. Journal De Chirurgie 2005, 142(2), 102–104. [Google Scholar] [CrossRef]
- Selvy, M.; et al. Intra-versus extracorporeal anastomosis in laparoscopic right colectomy: a meta-analysis of 3699 patients. International Journal of Colorectal Disease 2020, 35(9), 1673–1680. [Google Scholar] [CrossRef]
- Vaghiri, S.; et al. Intracorporeal Versus Extracorporeal Colo-colic Anastomosis in Minimally-invasive Left Colectomy: a Systematic Review and Meta-analysis. Journal of Gastrointestinal Surgery 2023, 27(12), 3024–3037. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, A.; et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World Journal of Gastroenterology 2018, 24(21), 2247–2260. [Google Scholar] [CrossRef] [PubMed]
- Kirk, RM; Winslet, M. Essential General Surgical Operations.
- Goulder, F. Bowel anastomoses: The theory, the practice and the evidence base. World Journal of Gastrointestinal Surgery 2012, 4(9), 208–213. [Google Scholar] [CrossRef]
-
Chassin’s Operative Strategy in General Surgery; Springer: Heidelberg, 2002.
- Symeonidis, D.; et al. Isoperistaltic vs antiperistaltic anastomosis after right hemicolectomy: A comprehensive review. World Journal of Clinical Cases 2023, 11(8), 1694–1701. [Google Scholar] [CrossRef]
- Ricci, C.; et al. A critical and comprehensive systematic review and meta-analysis of studies comparing intracorporeal and extracorporeal anastomosis in laparoscopic right hemicolectomy. Langenbeck’s Archives of Surgery 2017, 402(3), 417–427. [Google Scholar] [CrossRef]
- Goldstone, R.N.; Popowich, D.A. Laparoscopic Intracorporeal Anastomosis. Clinics in Colon and Rectal Surgery 2022, 36(1), 74–82. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; et al. Intracorporeal Versus Extracorporeal Anastomosis in Laparoscopic Right Colectomy: An Updated Systematic Review and Cumulative Meta-Analysis. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 2020, 30(4), 402–412. [Google Scholar]
- Iorio, T.; Blumberg, D. Totally intracorporeal laparoscopic colectomy (TILC) is associated with similar surgical outcomes in high and low operative risk patients. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2013, 23(2), 154–158. [Google Scholar] [CrossRef]
- Allaix, M.E.; et al. Intracorporeal or Extracorporeal Ileocolic Anastomosis After Laparoscopic Right Colectomy: A Double-blinded Randomized Controlled Trial. Annals of Surgery 2019, 270(5), 762–767. [Google Scholar] [CrossRef] [PubMed]
- Małczak, P.; et al. Bowel function after laparoscopic right hemicolectomy: a randomized controlled trial comparing intracorporeal anastomosis and extracorporeal anastomosis. Surgical Endoscopy 2022, 36(7), 4977–4982. [Google Scholar] [CrossRef]
- Vignali, A.; et al. Extracorporeal vs. Intracorporeal Ileocolic Stapled Anastomoses in Laparoscopic Right Colectomy: An Interim Analysis of a Randomized Clinical Trial. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 2016, 26(5), 343–348. [Google Scholar]
- Sorgato, N.; et al. Right colectomy with intracorporeal anastomosis for cancer: a prospective comparison between robotics and laparoscopy. Journal of Robotic Surgery 2022, 16(3), 655–663. [Google Scholar] [CrossRef]
- Harji, D.; et al. A multicentre, prospective cohort study of handsewn versus stapled intracorporeal anastomosis for robotic hemicolectomy. Colorectal Disease: The Official Journal of the Association of Coloproctology of Great Britain and Ireland 2022, 24(7), 862–867. [Google Scholar] [CrossRef]
- Guadagni, S.; et al. Ileo-colic intra-corporeal anastomosis during robotic right colectomy: a systematic literature review and meta-analysis of different techniques. International Journal of Colorectal Disease 2021, 36(6), 1097–1110. [Google Scholar] [CrossRef]
- D’Annunzio, E.; et al. Laparoscopic robot-assisted right colectomy with intracorporeal hand-sewn anastomosis. Journal of Visceral Surgery 2020, 157(6), 499–504. [Google Scholar] [CrossRef]
- Mari, G.; et al. Endoscopic Treatment of Anastomotic Bleeding in Laparoscopic Colorectal Surgery. Chirurgia (Bucharest, Romania: 1990) 2019, 114(2), 295–299. [Google Scholar] [CrossRef]
- MacRae, H.M.; McLeod, R.S. Handsewn vs. stapled anastomoses in colon and rectal surgery: a meta-analysis. Diseases of the Colon and Rectum 1998, 41(2), 180–189. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.M.; et al. Robotic NICE Procedure Using Handsewn Technique. Diseases of the Colon and Rectum 2022, 65(5), e324–e327. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; et al. Analysis of clinical efficacy and safety of hand-sewn anastomosis for the digestive tract with Da Vinci robot in rectal cancer surgery. World Journal of Surgical Oncology 2023, 21(1), 317. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.M.; Segelcke, D.; Schug, S.A. Postoperative pain-from mechanisms to treatment. Pain Reports 2017, 2(2), e588. [Google Scholar] [CrossRef] [PubMed]
- Kohl, B.A.; Deutschman, C.S. The inflammatory response to surgery and trauma. Current Opinion in Critical Care 2006, 12(4), 325–332. [Google Scholar] [CrossRef]
- Neutzling, C.B.; et al. Stapled versus handsewn methods for colorectal anastomosis surgery. The Cochrane Database of Systematic Reviews 2012, 2012(2), CD003144. [Google Scholar] [CrossRef]
- Blackmore, A.E.; Wong, M.T.C.; Tang, C.L. Evolution of laparoscopy in colorectal surgery: An evidence-based review. World Journal of Gastroenterology: WJG 2014, 20(17), 4926–4933. [Google Scholar] [CrossRef]
- Cauley, C.E.; Valente, M.A.; Champagne, B.J. Laparoscopic right colectomy: technique and atlas. Annals of Laparoscopic and Endoscopic Surgery 2019, 4(0). [Google Scholar] [CrossRef]
- The Art of Bowel Anastomosis - C. Chen. 2012.
- Wang, Z.-Q.; Hao, H.-K.; Hong, J. A novel method for intracorporeal end-to-end colorectal anastomosis using a linear stapler. Journal of Gastrointestinal Surgery 2025, 29(4), 101992. [Google Scholar] [CrossRef] [PubMed]
-
Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice, 21rst Edition; 2021.
- Hamamoto, H.; et al. Closure of enterotomy after side-to-side ileocolic anastomosis with two barbed sutures in totally laparoscopic colectomy for right-sided colon cancer. Surgery Today 2021, 51(3), 457–461. [Google Scholar] [CrossRef]
- Genser, L.; et al. Laparoscopic Roux-en-Y gastric bypass with hand-sewn gastro-jejunostomy. Journal of Visceral Surgery 2017, 154(1), 37–45. [Google Scholar] [CrossRef] [PubMed]
- Frasson, M.; et al. Risk Factors for Anastomotic Leak After Colon Resection for Cancer: Multivariate Analysis and Nomogram From a Multicentric, Prospective, National Study With 3193 Patients. Annals of Surgery 2015, 262(2), 321–330. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).