Submitted:
12 January 2026
Posted:
13 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Study Design and Ethics
2.2. Patient Selection
2.3. Preoperative Assessment
2.4. Surgical Technique
2.5. Outcome Measures and Statistical Analysis
3. Results
3.1. Demographics and Baseline Characteristics
3.2. Anatomical Outcomes
3.3. Functional Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | Autologous (neurosensory) Retinal Transplantation |
| BCVA | Best-Corrected Visual Acuity |
| bFGF | Basic Fibroblast Growth Factor |
| DONFL | Dissociated Optic Nerve Fiber Layer |
| EGF | Epidermal Growth Factor |
| hAM | Human Amniotic Membrane |
| ILM | Internal Limiting Membrane |
| logMAR | Logarithm of the Minimum Angle of Resolution |
| MH | Macular Hole |
| NGF | Nerve Growth Factor |
| OCT | Optical Coherence Tomography |
| PFCL | Perfluorocarbon Liquid |
| PPV | Pars Plana Vitrectomy |
| RD | Retinal Detachment |
| RPE | Retinal Pigment Epithelium |
| SD-OCT | Spectral-Domain Optical Coherence Tomography |
| SO | Silicone Oil |
| TGF-β | Transforming Growth Factor-beta |
References
- Bokor, Á.; Fodor, M. Surgical management of refractory full-thickness macular holes with emphasis on autologous retinal transplantation: A narrative review. Regen. Med. Rep. 2025, 3, 1–5. [Google Scholar] [CrossRef]
- Steel, D.H.W.; Dinah, C.; Habib, M.; White, K. ILM peeling technique influences the degree of a dissociated optic nerve fibre layer appearance after macular hole surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 691–698. [Google Scholar] [CrossRef]
- Hanai, M.; Amaral, D.C.; Jacometti, R.; et al. Large macular hole and autologous retinal transplantation: A systematic review and meta-analysis. Int. J. Retina Vitreous 2024, 10, 56. [Google Scholar] [CrossRef]
- Khaqan, H.A.; Sahyoun, J.Y.; Haider, M.A.; Buksh, H.M. Amniotic membrane graft for the treatment of large refractory macular hole. Retina 2022, 42, 1479–1483. [Google Scholar] [CrossRef]
- Teoh, C.S. An insight into current and novel treatment practices for refractory full-thickness macular hole. J. Clin. Transl. Ophthalmol. 2025, 3, 15. [Google Scholar] [CrossRef]
- Carlà, M.M.; Mateo, C. Autologous retina transplantation for refractory highly myopic macular holes: A long-term follow-up. Jpn. J. Ophthalmol. 2025, 69, 259–267. [Google Scholar] [CrossRef]
- Lorenzi, U.; Mehech, J.; Caporossi, T.; Romano, M.R.; De Fazio, R.; Parrat, E.; Matonti, F.; Mora, P. A retrospective, multicenter study on the management of macular holes without residual internal limiting membrane: The refractory macular hole (ReMaHo) study. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3837–3845. [Google Scholar] [CrossRef]
- Rojas-Juárez, S.; Cisneros-Cortés, J.; Ramirez-Estudillo, A.; Velez-Montoya, R. Autologous full-thickness retinal transplant for refractory large macular holes. Int. J. Retina Vitreous 2020, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Grewal, D.S.; Charles, S.; Parolini, B.; Kadonosono, K.; Mahmoud, T.H. Autologous retinal transplant for refractory macular holes: Multicenter international collaborative study group. Ophthalmology 2019, 126, 1399–1408. [Google Scholar] [CrossRef]
- Lumi, X.; Petrovic Pajic, S.; Sustar, M.; Fakin, A.; Hawlina, M. Autologous neurosensory free-flap retinal transplantation for refractory chronic macular hole - outcomes evaluated by OCT, microperimetry, and multifocal electroretinography. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Xu, J.; He, S.; et al. Quantitative evaluation of dissociated optic nerve fibre layer (DONFL) following idiopathic macular hole surgery. Eye 2022, 37, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Szurman, P.; Wakili, P.; Stanzel, B.V.; Siegel, R.; Boden, K.T.; Rickmann, A. Persistent macular holes - what is the best strategy for revision? Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Calis Karanfil, F.; Toklu, Y.; Arikan Yorgun, M. Amniotic membrane transplantation for macular hole closure. Beyoğlu Eye J. 2023, 8, 55–59. [Google Scholar] [CrossRef]
- Tartaro, R.; Caporossi, T.; Virgili, G.; Barca, F.; Giansanti, F.; Rizzo, S. Insights on the human amniotic membrane in clinical practice with a focus on the new applications in retinal surgery. Regen. Eng. Transl. Med. 2022, 8, 22–31. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Chen, G.; Han, F.; Jiang, W. Human amniotic membrane graft for refractory macular hole: A single-arm meta-analysis and systematic review. J. Fr. Ophtalmol. 2023, 46, 276–286. [Google Scholar] [CrossRef]
- Sharma, R.; Nappi, V.; Empeslidis, T. The developments in amniotic membrane transplantation in glaucoma and vitreoretinal procedures. Int. Ophthalmol. 2023, 43, 1771–1783. [Google Scholar] [CrossRef]
- Proença, H.; Antunes, M.; Ferreira, J.T.; Magro, P.; Faria, M.; Marques-Neves, C. Outcomes of amniotic membrane transplant for refractory macular hole - an optical coherence tomography and autofluorescence long term study. Graefes Arch. Clin. Exp. Ophthalmol. 2025, 263, 315–326. [Google Scholar] [CrossRef]
- Caporossi, T.; Scampoli, A.; Baldascino, A.; et al. Subretinal transplant of human amniotic membrane in advanced age-related macular degeneration. Life 2022, 12. [Google Scholar] [CrossRef]
- Ventre, L.; Marolo, P.; Reibaldi, M. A human amniotic membrane plug to treat persistent macular hole. Case Rep. Ophthalmol. 2020, 11, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Garcin, T.; Gain, P.; Thuret, G. Epiretinal large disc of blue-stained lyophilized amniotic membrane to treat complex macular holes: A 1-year follow-up. Acta Ophthalmol. 2022, 100, e598–e608. [Google Scholar] [CrossRef]
- Iannetta, D.; Chhablani, J.; Valsecchi, N.; Mesiani, M.; de Smet, M.D.; Fontana, L. Epiretinal implant of human amniotic membrane to treat highly myopic macular hole retinal detachments: A novel surgical technique. Eur. J. Ophthalmol. 2024, 34, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, T.; Governatori, L.; Gambini, G.; et al. Treatment of recurrent high myopic macular hole associated with retinal detachment using a human amniotic membrane. Jpn. J. Ophthalmol. 2022, 66, 518–526. [Google Scholar] [CrossRef]
- Özdemir Zeydanlı, E.; Özdek, Ş.; Yalçın, E.; Özdemir, H.B. Human amniotic membrane: A seal for complex retinal detachments. Turk. J. Ophthalmol. 2024, 54, 268–274. [Google Scholar] [CrossRef]
- Yamamoto, M.H.; Marin, A.I.; Tsui, I. Management of traumatic macular hole and retinal detachment using amniotic membrane transplantation: A case report. Am. J. Ophthalmol. Case Rep. 2025, 37, 102267. [Google Scholar] [CrossRef] [PubMed]
- Frisina, R.; Gius, I.; Tozzi, L.; Midena, E. Refractory full thickness macular hole: Current surgical management. Eye 2022, 36, 1344–1354. [Google Scholar] [CrossRef]
- Quiroz-Reyes, M.A.; Quiroz-Gonzalez, E.A.; Quiroz-Gonzalez, M.A.; Lima-Gomez, V. Safety and efficacy of human amniotic membrane plug transplantation in cases of macular hole. A scoping review. Int. J. Retina Vitreous 2024, 10, 82. [Google Scholar] [CrossRef]
- Ferreira, M.A.; Maia, A.; Machado, A.J.; Ferreira, R.E.A.; Hagemann, L.F.; Ribeiro Júnior, P.H.E.; Rezende, F.A. Human amniotic membrane for the treatment of large and refractory macular holes: a retrospective, multicentric, interventional study. Int. J. Retina Vitreous 2021, 7, 38. [Google Scholar] [CrossRef]
- Carlà, M.M.; Giannuzzi, F.; Hu, L.; Rizzo, C.; Crincoli, E.; Catania, F.; Gambini, G.; Caporossi, T.; Mateo, C.; Rizzo, S. The human amniotic membrane in vitreoretinal surgery: Applications, outcomes and limitations. Surv. Ophthalmol. 2025, 71, 14–24. [Google Scholar] [CrossRef]
- Huang, Y.H.; Tsai, D.C.; Wang, L.C.; Chen, S.J. Comparison between cryopreserved and dehydrated human amniotic membrane graft in treating challenging cases with macular hole and macular hole retinal detachment. J. Ophthalmol. 2020, 2020, 9157518. [Google Scholar] [CrossRef]
- Shroff, D.; Bhatia, G.; Kumar, S.; Grewal, D.S. Staining and sizing technique for human amniotic membrane graft in refractory macular holes. J. VitreoRetin. Dis. 2025, 9, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.Y.; Saab, M. Human amniotic membrane graft with viscodissection and intraoperative OCT for macular hole repair. Am. J. Ophthalmol. Case Rep. 2025, 38, 102302. [Google Scholar] [CrossRef] [PubMed]
- Gürelik, İ.G.; Özdemir, H.B.; Acar, A.B.; Aydın, B. Descemet’s membrane transplantation for the treatment of recurrent high myopic macular hole associated with retinal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2025, 263, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, V.; You, Q.; Me, R.; Lee, P.S.Y.; Le, K.; Tran, D.; Lin, X. Temporary amniotic membrane graft placement for treatment of refractory macular holes. Retin. Cases Brief Rep. 2026, 20, 39–42. [Google Scholar] [CrossRef] [PubMed]
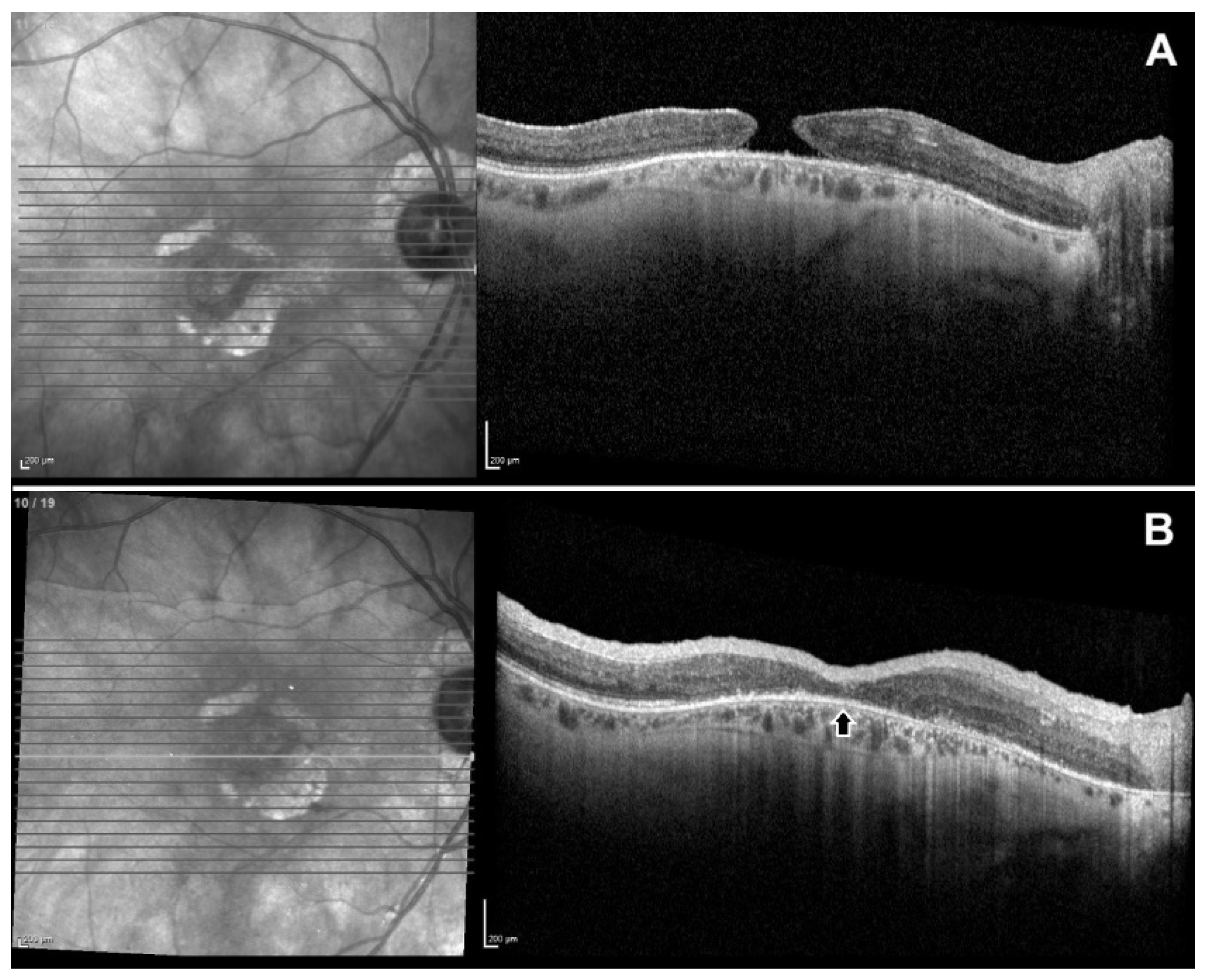
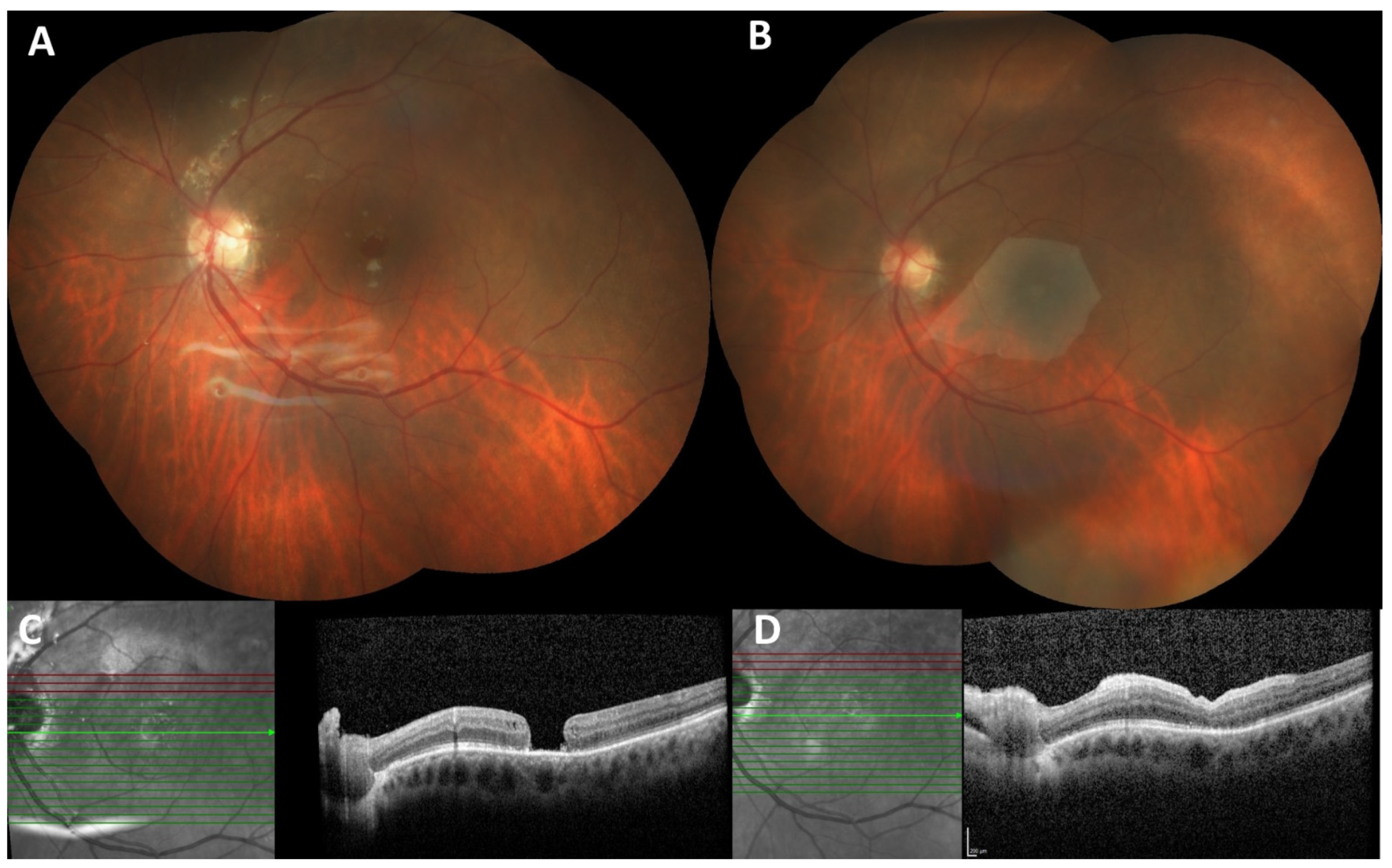
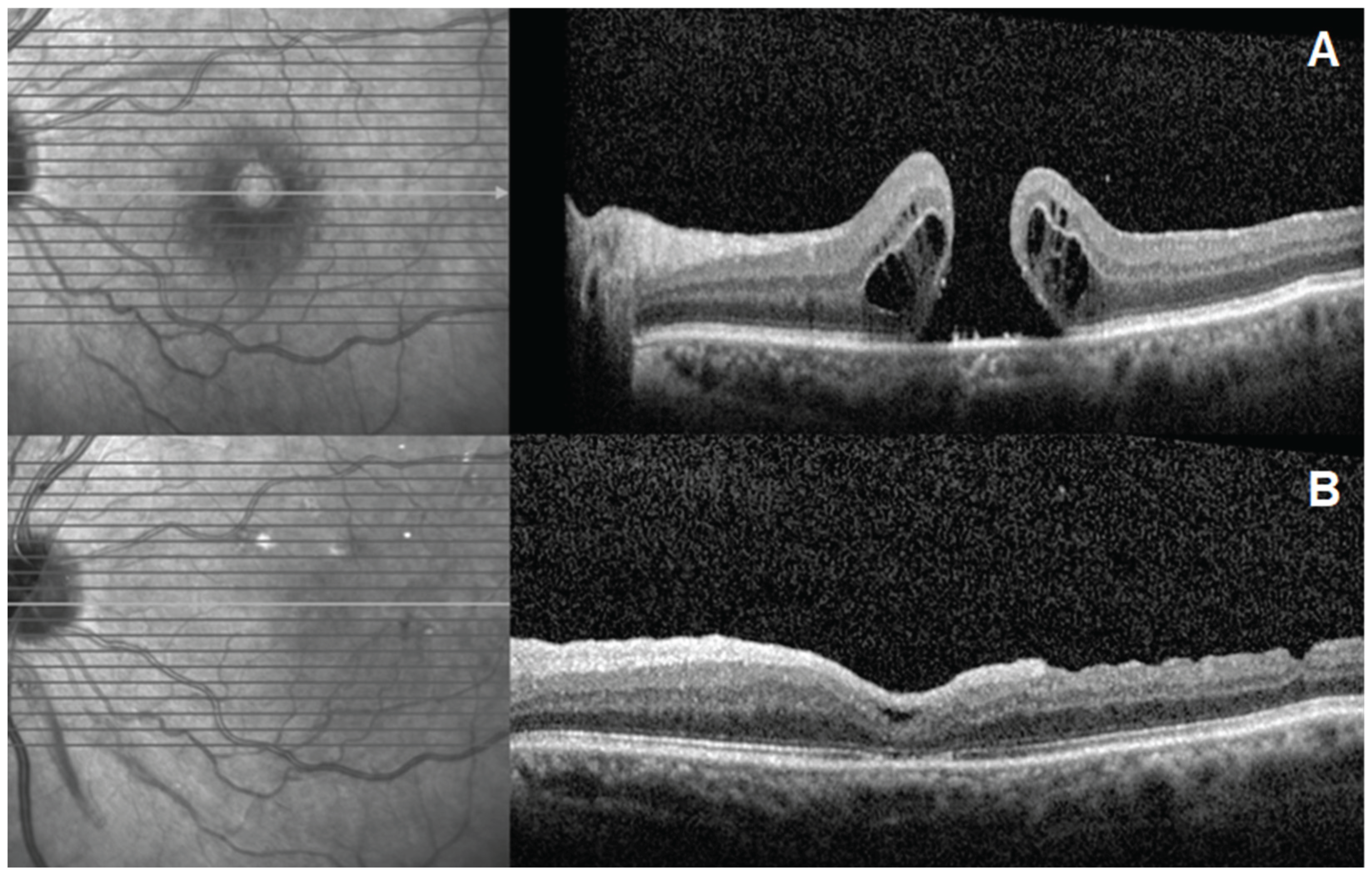
| ID | Age | Gender | Eye | Dx | Prior Surgery | AL (mm) | MH min (µm) | MH base (µm) | BCVA (logMAR) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | F | R | RMH | PPV + ILMp + C3F8 | 22.6 | 715 | 1115 | 1.6 |
| 2 | 58 | M | L | RMH | PPV + ILMp + C3F8 | 23.7 | 760 | 1234 | 1.4 |
| 3 | 59 | F | R | RMH | PPV + ILMp + C3F8 | 24.2 | 682 | 1087 | 1.2 |
| 4 | 50 | F | L | RD + RMH | PPV + ILMp + SO | 24.1 | 502 | 1205 | 1.8 |
| 5 | 63 | M | R | RMH | PPV + ILMp + C3F8 | 23.2 | 730 | 945 | 1.8 |
| 6 | 61 | M | L | RMH | PPV + ILMp + C3F8 | 22.3 | 712 | 1118 | 1.7 |
| 7 | 50 | F | R | RMH | PPV + ILMp + C3F8 | 22.7 | 812 | 1302 | 1.6 |
| 8 | 68 | F | R | RMH | PPV + ILMp + C3F8 | 23.0 | 612 | 989 | 1.7 |
| 9 | 50 | F | L | RMH | PPV + ILMp + C3F8 | 24.0 | 724 | 1023 | 1.6 |
| 10 | 55 | F | R | RMH | PPV + ILMp + C3F8 | 22.6 | 714 | 1145 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





