1. Introduction
Identification of reliable biomarkers of epileptogenesis, a dynamic process leading to transformation of the healthy brain to epileptic one, remains an urgent task. Search for early predictors of epileptogenesis, allowing to select patients with high risk of epilepsydevelopment after acute brain insults and to start preventive treatment, attracts growing attention. Network markers are a promising instrument for the purpose (Kramer 2012; Tracy, Doucet, 2015; Lam, Noebels, 2020). Pathophysiological mechanisms of epileptogenesis include widespread modification of inter-area functional interactions with recruitment of remote brain regions in the epileptic network. Pronounced network alterations have been reported during epileptogenesis in experimental animals (Lee et al., 2017; Bertoglio et al., 2019) and patients with epilepsy (Morgan et al., 2015; Haneef et al., 2015;Courtiol et al., 2020). However, very littleis known about network changes at the early stage of epileptogenesis having no obvious clinical correlates.
Preclinical studies have shown that spreading depolarization (SD), a wave of transient neuroglial depolarization (Somjen, 2001), appears as the earliest cortical event at the initial subclinical stage of epileptogenesis when other signs of cortical hyperexcitability are subtle or absent (Hatcher et al., 2020; Vinogradova et al., 2009). In the model of human glioma, spontaneous episodes of cortical SD appear at the earliest stage of tumor-related epileptogenesis before the emergence of epileptiform activity in the cortex (Hatcher et al., 2020). Cortical SD is reliably detected following repeated ictal episodes at the initial stage of chemical and audiogenic kindling (Koroleva et al. 1993; Vinogradova, 2015). Close association of seizures with cortical SD has been reported in clinical (Fabricius et al., 2008; Dreier e al., 2012) and preclinical (Tamim et al., 2021; Koroleva et al., 1993; Vinogradova, 2015) studies. However, inability of conventional EEG to detect SD makes it difficult to study a role of SD in epileptogenesis and the seizure-SD association in humans.
Our previous study in the audiogenic kindling model has reported significant changes in interhemispheric functional connectivity during the middle and late periods of cortical epileptogenesis (Medvedeva et al., 2023). In has been shown that bilateralization of cortical seizures at the final kindling stage was accompanied by an increase in hemispheric connectivity and the parietal cortex was very sensitive to the network changes (Medvedeva et al., 2023). Here, using the same experimental model, we focused on the connectivity changes in the parietal cortex at the early stage of kindling-related epileptogenesis. Well-known neuroanatomical substrates of audiogenic kindling and use of only sensory (sound) stimulation for seizure initiation makes the model especially valuable for studying network mechanisms of epileptogenesis. Primary epileptic focus (seizure-onset zone) of audiogenic seizures locates in the brainstem and repetition of the subcortically-driven seizures leads to gradual recruitment of the cerebral cortex in the epileptic network (Faingold, 2012: Marescaux et al., 1987). The model mimics epileptogenic changes associated with hypothalamic hamartoma, in which seizures are generated by subcortical lesion (hamartoma) and secondary spread to the cerebral cortex (Kerrigan et al., 2005; Scholly et al. 2017; Vinogradova, Grinenko, 2016).
The mild version of audiogenic kindling used in our studies produces slow stepwise progression of epileptogenic changes in the cortex. In the paradigm, a brief sound stimulation of susceptible rats induces a focal brainstem seizure behaviorally expressed as a brief episode of hyperkinetic seizure (unidirectional running). With repetition of subcortical seizures, the cortex is recruited in the epileptic network that manifests in appearance and gradual intensification of cortical seizures following brainstem seizure episodes (Vinogradova, 2015; Medvedeva et al., 2023). At the early kindling stage before development of the kindled cortical seizures, unilateral SD is reliably detected the cortex (Vinogradova, 2015). We suggested that interhemispheric functional connectivity starts to change early in kindling. To test the hypothesis, we compared long-term (interictal, resting state) and short-term (postictal) dynamics of hemispheric coupling before kindling and at the early kindling stage, using cortical SD as its electrographic marker. Time-related changes in functional connectivity were estimated by two measures - the mutual information (MI) function (Kraskov et al., 2004) and index of phase synchronization (PS) (Mormann et al., 2000) applied to LFP recordings from homotopic sites of the parietal cortex. We found that interhemispheric hypoconnectivity is a reliable manifestation of early epileptogenic changes in the cortex.
2. Materials and Methods
2.1. Animals
Adult male Wistar rats susceptible to audiogenic seizures (3-5 months, 350-450 g) (Stolbovaya breeding center, Russia) were used for the experiments. Rats were housed in individual cages under controlled environmental conditions (a 12-h light-dark cycle, lights on at 7:00 A.M., 20-23C) with free access to food and water. All experimental procedures were conducted in accordance with the ARRIVE guidelines and Council Directive 2010/63EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes. The study protocol was approved by the Institutional Animal Care Committee (protocol N1, 01.02.2022). Every effort was made to minimize animal suffering.
2.2. Surgery
Electrodes for recording of wideband cortical activity (screws/glass pipette with inner carbon fiber) were implanted bilaterally in symmetrical points of the parietal cortex under chloral hydrate (360 mg/kg, i.p.) anesthesia. Coordinates of implantation were (mm from bregma): posterior 2.0 and lateral 3.0 (Paxinos, Watson, 2005). A stainless-steel screw positioned over the cerebellum was used as reference electrode. All electrodes were soldered to a pin connector and secured with acrylic cement. The experiment began two weeks after surgery.
2.3. Experimental Design
Experiments were performed in awake freely moving rats with simultaneous video-monitoring of behavior and wideband monopolar recording of local field potentials (LFP). Each rat was individually placed in a shielded experimental chamber (60x40x40 cm) and the implanted connector was attached to the recording cable. After a 5-min period of habituation in the chamber and a 10-min period of baseline recording of cortical activity, an episode of hyperkinetic seizure was induced by broadband sound stimulation (50-60 dB, 13-85 kHz) lasting until the seizure onset. Electrical activity of the cortex (1 kHz sampling rate) was recorded with a four-channel, high-input impedance (1G) DC amplifier and A/D converter (E14-440, L-Card, Russia) and stored on the computer for offline analysis.
2.4. Audiogenic Kindling Procedure
To induce epileptogenesis (kindling), each rat was subjected to repeated sound stimulations once a day at 3-4-day intervals. Sound triggered a single episode of hyperkinetic seizure (paroxysmal running). The seizure was the only response to acoustic stimulation before kindling and at the early kindling stage. At the middle and final stages of kindling, the sound-induced hyperkinetic seizure was followed by limbic clonus (facial automatisms, ears/vibrissae clonus, head nodding) of growing duration and intensity. Data for the late kindling stages have been reported in our previous paper (Medvedeva et al., 2023). Here, we studied dynamics of functional connectivity at the early stage of kindling before development of limbic seizures. We used cortical SD as a marker of growing cortical excitability during audiogenic kindling. Two types of audiogenic seizures were analyzed - (1) non-kindled subcortical seizures (an episode of running) induced by the first and second sound stimulation, (2) slightly kindled subcortical seizures induced by repeated sound stimulation and accompanied by cortical SD. The seizure types were classified by two experienced observers who marked the onset and termination of hyperkinetic seizure and SD based on the LFP recording verified with video data. The LFP recordings in each group were analyzed by a person, blinded to the group. SD was identified by a characteristic high-amplitude negative DC potential shift. Latency of SD was determined by the time that elapsed between the termination of a hyperkinetic seizure and the onset of negative DC shift.
2.5. Data Processing
The segments were filtered with a highpass (1 Hz highcut) and bandstop (48 Hz lowcut and 52 Hz highcut) Butterworth digital filters. Recordings filtered with a lowpass (0-48 Hz) filter were used for identification of SD. Further, the 300-s epochs were divided into 10-s length intervals and the mean power for each interval in each frequency band was evaluated without overlapping. Spectral power was computed using a Fast Fourier Transform (FFT) routine for five frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–25 Hz) and gamma (25–50 Hz). Spectrograms were obtained with 2048 data points (approximately 2 s) used in each block for the FFT and overlapping of 90%.
2.6. Functional Connectivity Analysis
Artifact-free 300-s epochs of baseline and post-seizure electrographic recordings from the homotopic sites of the parietal cortex before kindling and at the early kindling stage were selected by a trained member of the study who was blinded to the groups. The segments were band-pass filtered (1-100 Hz, Fourier transform, no phase shift) and analyzed off-line. The LFP recordings immediately following the hyperkinetic seizure offset were used to calculate connectivity levels for the postictal period. The LFP recordings during the quiet period before seizure induction were selected to calculate baseline level of connectivity. Further, these epochs were divided into 10-s length intervals and the connectivity measures were evaluated for each interval independently without overlapping. To analyze the coupling dynamics between the left and right hemispheres, the mutual information function (Kraskov et al., 2004) and the index of phase synchronization (Kreuz et al., 2007) were used.
Mutual information is a statistical function measuring mutual dependence between two (random) variables
and
. Its dimension is the same as for information entropy (Shannon entropy) and practically it determines the amount of information. Here, we used a technique based on a number of nearest neighbors on the
proposed by Kraskov et al., 2004. X-Y plane is a plane at which analytical signal is plotted, with X-axis corresponding to original series and Y-axis corresponding to conjugate signal constructed via Hilbert’s transform. This technique uses so called Kozachenko-Leonenko entropy (Kozachenko & Leonenko, 1987), just another definition of Shannon’s entropy. We used the calculation formula (2):
where
is a length of time window in points,
nd
are the number of neighbors of the
-th data point
on the plane
such that the distance to them along either
or
-axis is less than the distance to the nearest neighbor of
-th point,
is digamma function. The formula (1) provides asymptotically unbiased estimates, which for
are very precise.
is a classical measure for detection of nonlinear similarities of the signals if actual mechanisms of coupling are not clear or cannot be revealed on the existing level of experimental technique. Since is the information measure of entropy, it reflects the overall intensity of information transfers between the considered objects in all frequency bands including mediated interactions and nonlinear interactions when the objects use different frequency bands for crosstalk. It has been shown to offer significant advantages compared to the correlation-based measures (Pregowska et al., 2015; Grishchenko et al., 2017).
Mean phase synchronization index is a numerical indicator quantitatively characterizing simultaneity of phase changes of two signals proposed by Mormann et al., 2000, see Eq. (3).
where
and
are phases of the signals
and
respectively and
is the phase difference. The phase was calculated for the signal rather than for different harmonics separately; therefore, such a phase is called “a mean phase”. Analytical signal was calculated via Hilbert’s transform, then the phase was found as arc tangent in the complex plane. There was a center of rotation in the complex plane for the analytical signal after filtration and Hilbert’s transform, which provides sufficient evidence that the phase can be established properly. Band separation provided the possibility to establish a single mean phase for each band. Mean phase occurred to be correct for almost all-time segments for both signals for the delta and theta ranges, with some errors in the beta and gamma bands.
In this study the signals were filtered by band-pass filter using Fourier transform in five frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–25 Hz) and gamma (25–50 Hz) as for spectral analysis.
2.7. Statistical Analysis
The durations of electrographic epileptic discharges were expressed as mean ± S.E.M. To establish changes in time we calculated the median value and its interquartile range over all considered recordings. The statistical hypothesis was that the median values of a measure at some point did not differ from baseline values. So, we tried to refute this hypothesis at some reasonable probability .
Then, we performed statistical evaluation to correct the results for multiple testing using an algorithm proposed in (Maris &Oostenveld, 2007). The idea of this approach was to consider multiple significances for
and
corresponding to some longer time interval. If
points significantly differed from the baseline at the level of
, this mean that at least one of them was significant at the level of
where
was the number of points in the entire interval (
in our case). This approach reduces the temporal resolution but provides high reliability. Since
provided
, which was not enough, we used
provided
(marked by color in figures).We referred connectivity changes to be significant in the corresponding time interval if we had at least three points significantly differed from the baseline at the
of
or
. Cases of
provided the level of
.
Baseline and ictal connectivity levels for different seizure types were compared by Mann-Whytney test.
2.8. Software and Algorithms
The data analysis was conducted utilizing custom-developed scripts in Python, incorporating the following scientific libraries: Matplotlib (Hunter, 2007) and NumPy (Harris et al.) and SciPy (Virtanen et al., 2020).
3. Results
3.1. Electrographic Characteristics of Audiogenic Seizures at the Early Kindling Stage
In susceptible freely behaving rats, sound elicited a single hyperkinetic seizure – a brief (3-9 s) episode of self-sustained unidirectional running. In repeated tests, the direction of repeated running seizures remained consistent. After 9.0±1.4 (range 6-15) repetitions, hyperkinetic seizures began to be followed by a unilateral SD detected in the cortex ipsilateral to the direction of running. In the parietal cortex, SD appeared 92.5±4.7 s (n=12) after termination of hyperkinetic seizures. Behavioral phenotype, intensity and duration of seizures with and without cortical SD were identical – 6.7±0.6 s vs 6.8±0.4 s, respectively (n=12 per group). Hyperkinetic seizures terminated abruptly and were followed by postictal behavioral immobility. Its duration did not differ for seizures without SD (243±27 s, n=12) and with cortical SD (208±21 s, n=12, p=0.3013, Mann-Whytney test).
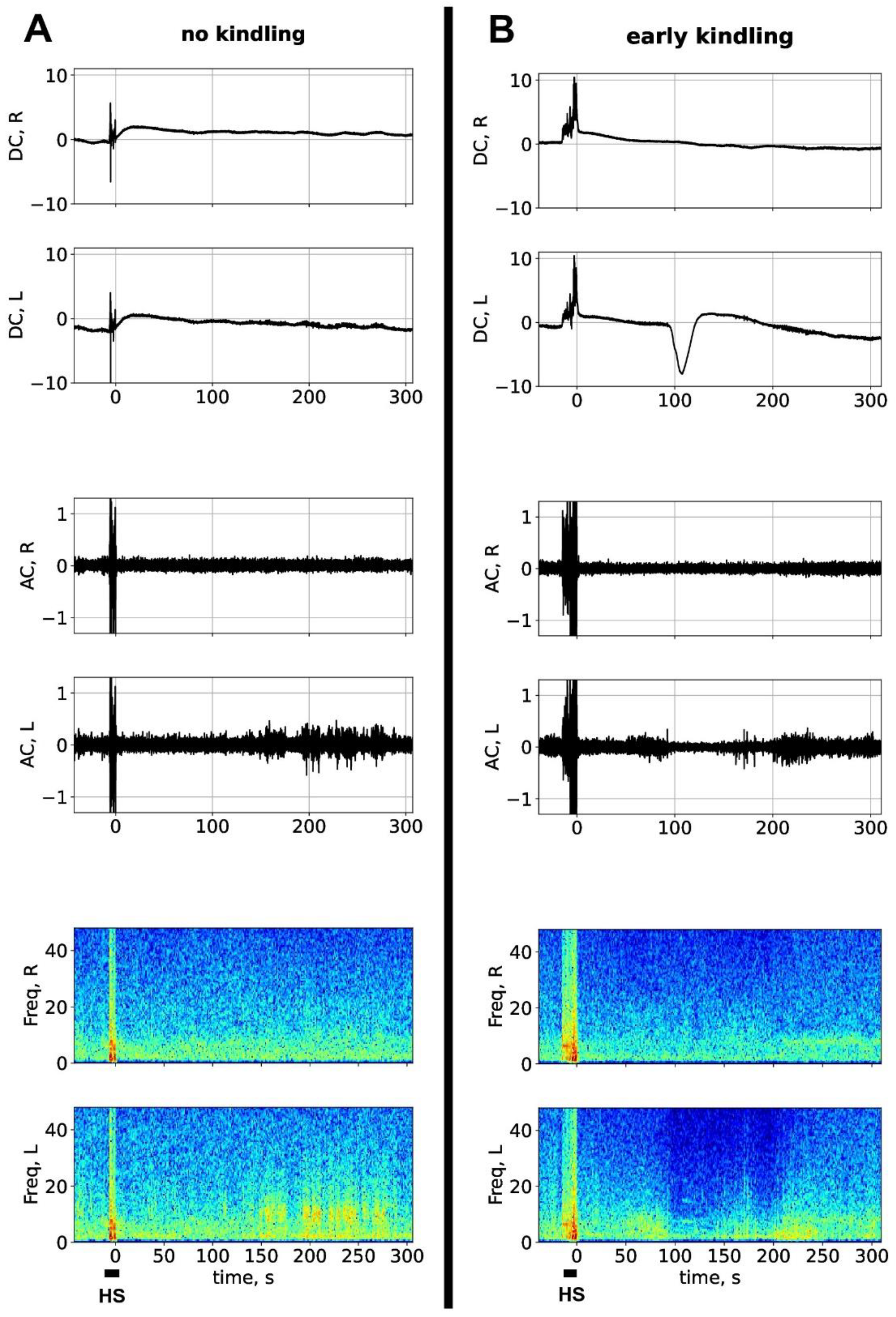
Figure 1 shows representative DC/AC recordings of hyperkinetic seizures induced by the first sound stimulation in a seizure-naïve rat (A) and by the ninth acoustic stimulation in the same rat (B). The recordings were obtained in the homotopic regions of the parietal cortex of the two hemispheres during peri-ictal period. High-amplitude artefacts mark period of hyperkinetic seizure. After the ninth seizure (early kindling), a large negative DC potential shift (a signature of SD) appeared in the left cortex at about 90 s (
Figure 1B). As seen in spectrogram, non-kindled hyperkinetic seizure did not induce significant postictal changes in cortical activity (
Figure 1A) but slightly kindled seizures associated with cortical SD produced transient depression of electrical activity, mainly in the left cortex affected by SD.
3.2. Postictal Depression of Cortical Activity at the Early Kindling Stage
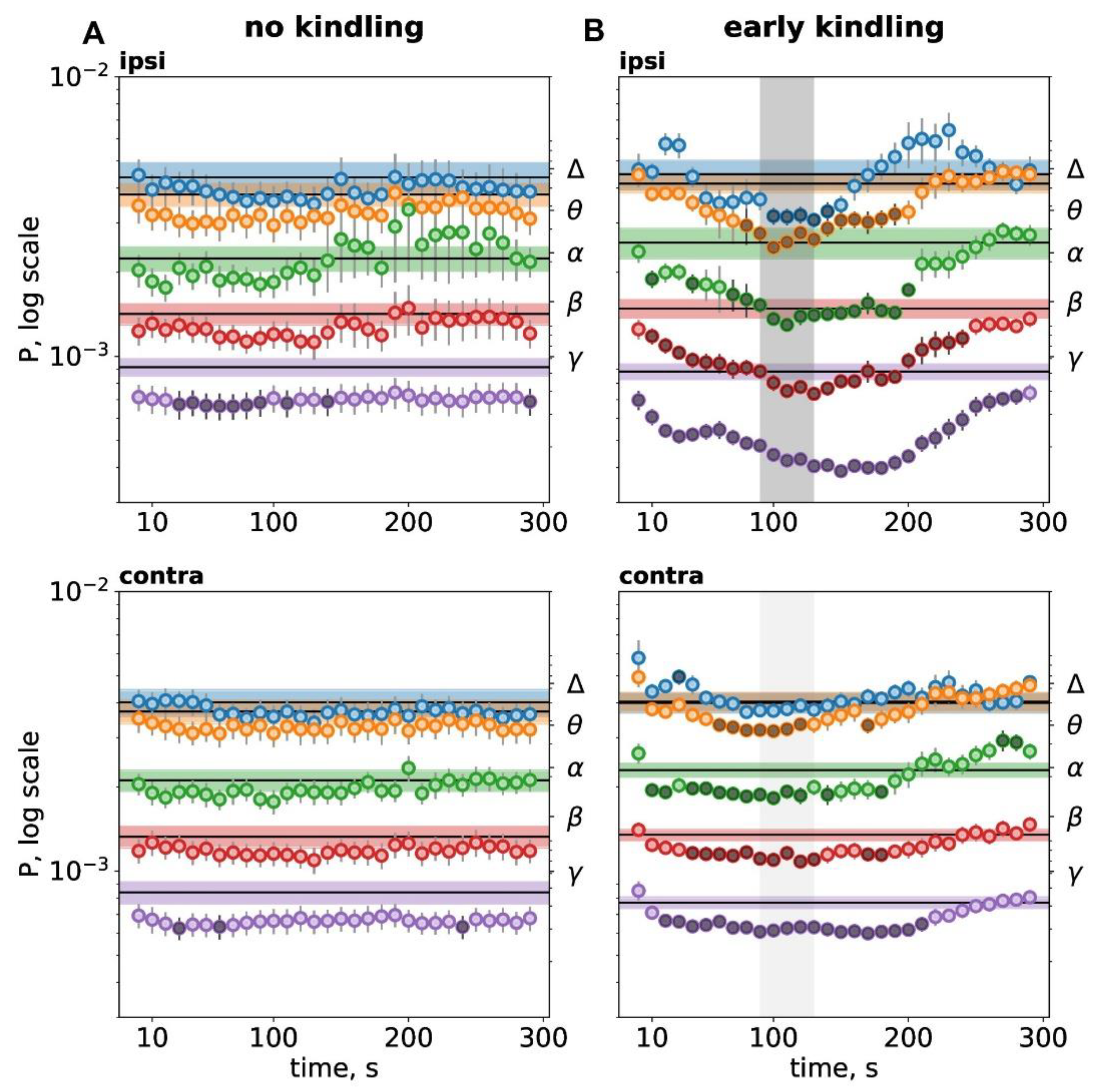
Spectral analysis showed that non-kindled subcortical seizures produced mild postictal depression of high-frequency gamma oscillations without changes in other frequency bands (
Figure 2A). The gamma depression lasted longer in the cortex of one hemisphere (90 s in total) than in another (20 s). The asymmetry of the gamma depression corresponded to motor asymmetry of hyperkinetic seizures - depression was longer in the cortex ipsilateral to direction of running and homolateral to the brainstem seizure focus. This was the cortex where SD and kindled seizure appeared first during kindling.
At the early kindling stage when unilateral SD appeared in the cortex, the postictal depression became strong and wideband. Fast (beta-gamma) cortical oscillations showed longer (230-280 s) and stronger depression compared to slow cortical activity. It the cortex affected by SD, beta-gamma depression started long before SD arrival to the recording site and subsided after SD termination. Power of the slowest delta activity reduced only during SD and only in the cortex affected by SD. In the contralateral cortex unaffected by SD, cortical oscillations in all frequency bands except delta were also depressed though milder than in the SD-affected cortex.
3.3. Homotopic Functional Connectivity During Postictal and Interictal (Baseline) Periods Before Kindling and at the Early Kindling Stage
3.3.1. Mutual Information
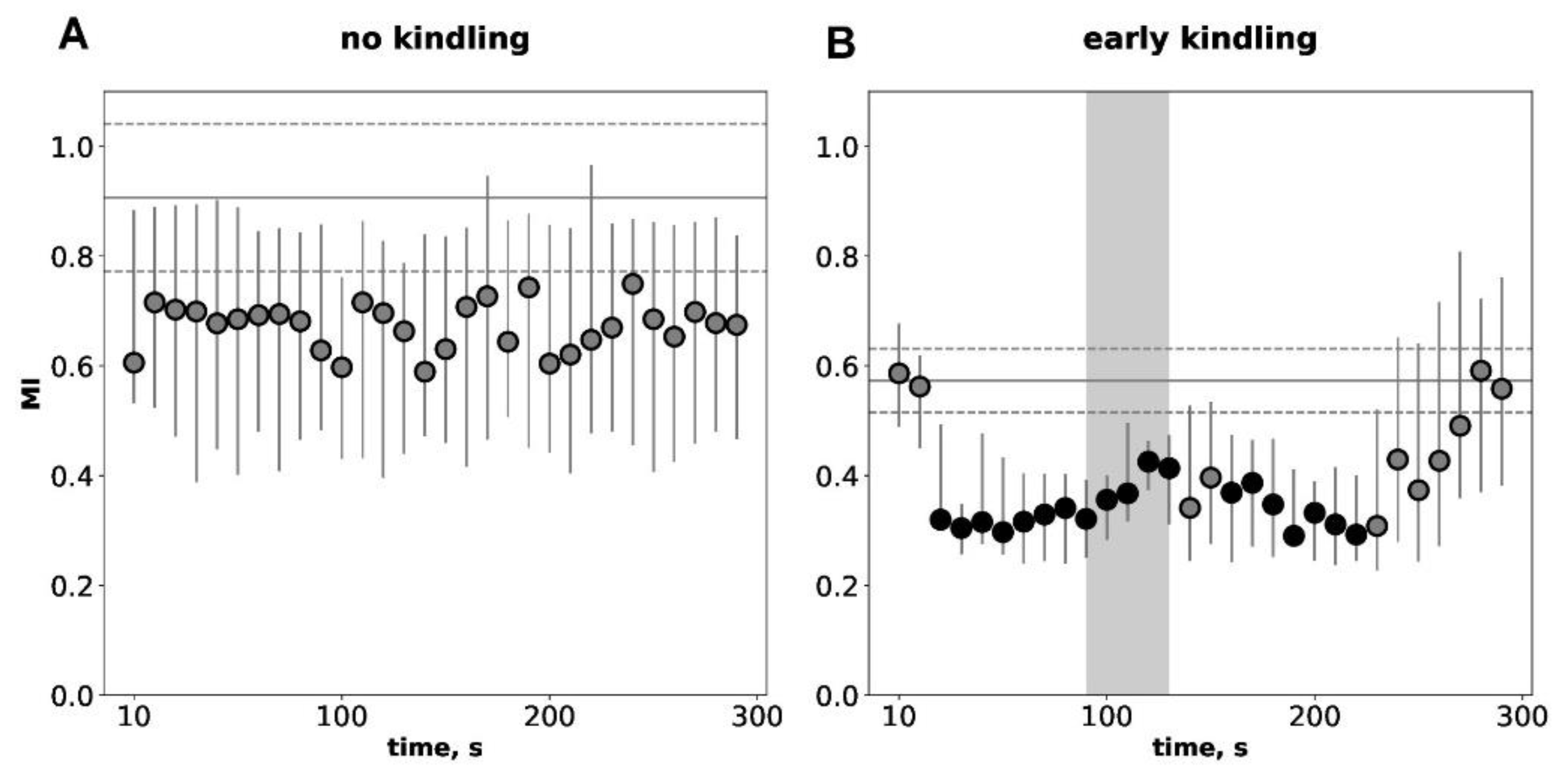
Temporal dynamics of mutual information (MI) values during 300-s immediate postictal period compared to baseline preictal levels for non-kindled and slightly kindled seizures are shown in
Figure 3. Before kindling, hyperkinetic seizures did not change baseline level of MI (
Figure 3A). At the early kindling stage, audiogenic seizures produced an abrupt twofold decrease in connectivity strength for several minutes (
Figure 3B). The MI drop had two-wave pattern with the first wave (30 -140 s) starting before SD arrival to the recording site and the second wave (170 – 230 s) developing after termination of cortical SD. Baseline MI level was significantly lower in slightly kindled animals compared to seizure-naïve ones (p<0.05, p=0.03791;Mann-Whytney test,
Figure 3). Thus, MI analysis shows that the early stage of epileptogenesis is associated with pronounced decrease in interhemispheric functional connectivity both during interictal and postictal periods.
3.3.2. Phase Synchronization
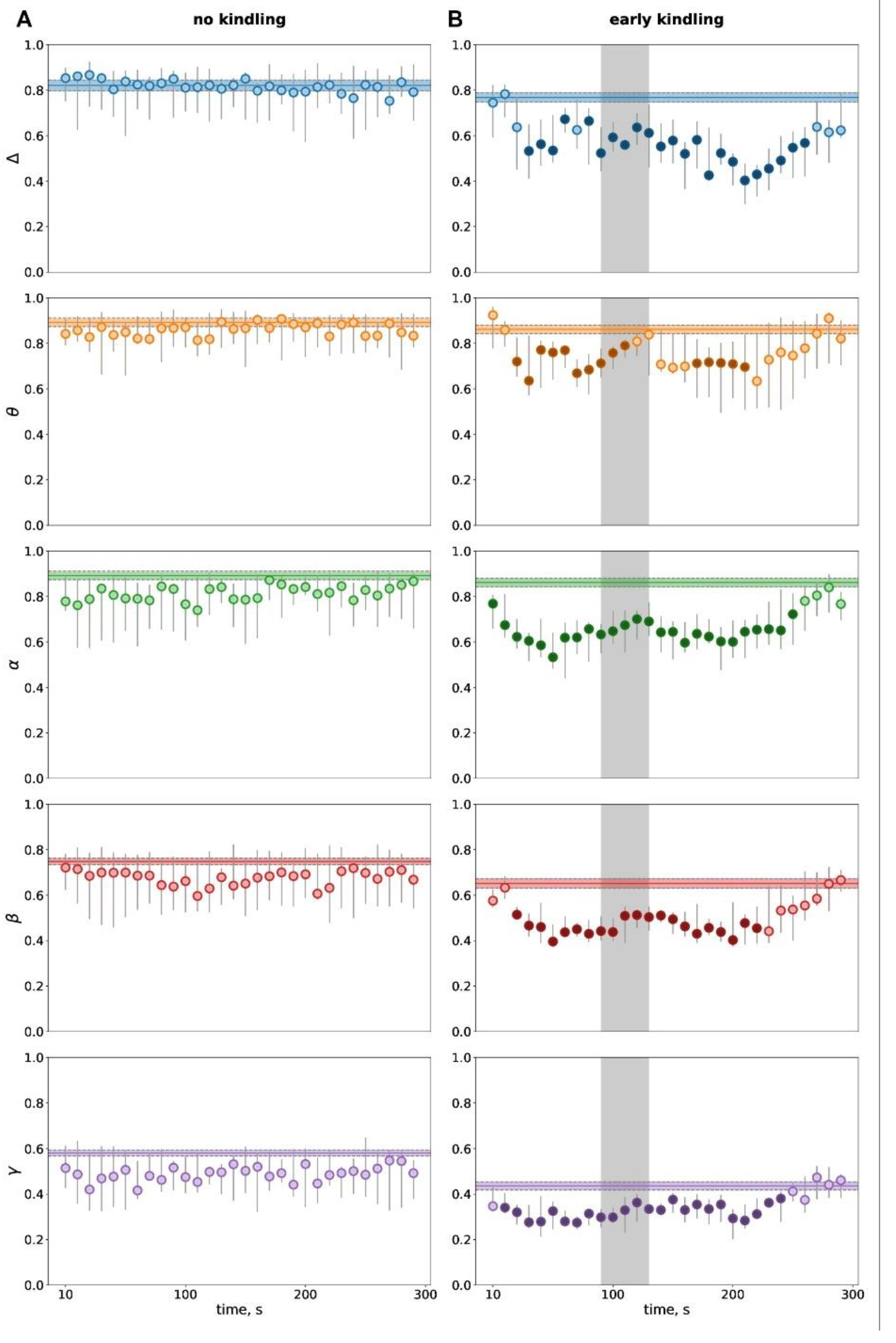
Interhemispheric phase synchronization (PS) showed no changes following non-kindled subcortically-driven seizures (Fi. 4A). With recruitment of the cortex in the epileptic network and appearance of cortical SD, hyperkinetic seizures started to induce transient wideband loss of interhemispheric synchronization during postictal period (
Figure 4B). The hemispheric desynchronization started very soon after seizure termination – immediately in the alpha band and a bit later (since 20-40 s) in other frequency bands. The drop of interhemispheric synchronization lasted till 220-270 s.
Comparison of baseline levels of interhemispheric synchronization in seizure-naïve and slightly kindled animals showed no significant difference in the delta (p=0.178), theta (p=0.307), and alpha (p=0.307) frequency bands but a tendency to lowering in the beta band (p=0.066) and a significant reduction in the gamma band (p=0.004) at the early kindling stage.
4. Discussion
4.1. Reduced Resting-State Hemispheric Connectivity at the Early Stage of Epileptogenesis
Our previous study in the audiogenic kindling model has shown that resting-state hemispheric connectivity significantly changes during late-stage kindling associated with development of cortical seizures (Medvedeva et al., 2023). The present findings demonstrate very early onset of the network reorganization - long before the epileptic activation of the cortex, baseline interhemispheric connectivityreduces compared to its pre-kindling level. Given the data, we can trace evolution of the resting-state connectivity over the duration of kindling-related epileptogenesis. Baseline MI level reduces early in kindling (present study) and remains to be reduced later (Medvedeva et al., 2023) that indicates persistent functional decoupling the two cortices during epileptogenesis. The result is in line with clinical data from resting-state fMRI studies showing reduced functional connectivity between homologous temporal lobes of the two hemispheres in patients with temporal lobe epilepsy as compared to healthy controls (Maccotta et al., 2013; Morgan et al., 2014; Sirin et al., 2020).
Our analysis of phase synchronization has shown that the hemispheric synchrony in gamma frequency band is the most sensitive marker of epileptogenesis compared to other bands. As shown in our previous study, baseline gamma connectivity between the hemispheres increases at the final stages of kindling, when cortical seizures became bilateral,that suggests a role of the gamma hypercoupling in facilitated spread of epileptic activity between the cortex of the two hemispheres (Medvedeva et al., 2023). The present study demonstrates reduced gamma synchronization of the hemispheres at the early kindling stage. Therefore, the data from our present and previous studies show that kindling progression is associated with early hemispheric gamma-band hyposynchrony followed by gamma hypersynchronization. Similar alterations of resting-state fMRI connectivity with transformation from hyposynchrony to hypersynchrony has been described during epileptogenesis in post-status animal models (Bertoglio et al., 2019). The interhemispheric synchronization in the gamma frequency band may be a very sensitive marker of epileptogenic changes in the cortex. Given that cortical gamma activity plays the critical role in cognition, the alterations of gamma synchrony may underlie cognitive dysfunction in epileptic patients.
Alterations in baseline hemispheric connectivity reflect long-term plasticity of neural networks that can either promote or prevent expansion of the epileptic network. We suggest an adaptive role of the early functional disconnection of the hemispheres. The sustained decrease in hemispheric communication early in epileptogenesis is likely to represent homeostatic network mechanism preventing or hindering seizure spread within the brain. This is in line with clinical studies reported reduced LFP synchrony between seizure-onset zone and surrounding brain regions (Warren et al., 2010). Authors suggested that the hyposynchrony produces functional isolation of the epileptic focus from other brain regions.
4.2. Postictal Dynamics of Interhemispheric Connectivity at the Early Stage of Epileptogenesis
At the early kindling stage, in addition to the sustained interhemispheric hypoconnectivity, seizures began to be followed by transient postictal drop of hemispheric coupling. In non-kindled animals, brainstem seizures do not change interhemispheric connectivity during the postictal period. In slightly kindled rats, similar seizures are followed by a two-fold drop of hemispheric connectivity (both MI and PS) for several minutes. Given our previous findings (Medvedeva et al., 2023), we observe dynamic modification of postictal connectivity patterns during epileptogenesis. Postictal MI drop appears at the early stage of kindling, disappears at the middle stage (no changes after focal cortical seizures) and is replaced by postictal hyperconnectivity at the final kindling stage (increased MI after bilateral cortical seizures). Postictal PS levels show similar dynamics during epileptogenesis – wideband drop early in kindling, which becomes milder or disappears later and progresses to delta hypercoupling at the final stage of kindling (Medvedeva et al., 2023). Thus, postictal changes in interhemispheric connectivity evolve from hypo- to hyperconnectivity during epileptogenesis. Postictal alterations in brain activity are thought to reflect reversible seizure-induced changes in network activity that may include adaptive mechanisms of short-term plasticity directed at restoration of brain function. We suggest that the homeostatic mechanisms are activated early during kindling in relatively normal brain but exhaust with recurrent seizures and finally convert to maladaptive changes promoting seizure propagation in the hyperexcitable brain. The post-seizure breakdown of hemispheric connectivity and disruption of functional integrity of brain networks may underlie reversible deficits in perceptual, executive and cognitive function during postictal period.
4.3. Postictal Depression of Cortical Activity Before Kindling and at the Early Kindling Stage
Transient neurological deficit and EEG depression are well-recognized postictal events in epileptic patients. In our study, subcortically-driven seizures are followed by mild depression of cortical gamma oscillations before kindling and by strong wideband depression early in kindling. For both non-kindled and kindled seizures, maximal postictal depression was found in high-frequency gamma band. Cortical gamma activity plays the crucial role in higher brain function, including perception and arousal. Suppression of gamma oscillations in the cortex is associated with a decrease in arousal (Garcia-Rill et al., 2019). As shown in the present study, audiogenic seizures werealways followed by postictal behavioral immobility. Behavioral arrest in rodents is thought toreflect dysfunction of brainstem mechanisms controlling movement initiation (Klemm, 2001).Maintenance of gamma activity in the cortex is related to cortical networks and ascending drive from the pedunculopontine nucleus (Garcia-Rill et al., 2016, 2019). The brainstem nucleus is a part of the reticular activating system and mesencephalic locomotor regions, that playing a role in regulation of arousal state and locomotion (Faingold, 2012; Garcia-Rill et al., 2019). Deficient cortical gamma activity during postictal period found in our study may reflect abnormalities in the rhythm-generating networks. An important role of the networks in control of arousal and movement initiation may explain association of the gamma depression with the decrement in arousal levels and behavioral arrest during postictal period.
4.4. Cortical SD is an Electrographic Marker of Early Epileptogenic Changes and Acontributing Factor in Postictal Alterations of Cortical Activity
Cortical spreading depolarization (SD) develops in response to different brain insults, including epileptic seizures (Somjen, 2001; Drier et al., 2012; Tamim et al., 2021; Koroleva et al., 1993; Samotaeva et al., 2013). Co-occurrence of cortical SD and seizures has been reported in clinical (Fabricius et al., 2008; Dreier e al., 2012) and preclinical (Tamim et al., 2021; Koroleva et al., 1993; Vinogradova, 2015) studies. It is suggested that SD triggered by focal cortical seizures can act as endogenous anti-seizure mechanism (Tamim et al., 2021). In preclinical models of epileptogenesis, cortical SD starts to develop at the initial subclinical stage of epileptogenesis before the emergence of pronounced epileptiform activity in the cortex (Hatcher et al., 2020; Vinogradova et al., 2009).
In audiogenic kindling, cortical SD can be triggered either by ascending drive from the brainstem seizure focus or by mild epileptic activation of the cortex during brainstem seizures. In either case, triggering cortical SD indicates hyperexcitable state of the cortex. In audiogenic kindling, cortical SD appears with the large time lag (90-100 s) after terminationof a brainstem-driven seizure. It looks as if SD arises in a remote circumscribed regionof the cortex and slowly propagates to the parietal cortex (velocity of SD propagation over the cortex is 3-6 mm/min). Unilateral pattern of cortical SD confirms the focal triggering cortical SD and suggests non-symmetric growing excitability of the cortex of the two hemispheres during audiogenic kindling.
Postictal changes in cortical activity at the early kindling stage seem to represent interference of depressive effects of seizures and seizure-induced SD. Pattern of postictal depression after slightly kindled seizures - strong silencing fast (beta-gamma) activity and mild suppression of slow cortical oscillations - is similar to that induced by cortical SD in healthy awake rats (Medvedeva et al., 2024). A role of SD in postictal depression is also supported by the longer and stronger suppression of spontaneous electrical activity in the cortex affected by SD. On the other hand, rapid onset of postictal depression and involvement of the cortex unaffected by SD also indicates contribution of ictal activity. Both seizure and seizure-induced SD are likely to contribute to postictal hemispheric disconnection. It has two-wave pattern. The first wave develops before SD arrival to the recording point and seems to result from ictal excitation of the cortex. Timing of the second wave corresponds well to a network signature of unilateral cortical SD - transient (100 s) interhemispheric decoupling which starts after termination of SD (Vinogradova et al., 2021, Lachinova et al., 2024).
5. Conclusions
Traditionally, epilepsy is considered as a disorder of excessive synchronization and hyperconnectivity. Our findings have revealed that the earliest phase of epileptogenesis is associated with reduced synchronization and breakdown of normal long-range communication (decoupling of the hemispheres) during interictal and postictal periods. The initial reduction in hemispheric communication may reflect homeostatic long- and short-term plastic alterations within brain networks and an active attempt of the brain to restrict epileptogenic process and to isolate functionally the cortical regions experiencing seizure or subcortical drive at the initial stage of epileptogenesis. Enhanced synchronization and hypercoupling might be local or develop later during epileptogenic process. Our findings suggest that seizure-induced SD (spreading depolarization) contributes to hemispheric decoupling and depression of cortical activity during postictal period. This indicates that postictal changes reflect not just neural exhaustion but result from active events triggered by seizures. We think that hemispheric hypoconnectivity (resting-state and postictal) may be an early marker of epileptogenesis.
Author Contributions
Tatiana Medvedeva: Formal analysis, Data curation; Software, Methodology, Visualization, Drafting maniscript; Lyudmila Vinogradova: Conceptualization; Data acquisition; Methodology; Validation; Drafting and writing manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 22-15-00327-P.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Institute of Higher Nervous Activity and Neurophysiology of Russian Academy of Sciences (protocol code N1, 01.02.2022).
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bertoglio, D.; Jonckers, E.; Ali, I.; Verhoye, M.; Van der Linden, A.; Dedeurwaerdere, S. In vivo measurement of brain network connectivity reflects progression and intrinsic disease severity in a model of temporal lobe epilepsy. Neurobiol Dis 2019, 127, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Courtiol, J; Guye, M; Bartolomei, F; Petkoski, S; Jirsa, VK. Dynamical Mechanisms of Interictal Resting-State Functional Connectivity in Epilepsy. J Neurosci 2020, 40(29), 5572–5588. [Google Scholar] [CrossRef] [PubMed]
- Dreier, JP; Major, S; Pannek, HW; Woitzik, J; Scheel, M; Wiesenthal, D; Martus, P; Winkler, MK; Hartings, JA; Fabricius, M; et al. COSBID study group. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain 2012, 135 Pt 1, 259-75. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, M; Fuhr, S; Willumsen, L; Dreier, JP; Bhatia, R; Boutelle, MG; Hartings, JA; Bullock, R; Strong, AJ; Lauritzen, M. Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin Neurophysiol. 2008, 119(9), 1973-84. [Google Scholar] [CrossRef]
- Faingold, CL. Brainstem Networks: Reticulo-Cortical Synchronization in Generalized Convulsive Seizures. In Jasper’s Basic Mechanisms of the Epilepsies [Internet], 4th ed.; Noebels, JL, Avoli, M, Rogawski, MA, Olsen, RW, Delgado-Escueta, AV, Eds.; National Center for Biotechnology Information (US): Bethesda (MD), 2012. [Google Scholar] [PubMed]
- Garcia-Rill, E; D’Onofrio, S; Mahaffey, S. Bottom-up Gamma: the Pedunculopontine Nucleus and Reticular Activating System. Transl Brain Rhythm 2016, 1(2), 49–53. [Google Scholar] [CrossRef]
- Garcia-Rill, E; Mahaffey, S; Hyde, JR; Urbano, FJ. Bottom-up gamma maintenance in various disorders. Neurobiol Dis. 2019, 128, 31–39. [Google Scholar] [CrossRef]
- Grishchenko, AA; van Rijn, CM; Sysoev, IV. Comparative Analysis of Methods for Estimation of Undirected Coupling from Time Series of Intracranial EEGs of Cortex of Rats-Genetic Models of Absence Epilepsy. Math. Biol. Bioinf 2017, 12(2), 317–326. [Google Scholar] [CrossRef]
- Haneef, Z; Chiang, S; Yeh, HJ; Engel, J, Jr.; Stern, JM. Functional connectivity homogeneity correlates with duration of temporal lobe epilepsy. Epilepsy Behav 2015, 46, 227–233. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Hatcher, A; Yu, K; Meyer, J; Aiba, I; Deneen, B; Noebels, JL. Pathogenesis of peritumoral hyperexcitability in an immunocompetent CRISPR-based glioblastoma model. J Clin Invest 2020, 130(5), 2286–2300. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Kerrigan, JF; Ng, YT; Chung, S; Rekate, HL. The hypothalamic hamartoma: a model of subcortical epileptogenesis and encephalopathy. SeminPediatr Neurol 2005, 12(2), 119–131. [Google Scholar] [CrossRef] [PubMed]
- Klemm, WR. Behavioral arrest: in search of the neural control system. Prog Neurobiol 2001, 65(5), 453–471. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, VI; Vinogradova, LV; Bures, J. Reduced incidence of cortical spreading depression in the course of pentylenetetrazol kindling in rats. Brain Res 1993, 608(1), 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kozachenko, LF; Leonenko, NN. Sample estimate of the entropy of a random vector. 168 Problems of Information Transmission 1987, 23(2), 9–16. [Google Scholar]
- Kramer, MA; Cash, SS. Epilepsy as a disorder of cortical network organization. Neuroscientist 2012, 18(4), 360–372. [Google Scholar] [CrossRef]
- Kraskov, A.; Stogbauer, H.; Grassberger, P. Estimating mutual information. Phys.Rev.E Volume 2004, 69, 066138. [Google Scholar] [CrossRef]
- Kreuz, T; Mormann, F; Andrzejak, RG; Kraskov, A; Lehnertz, K; Grassberger, P. Measuring synchronization in coupled model systems: A comparison of different approaches. Physica D: Nonlinear Phenomena 2007, 225(1), 29–42. [Google Scholar] [CrossRef]
- Lachinova, DA; Smirnova, MP; Pavlova, IV; Sysoev, IV; Vinogradova, LV. Transient destabilization of interhemispheric functional connectivity induced by spreading depolarization. NetwNeurosci 2024, 8(4), 1383–1399. [Google Scholar] [CrossRef]
- Lam, AD; NoebelsJ. Night Watch on the Titanic: Detecting Early Signs of Epileptogenesis in Alzheimer Disease. Epilepsy Curr. 2020, 20(6), 369–374. [Google Scholar] [CrossRef]
- Lee, H; Jung, S; Lee, P; Jeong, Y. Altered intrinsic functional connectivity in the latent period of epileptogenesis in a temporal lobe epilepsy model. Exp Neurol 2017, 296, 89–98. [Google Scholar] [CrossRef]
- Maccotta, L; He, BJ; Snyder, AZ; Eisenman, LN; Benzinger, TL; Ances, BM; Corbetta, M; Hogan, RE. Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin. 2013, 2, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Marescaux, C; Vergnes, M; Kiesmann, M; Depaulis, A; Micheletti, G; Warter, JM. Kindling of audiogenic seizures in Wistar rats: an EEG study. Exp Neurol 1987, 97(1), 160-8. [Google Scholar] [CrossRef] [PubMed]
- Maris, E; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, TM; Sysoeva, MV; Sysoev, IV; Vinogradova, LV. Intracortical functional connectivity dynamics induced by reflex seizures. Exp Neurol 2023, 368, 114480. [Google Scholar] [CrossRef]
- Medvedeva, TM; Smirnova, MP; Pavlova, IV; Vinogradova, LV. Different vulnerability of fast and slow cortical oscillations to suppressive effect of spreading depolarization: state-dependent features potentially relevant to pathogenesis of migraine aura. J Headache Pain 2024, 25(1), 8. [Google Scholar] [CrossRef]
- Morgan, VL; Abou-Khalil, B; Rogers, BP. Evolution of functional connectivity of brain networks and their dynamic interaction in temporal lobe epilepsy. Brain Connect 2015, 5(1), 35–44. [Google Scholar] [CrossRef]
- Mormann, F; Lehnertz, K; David, P; Elder, CE. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D: Nonlinear Phenomena 2000, V. 144, 358–369. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinate, fifth ed.; Elsevier Academic Press: USA, 2005. [Google Scholar]
- Pregowska, A; Szczepanski, J; Wajnryb, E. Mutual information against correlations in binary communication channels. BMC Neuroscience 2015, 16. [Google Scholar] [CrossRef]
- Samotaeva, IS; Tillmanns, N; van Luijtelaar, G; Vinogradova, LV. Intracortical microinjections may cause spreading depression and suppress absence seizures. Neuroscience 2013, 230, 50–55. [Google Scholar] [CrossRef]
- Somjen, GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001, 81(3), 1065-96. [Google Scholar] [CrossRef] [PubMed]
- Scholly, J; Staack, AM; Kahane, P; Scavarda, D; Régis, J; Hirsch, E; Bartolomei, F. Hypothalamic hamartoma: Epileptogenesis beyond the lesion? Epilepsia 2017, 58 Suppl 2, 32–40. [Google Scholar] [CrossRef]
- Sirin, NG; Kurt, E; Ulasoglu-Yildiz, C; Kicik, A; Bayram, A; Karaaslan, Z; Bebek, N; Baykan, B; Demiralp, T; Gurses, C. Functional connectivity analysis of patients with temporal lobe epilepsy displaying different ictal propagation patterns. Epileptic Disord. 2020, 22(5), 623–632. [Google Scholar] [CrossRef] [PubMed]
- Tamim, I; Chung, DY; de Morais, AL; Loonen, ICM; Qin, T; Misra, A; Schlunk, F; Endres, M; Schiff, SJ; Ayata, C. Spreading depression as an innate antiseizure mechanism. Nat Commun 2021, 12(1), 2206. [Google Scholar] [CrossRef] [PubMed]
- Tracy, JI; Doucet, GE. Resting-state functional connectivity in epilepsy: growing relevance for clinical decision making. CurrOpin Neurol 2015, 28(2), 158–165. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, LV; Kuznetsova, GD; Coenen, AM. Unilateral cortical spreading depression induced by sound in rats. Brain Res. 2009, 1286, 201–207. [Google Scholar] [CrossRef]
- Vinogradova, LV. Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: Implications for the pathophysiology of migraine aura. Cephalalgia 2015, 35(11), 979–986. [Google Scholar] [CrossRef]
- Vinogradova, LV; Grinenko, OA. Ictal electrographic pattern of focal subcortical seizures induced by sound in rats. Brain Res. 2016, 1635, 161–168. [Google Scholar] [CrossRef]
- Vinogradova, LV; Suleymanova, EM; Medvedeva, TM. Transient loss of interhemispheric functional connectivity following unilateral cortical spreading depression in awake rats. Cephalalgia 2021, 41(3), 353–365. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Warren, CP; Hu, S; Stead, M; Brinkmann, BH; Bower, MR; Worrell, GA. Synchrony in normal and focal epileptic brain: the seizure onset zone is functionally disconnected. J Neurophysiol. 2010, 104(6), 3530–3539. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |