1. Introduction
Normal pressure hydrocephalus (NPH) has been recognized as a treatable neurological disorder that causes brain dysfunction mostly in the elderly population [
1]. It represents one of the few potentially reversible causes of gait disturbance, cognitive decline and urinary incontinence [
2]. NPH is classified as a form of communicating hydrocephalus and is characterized by an expansion of the ventricles, while the intracranial pressure (ICP) stays within normal values (7-15 mmHg) [
3].
The diagnosis of NPH, can be challenging, particularly due to the multiple comorbidities that this population of elderly patients presents. The diagnosis of NPH is primarily based on the combination of clinical presentation and radiological imaging findings obtained by magnetic resonance imaging (MRI) or computed tomography (CT). Typically seen with imaging technique is ventriculomegaly disproportionate to cortical atrophy, enlargement of the lateral ventricles, and associated signs such as narrowing of the high-convexity sulci or periventricular signal changes [
4]. Lateral ventricle enlargement is quantified using the Evans index [
4,
5]. An Evans index greater than 0.30 is considered indicative of ventriculomegaly [
4]. It is calculated as the ratio of the maximal frontal horn width to the maximum inner skull diameter [
4,
5]. The specific triad of symptoms, with which NPH commonly presents is the Hakim-Adams triad, and includes gait disturbance, cognitive disorders and urinary incontinence [
2]. The complete triad of symptoms is only present in approximately half of the cases, making the diagnosis challenging due to its varied presentation and overlap with other neurodegenerative disorders common in the elderly [
2]. The differential diagnosis includes cortical dementias, such as Alzheimer’s disease and frontotemporal dementia, and subcortical dementias - Lewy-body dementia, Parkinson’s disease and vascular parkinsonism [
6].
The exact pathophysiologic pathway for NPH remains unclear [
7]. The characteristic clinical triad of NPH is thought to result from ventricular enlargement and altered cerebrospinal fluid dynamics affecting vulnerable periventricular and frontal–subcortical pathways [
8]. Gait disturbance, often the earliest and most prominent symptom, is associated with involvement of corticospinal fibers adjacent to the lateral ventricles, while cognitive impairment reflects disruption of frontal–subcortical circuits [
8]. Urinary incontinence is attributed to early involvement of periventricular pathways responsible for supraspinal bladder control [
8].
Surgical treatment of NPH is based on cerebrospinal fluid (CSF) diversion through the insertion of a ventriculoperitoneal shunt (VPS) [
9]. Although alternative CSF diversion techniques such as ventriculoatrial and ventriculopleural shunting exist, their use in elderly patients is thus limited by higher systemic complication risks [
10]. Their use mainly stems from recurrent VPS failures [
10]. VPS remains the gold standard treatment option and represents the most effective intervention capable of reversing or stabilizing symptoms in Hakim-Adams triad in appropriately selected patients [
9]. In Japan however, lumboperitoneal shunting has historically been used as the predominant surgical treatment for NPH even more frequently than VPS in national practice patterns [
11]. A nationwide Japanese study reported that lumboperitoneal shunts constituted approximately 55% of all procedures for NPH, compared with 43% for VPS [
11]. This practice is based on the perception that lumboperitoneal shunting is less invasive, as it does not require cranial entry [
11]. However, VPS was associated with a more stable profile regarding mechanical complications, as lumboperitoneal shunt-treated patients required more frequent surgical revisions due to tube displacement or blockage, as demonstrated in the SINPHONI-2 trial [
11]. Because not all patients benefit from either surgical treatment, preoperative predictive tests to assess the likelihood of shunt responsiveness are routinely recommended [
12]. The most commonly used predictive method is a large-volume lumbar puncture, during which a sufficient amount of CSF is removed to evaluate short-term clinical improvement [
12]. Following shunt implantation, improvement in one or more components of the clinical triad has been reported in approximately 60–90% of patients, although the degree and durability of symptom relief vary across studies [
13].
Despite the relative frequency of NPH in aging populations, data on surgical outcomes remain limited, particularly in Slovenia. This lack of regional evidence prompted us to conduct a retrospective analysis of elderly patients (aged 65 years and older) who underwent VPS implantation for NPH at the University Medical Centre Ljubljana between 2015 and 2025. The aim of this study was to evaluate pre- and postoperative clinical outcomes, associated comorbidities, and the incidence of postoperative complications.
2. Materials and Methods
2.1. Study Design and Patient Selection
The study was designed as a single-center retrospective observational analysis and included all elderly patients, aged 65 or older, who underwent VPS implantation for NPH at the University Medical Centre Ljubljana between January 2015 and December 2025. All eligible patients treated during the study period were consecutively included to minimize selection bias and to reflect routine clinical practice. Data were collected retrospectively from hospital medical records and electronic databases. For each patient, we recorded demographic data (age, sex), time from symptom onset to surgery, number and duration of hospitalizations, pre- and postoperative clinical status, comorbidities, and postoperative complications. Patients with incomplete medical documentation, patients in whom the shunt was inserted for other indications, patients who were not treated at University Medical Centre Ljubljana, and patients who had their first procedure before reaching the age of 65, were excluded from the study.
2.2. Diagnostic Criteria and Data Characteristics
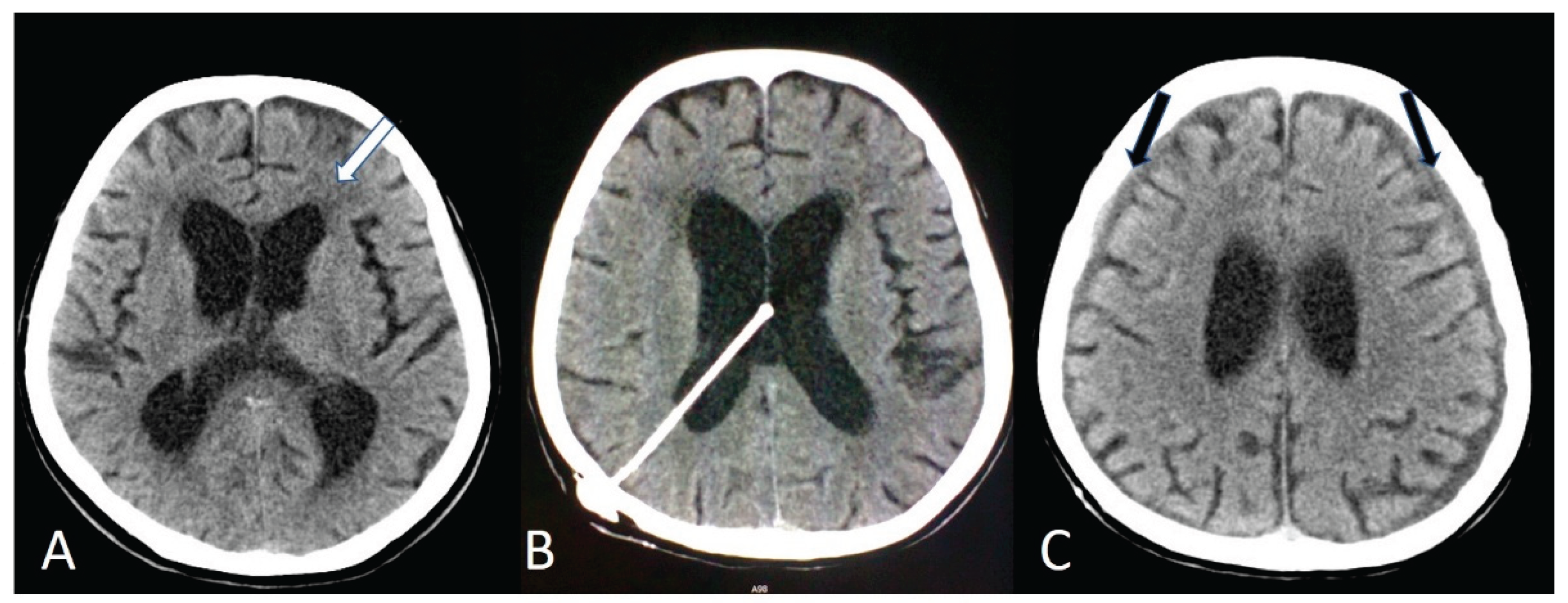
The diagnosis of NPH was based on the clinical presentation and MRI/CT findings. Imaging assessment focused on ventriculomegaly disproportionate to cortical atrophy and enlargement of the lateral ventricles (
Figure 1A).
The presence of components of the Hakim–Adams triad, including gait disturbance, cognitive impairment, and/or urinary incontinence, was evaluated both preoperatively and postoperatively. Gait disturbance was assessed using Eide’s revised clinical gait grading scale, which ranges from grade 1 (severe gait impairment with inability to walk independently) to grade 5 (normal, independent gait). Cognitive impairment was evaluated clinically based on neurological examination and documented changes in memory, attention, and executive function, as reported in medical records. Urinary incontinence was defined as the presence of involuntary urine leakage requiring protective measures, reported during the clinical examination. Postoperative improvement in gait, cognition, and urinary continence was determined through follow-up clinical evaluations and categorized dichotomously as improved or not improved, based on documented physician assessment and patient or caregiver reports. Comorbidities were categorized into vascular, cardiovascular, neurological, and other subgroups. Postoperative complications and comorbidities were recorded as present or absent. Postoperative complications were identified during a follow-up postoperative clinical examination.
2.3. Preoperative Evaluation
As part of the standard preoperative evaluation, all patients underwent a cerebrospinal fluid tap test to support the diagnosis and assess the likelihood of responsiveness to shunt surgery. The procedure consisted of a standard lumbar puncture with removal of a large volume of cerebrospinal fluid, typically ranging between 30 and 50 mL [
14]. Clinical reassessment focused primarily on gait performance, cognitive status, and urinary symptoms and was conducted within hours to days following the procedure. In patients with inconclusive or borderline responses, repeat or extended lumbar drainage was performed at the discretion of the treating physician.
2.4. Surgical Technique and Perioperative Management
All patients underwent VPS implantation under general anesthesia. A medium-pressure fixed differential valve was used in all cases to ensure procedural standardization. The ventricular catheter was inserted into either the frontal or occipital horn of the lateral ventricle (
Figure 1B) based on surgeon preference and patient-specific anatomical considerations. The distal catheter was tunneled subcutaneously and placed into the peritoneal cavity. Standard perioperative antibiotic prophylaxis was administered according to institutional protocol. Postoperative cranial imaging was routinely performed to verify the catheter position and to assess for early complications.
2.5. Data Analysis
Descriptive statistics (mean, median, standard deviation, proportions, frequency distribution) were used to analyze the data. Gender-related differences were assessed using the Mann–Whitney U test. A p-value of less than 0.05 was considered statistically significant. Missing data were not imputed and were excluded from analyses involving the affected variables.
3. Results
3.1. Patient Characteristics
From 2015 to 2025, 124 patients with the diagnosis of NPH were hospitalized at the University Medical Centre Ljubljana. Of these, 98 patients (79.0%) were aged 65 years or older and were therefore eligible for inclusion in our study. Male patients were 60/98 (61.2%) and females were 38/98 (38.8%). The mean age was 73.84 ± 5.59 years. Data on the interval from symptom onset to surgery were available for 54/98 (55.1%) patients. This interval ranged from 3 months to several years, with most patients undergoing surgery within the first 12 months from the onset of symptoms. The mean time from the onset of symptoms to surgery was 6.51 months.
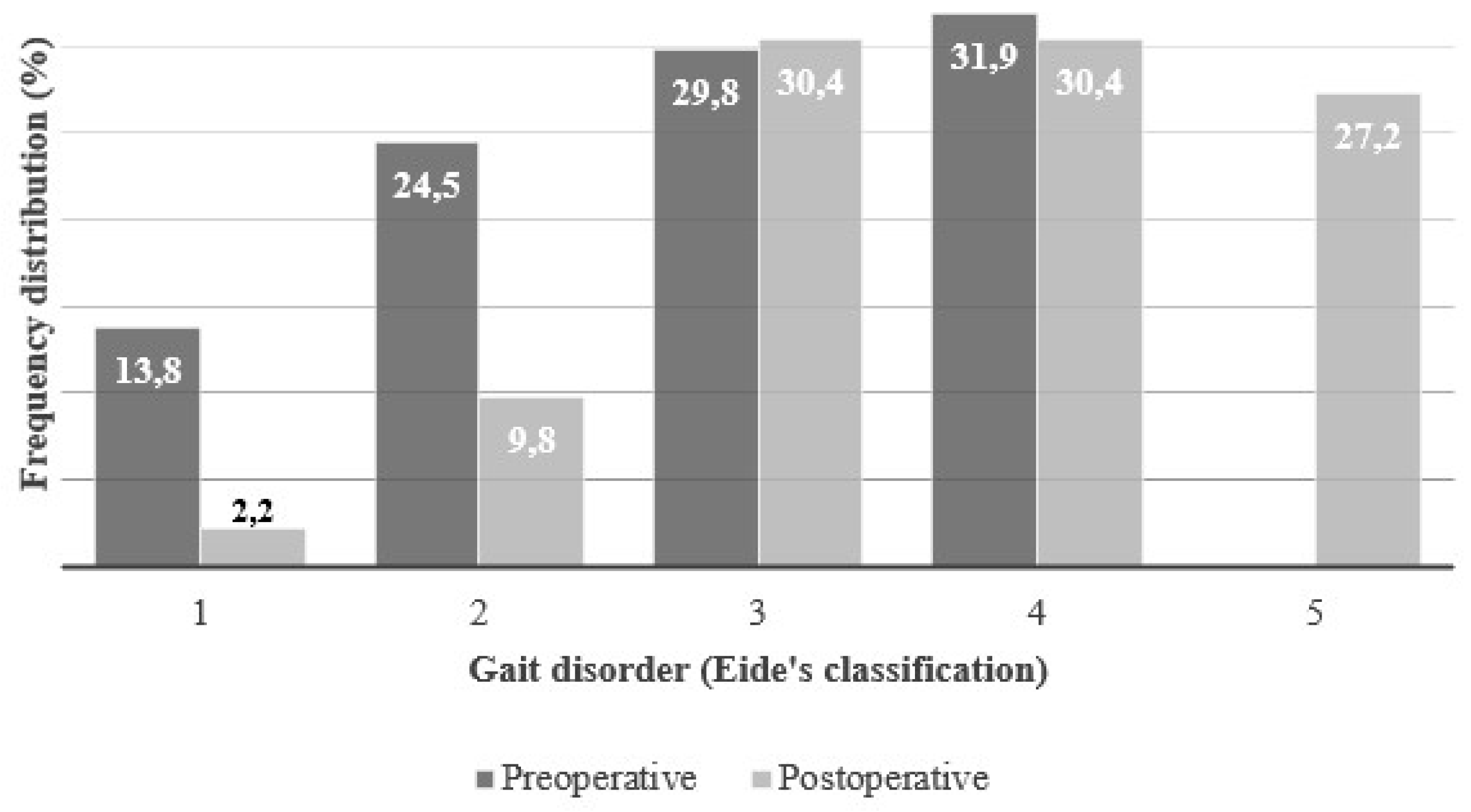
Nearly all patients, 94/98 (95.9%), presented with gait disturbances, classified according to Eide’s grading scale from 1 to 5 (
Figure 2).
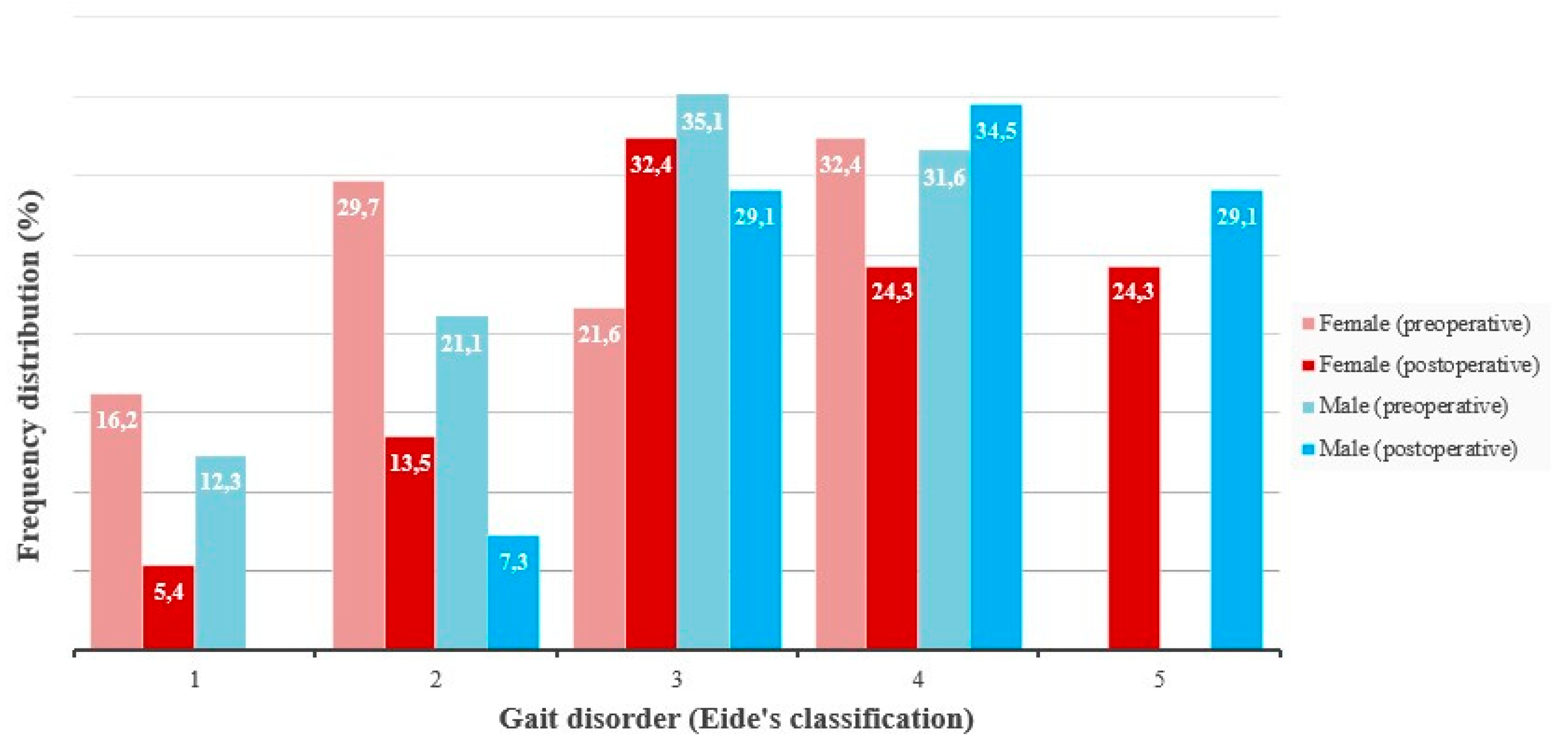
The frequency distribution of patients by Eide gait grade was as follows: grade 1 (13.8%), grade 2 (24.5%), grade 3 (29.8%), grade 4 (31.9%), and grade 5 (0.0%). No statistically significant difference in gait disturbance was observed between genders (men: n = 57, female: n = 37, U = 972.00, p = 0.5091) (
Figure 3).
Cognitive impairment was present in 80/98 (81.6%) patients, and urinary incontinence in 61/98 (62.2%). The complete Hakim triad was observed in 56/98 (57.1%) patients.
3.2. Surgical Procedure
All patients underwent VPS placement using a medium-pressure differential valve.
The ventricular catheter was placed in the frontal or occipital horn (
Figure 1B), based on the preference of each surgeon, or on the specific anatomical conditions of each patient. The average length of hospital stay was 10.81.
3.3. Postoperative Outcomes
Postoperative gait data were available for 92/98 (93.8%) patients. The frequency distribution according to Eide grading was: grade 1 (2.2%), grade 2 (9.8%), grade 3 (30.4%), grade 4 (30.4%), and grade 5 (27.2%) (
Figure 2). The proportion of patients in Eide’s stages 1 and 2 (those who were unable to walk independently), decreased from 38.3% to 12.0%. At the same time, 27.2% of patients improved to stage 5 (meaning they were able to walk completely independently). No statistically significant difference in gait disturbance was observed between genders (men: n = 57, female: n = 37, U = 842.50, p = 0.1480) (
Figure 3).
Postoperative cognitive outcomes were available for 80/98 patients (81.6%), among whom 63/80 (78.8%) demonstrated cognitive improvement. Postoperative urinary incontinence data were available for 55/98 patients (56.1%), and improvement in urinary incontinence was observed in 34/55 patients (61.8%). Following surgery, the complete Hakim triad persisted in only 5.4% of patients.
3.4. Complications
Postoperative shunt infections occurred in 2/98 (2.0%) patients. An intracerebral bleeding occurred in 6/98 (6.1%) patients. Revision surgery was required in 14/98 (14.3%) patients and it was most commonly due to overdrainage, which manifested as headache in 9/14 (64.3%) patients, dizziness in 3/14 (21.4%), and bilateral hygromas (
Figure 1C) in 5/14 (35.7%). Rare complications following VPS placement included hearing loss, wound dehiscence, and gastrointestinal issues, each occurring in a single patient. A postoperative prophylactic antiepileptic treatment with Levetiracetam was given to 9/98 (9.2%) patients and no seizures were reported in any patient after surgery.
3.5. Comorbidities
Comorbidities were documented in 94/98 (95.9%) patients across several organ systems (
Table 1). Multiple comorbidities (more than two) were present simultaneously in 52/98 (53.1%) patients.
4. Discussion
The demographic characteristics of our cohort are consistent with large epidemiological studies of normal pressure hydrocephalus, which consistently report a predominance of elderly patients with a slight male preponderance. Previous population-based studies from Scandinavia and Japan have reported mean ages ranging from 72 to 76 years, closely matching the mean age of 73.84 years observed in our cohort [
11,
15,
16]. This demographic alignment supports the external validity of our findings and confirms that the Slovenian NPH population does not differ substantially from those reported in larger international series.
The high prevalence of gait disturbance (95.9%) and the fact that 38.3% of patients presented with severe ambulation impairment (Eide Grades 1 and 2) underscores the disease's impact on mobility [
14,
17]. Gait disturbance was the most prevalent presenting symptom in our study, consistent with the literature identifying gait impairment as the earliest and most sensitive clinical marker of NPH [
15,
17,
18].
Furthermore, the short mean time from symptom onset to surgery (6.51 months) suggests that a shorter symptom duration correlates with improved post-operative outcomes [
19,
20,
21]. In contrast, several large observational studies describe diagnostic delays ranging from 12 to 36 months, largely attributable to symptom overlap with Alzheimer’s disease, Parkinsonian syndromes, and vascular dementia [
19,
20,
21,
22]. Andrén et al. demonstrated that prolonged symptom duration prior to shunting is a significant negative predictor of postoperative improvement, particularly for gait and cognition [
23]. Similarly, Toma et al. emphasized that earlier surgical intervention is associated with superior functional recovery and sustained benefit over time [
19]. The comparatively prompt diagnosis and referral observed in our cohort may therefore explain the high postoperative response rates across all components of the Hakim–Adams triad.
The surgical management of NPH through VPS remains the most effective intervention for reversing the symptoms of the Hakim triad [
11,
24]. Data from our cohort robustly support this therapeutic efficacy, particularly in the context of standardized fixed-pressure shunting.
Following VPS placement, the clinical results demonstrated significant functional recovery. The proportion of patients unable to walk independently (Eide Grades 1 and 2) decreased from 38.3% to 12.0%. Moreover, the attainment of completely independent gait (Eide grade 5) in 27.2% of patients exceeds rates reported in several earlier cohorts, where full gait normalization was observed in approximately 15–25% of cases [
17]. This marked improvement, coupled with high rates of cognitive (78.8%) and urinary (61.8%) improvement, resulted in the complete Hakim triad persisting after surgery in only 5.4% of patients. These outcomes are at the upper range of reported success rates in the literature, which typically cites overall improvement between 60% and 90% [
15,
25,
26]. Importantly, cognitive response to shunting has been shown to correlate inversely with the presence of concomitant neurodegenerative pathology, particularly Alzheimer’s disease [
27,
28,
29]. Despite a high burden of neurological comorbidities in our cohort, the observed cognitive benefit suggests that careful preoperative selection, including CSF tap testing, remains effective even in clinically complex elderly populations. The relatively lower responsiveness of urinary symptoms compared to gait and cognition has been attributed to irreversible damage to supraspinal bladder control pathways in advanced disease stages.
All patients in this cohort were treated with a standardized medium-pressure differential valve. The rationale for this fixed-pressure approach lies in its mechanical simplicity and inherent reliability, which may contribute to the low incidence of hardware failure, though it contrasts sharply with current practices that favor programmable valves, for their capacity to regulate CSF drainage non-invasively [
30,
31,
32]. The limitations of the fixed-pressure system might be reflected by the complication profile. While the shunt infection rate of 2.0% (2/98) is comparable to the rate of 2–5% often cited in major series, the revision rate of 14.3% (mainly due to overdrainage) is noteworthy [
20]. Overdrainage is a recognized consequence of a differential pressure valve and by means of adjustable valves these complications might be managed by adjusting the valve resistance, potentially lowering the need for revision surgery. Anzai et al., a SRMA publication of four comparative studies, failed to demonstrate clear superiority of programmable over fixed-pressure valves in terms of long-term clinical outcomes, despite lower revision rates in some series [
32,
33,
34].
The proactive postoperative use of Levetiracetam in 9.2% of patients is a point of specific interest. The absence of reported seizures in the entire cohort suggests the efficacy of this prophylactic measure, although further investigation is required to establish whether routine anti-epileptic prophylaxis is universally warranted in NPH shunting.
The high prevalence of comorbidities in this cohort reflects the clinical reality of NPH in the elderly. More than half of patients had multiple comorbid conditions, with vascular and neurological disorders being particularly common. Vascular risk factors such as arterial hypertension and diabetes mellitus have been implicated in the pathophysiology of NPH through mechanisms involving reduced cerebrovascular compliance and impaired CSF absorption [
35,
36,
37]. Nevertheless, several studies have demonstrated that the presence of comorbidities does not preclude meaningful improvement after shunt surgery, provided that NPH is correctly diagnosed and appropriately treated [
37].
5. Conclusions
This retrospective analysis strongly confirms that normal pressure hydrocephalus is a quite common diagnosis in the elderly, yet it is often overlooked. We analyzed the medical documentation of 98 patients in the period of 10 years that were hospitalized and treated for NPH. The data indicate that most patients underwent treatment fairly quickly - within a year of symptom onset, with satisfactory outcomes reflected in the postoperative absence of the Hakim's triad. A low complication frequency was noted as well as a low incidence of re-operations. Apart from the overall predominance of males among patients, no significant gender differences were observed in symptom presentation, treatment or postoperative outcome. Almost all patients had previously known comorbidities, mostly vascular and neurological, highlighting the complexity of treating these patients and emphasizing the importance of a multidisciplinary approach. Due to the lack of a conservative approach that can successfully manage this condition, our data hereby aligns with foreign study findings, confirming that surgical intervention is both essential and relatively safe and effective, even in this fragile patient population.
Author Contributions
Conceptualization, P.S.; methodology, P.S., A.Š.J.; software, A.Š.J.; validation, P.S.; formal analysis, A.Š.J., K.L. and M.L.; investigation, A.Š.J., K.L. and M.L.; resources, P.S..; data curation, A.Š.J., K.L. and M.L.; writing—original draft preparation, A.Š.J.; writing—review and editing, P.S.; visualization, A.Š.J.; supervision, P.S.; project administration, P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of UMC LJUBLNANA (protocol code 10/2025 – 12th November 2025.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| NPH |
Normal pressure hydrocephalus |
| VPS |
Ventriculoperioneal shunt |
| CSF |
Cerebrospinal fluid |
| MRI |
Magnetic resonance imaging |
| CT |
Computed tomography |
| AH |
Arterial hypertension |
| T2DM |
Type 2 diabetes mellitus |
| HLP |
Hyperlipidemia |
| AF |
Atrial fibrilation |
| BPH |
Benign prostate hyperplasia |
| SRMA |
Systematic review and meta-analysis |
References
- Liew, BS; Takagi, K; Kato, Y; Duvuru, S; Thanapal, S; Mangaleswaran, B. Current updates on idiopathic normal pressure hydrocephalus. Asian J Neurosurg. 2019, 14(3), 648–56. [Google Scholar] [CrossRef]
- Gavrilov, GV; Gaydar, BV; Svistov, DV; Korovin, AE; Samarcev, IN; Churilov, LP; et al. Idiopathic Normal Pressure Hydrocephalus (Hakim-Adams Syndrome): Clinical Symptoms, Diagnosis and Treatment. Psychiatr Danub 2019, 31 (Suppl 5), 737–44. [Google Scholar] [PubMed]
- Sahuquillo, J; Rubio, E; Codina, A; et al. Reappraisal of the intracranial pressure and cerebrospinal fluid dynamics in patients with the so-called “normal pressure hydrocephalus” syndrome. Acta Neurochir (Wien) 1991, 112, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X; Xia, J. Application of Evans Index in normal pressure hydrocephalus patients: a mini review. Front Aging Neurosci. 2022, 13, 783092. [Google Scholar] [CrossRef] [PubMed]
- Toma, AK; Holl, E; Kitchen, ND; Watkins, LD. Evans' index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery 2011, 68(4), 939–44. [Google Scholar] [CrossRef]
- Kiefer, M; Unterberg, A. The differential diagnosis and treatment of normal-pressure hydrocephalus. Dtsch Arztebl Int. 2012, 109(1-2), 15–25, quiz 26. [Google Scholar] [CrossRef]
- Bateman, GA. The reversibility of reduced cortical vein compliance in normal-pressure hydrocephalus following shunt insertion. Neuroradiology 2003, 45(2), 65–70. [Google Scholar] [CrossRef]
- Bradley, WG. Normal pressure hydrocephalus: new concepts on etiology and diagnosis. AJNR Am J Neuroradiol 2000, 21(9), 1586–90. [Google Scholar]
- Nakajima, M; Yamada, S; Miyajima, M; Ishii, K; Kuriyama, N; Kazui, H; et al. Guidelines for Management of Idiopathic Normal Pressure Hydrocephalus (Third Edition): Endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurol Med Chir (Tokyo) 2021, 61(2), 63–97. [Google Scholar] [CrossRef]
- Yerragunta, T; Manda, VS; Yerramneni, VK; Reddy Kanala, RN. A brief review of ventriculoatrial and ventriculopleural shunts. Neurol India 2021, 69 (Suppl), S476–S480. [Google Scholar] [CrossRef]
- Kazui, H; Miyajima, M; Mori, E; Ishikawa, M. SINPHONI-2 Investigators. Lumboperitoneal shunt surgery for idiopathic normal pressure hydrocephalus (SINPHONI-2): an open-label randomised trial. Lancet Neurol. 2015, 14(6), 585–594. [Google Scholar] [CrossRef] [PubMed]
- Bluett, B; Acosta, LM; Ash, E; Bloem, BR; Espay, AJ; Farheen, A; et al. International Parkinson and Movement Disorders Society Normal Pressure Hydrocephalus (MDS-NPH) Study Group. Standardizing the large-volume tap test for evaluating idiopathic normal pressure hydrocephalus: a systematic review. J Neurosurg Sci. 2025, 69(1), 46–63. [Google Scholar] [CrossRef]
- Williams, MA; Relkin, NR. Diagnosis and management of idiopathic normal-pressure hydrocephalus. Neurol Clin Pract. 2013, 3(5), 375–385. [Google Scholar] [CrossRef]
- Öztop-Çakmak, Ö; Akar, K; Youssef, H; Kahraman, AD; Özkan, E; Samancı, MY; Vural, A. Gait analysis in idiopathic normal pressure hydrocephalus: a single centre experience. Istanbul Med J. 2023, 24(1), 36–39. [Google Scholar] [CrossRef]
- Nassar, BR; Lippa, CF. Idiopathic normal pressure hydrocephalus: a review for general practitioners. Gerontol Geriatr Med 2016, 2, 2333721416643702. [Google Scholar] [CrossRef]
- Constantinescu, C; Wikkelsø, C; Westman, E; Ziegelitz, D; Jaraj, D; Rydén, L; et al. Prevalence of possible idiopathic normal pressure hydrocephalus in Sweden: a population-based MRI study in 791 70-year-old participants. Neurology 2024, 102(2), e208037. [Google Scholar] [CrossRef] [PubMed]
- Katzen, H; Ravdin, LD; Assuras, S; Heros, R; Kaplitt, M; Schwartz, TH; Fink, M; Levin, BE; Relkin, NR. Postshunt cognitive and functional improvement in idiopathic normal pressure hydrocephalus. Neurosurgery 2011, 68(2), 416–9. [Google Scholar] [CrossRef]
- Salzman, B. Gait and balance disorders in older adults. Am Fam Physician 2010, 82(1), 61–8. [Google Scholar]
- Toma, AK; Papadopoulos, MC; Stapleton, S; Kitchen, ND; Watkins, LD. Systematic review of the outcome of shunt surgery in idiopathic normal-pressure hydrocephalus. Acta Neurochir (Wien) 2013, 155(10), 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Giordan, E; Palandri, G; Lanzino, G; Murad, MH; Elder, BD. Outcomes and complications of different surgical treatments for idiopathic normal pressure hydrocephalus: a systematic review and meta-analysis. J Neurosurg. 2018, 131(4), 1024–1036. [Google Scholar] [CrossRef]
- de Oliveira, MF; Sorte, AAB, Jr.; Emerenciano, DL; Rotta, JM; Mendes, GAS; Pinto, FCG. Long-term follow-up of shunted idiopathic normal pressure hydrocephalus patients: a single-center experience. Acta Neurol Belg 2021, 121(6), 1799–1806. [Google Scholar] [CrossRef]
- Thomas, G; McGirt, MJ; Woodworth, G; Heidler, J; Rigamonti, D; Hillis, AE; Williams, MA. Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2005, 20(2–3), 163–8. [Google Scholar] [CrossRef]
- Andrén, K; Wikkelsø, C; Sundström, N; Israelsson, H; Agerskov, S; Laurell, K; et al. Survival in treated idiopathic normal pressure hydrocephalus. J Neurol. 2020, 267(3), 640–648. [Google Scholar] [CrossRef]
- Shprecher, D; Schwalb, J; Kurlan, R. Normal pressure hydrocephalus: diagnosis and treatment. Curr Neurol Neurosci Rep. 2008, 8(5), 371–376. [Google Scholar] [CrossRef]
- Saito, M; Nishio, Y; Kanno, S; Uchiyama, M; Hayashi, A; Takagi, M; Kikuchi, H; Yamasaki, H; Shimomura, T; Iizuka, O; Mori, E. Cognitive profile of idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Dis Extra 2011, 1(1), 202–11. [Google Scholar] [CrossRef]
- Sakakibara, R; Kanda, T; Sekido, T; Uchiyama, T; Awa, Y; Ito, T; Liu, Z; Yamamoto, T; Yamanishi, T; Yuasa, T; Shirai, K; Hattori, T. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2008, 27(6), 507–10. [Google Scholar] [CrossRef] [PubMed]
- Duinkerke, A; Williams, MA; Rigamonti, D; Hillis, AE. Cognitive recovery in idiopathic normal pressure hydrocephalus after shunt. Cogn Behav Neurol 2004, 17(3), 179–84. [Google Scholar] [CrossRef]
- Hellström, P; Edsbagge, M; Blomsterwall, E; Archer, T; Tisell, M; Tullberg, M; Wikkelsø, C. Neuropsychological effects of shunt treatment in idiopathic normal pressure hydrocephalus. Neurosurgery 2008, 63(3), 527–35; discussion 535–6. [Google Scholar] [CrossRef] [PubMed]
- Picascia, M; Zangaglia, R; Bernini, S; Minafra, B; Sinforiani, E; Pacchetti, C. A review of cognitive impairment and differential diagnosis in idiopathic normal pressure hydrocephalus. Funct Neurol. 2015, 30(4), 217–228. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, N; Malm, J; Laurell, K; Lundin, F; Kahlon, B; Cesarini, KG; Leijon, G; Wikkelso, C. Incidence and outcome of surgery for adult hydrocephalus patients in Sweden. Br J Neurosurg. 2017, 31, 21–27. [Google Scholar] [CrossRef]
- Grasso, G; Torregrossa, F; Leone, L; Frisella, A; Landi, A. Long-term efficacy of shunt therapy in idiopathic normal pressure hydrocephalus. World Neurosurg 2019, 129, e458–e463. [Google Scholar] [CrossRef] [PubMed]
- Anzai, A; Utino, A; Katayama, H; Spir, IAZ; Nery, MM; Anhesini, M; et al. Use of programmable valve versus fixed pressure valve in the treatment of idiopathic normal pressure hydrocephalus: a systematic review and meta-analysis. Rev Assoc Med Bras (1992) 2023, 69(2), 207–212. [Google Scholar] [CrossRef]
- Rinaldo, L; Bhargav, AG; Nesvick, CL; Lanzino, G; Elder, BD. Effect of fixed-setting versus programmable valve on incidence of shunt revision after ventricular shunting for idiopathic normal pressure hydrocephalus. J Neurosurg. 2019, 133(2), 564–572. [Google Scholar] [CrossRef]
- Serarslan, Y; Yilmaz, A; Çakır, M; Güzel, E; Akakin, A; Güzel, A; et al. Use of programmable versus nonprogrammable shunts in the management of normal pressure hydrocephalus: a multicenter retrospective study with cost-benefit analysis in Turkey. Medicine (Baltimore) 2017, 96(39), e8185. [Google Scholar] [CrossRef] [PubMed]
- Pearce, RKB; Gontsarova, A; Richardson, D; Methley, AM; Watt, HC; Tsang, K; Carswell, C. Shunting for idiopathic normal pressure hydrocephalus. Cochrane Database Syst Rev. 2024, 8(8), CD014923. [Google Scholar] [CrossRef]
- Virhammar, J; Fasth, O; Vedung, F. When and how are complications suspected after shunt surgery in patients with normal pressure hydrocephalus? Acta Neurochir (Wien) 2025, 167(1), 6. [Google Scholar] [CrossRef]
- Andrén, K; Wikkelsö, C; Sundström, N; Agerskov, S; Israelsson, H; Laurell, K; et al. Long-term effects of complications and vascular comorbidity in idiopathic normal pressure hydrocephalus: a quality registry study. J Neurol. 2018, 265(1), 178–186. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).