Submitted:
09 January 2026
Posted:
12 January 2026
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
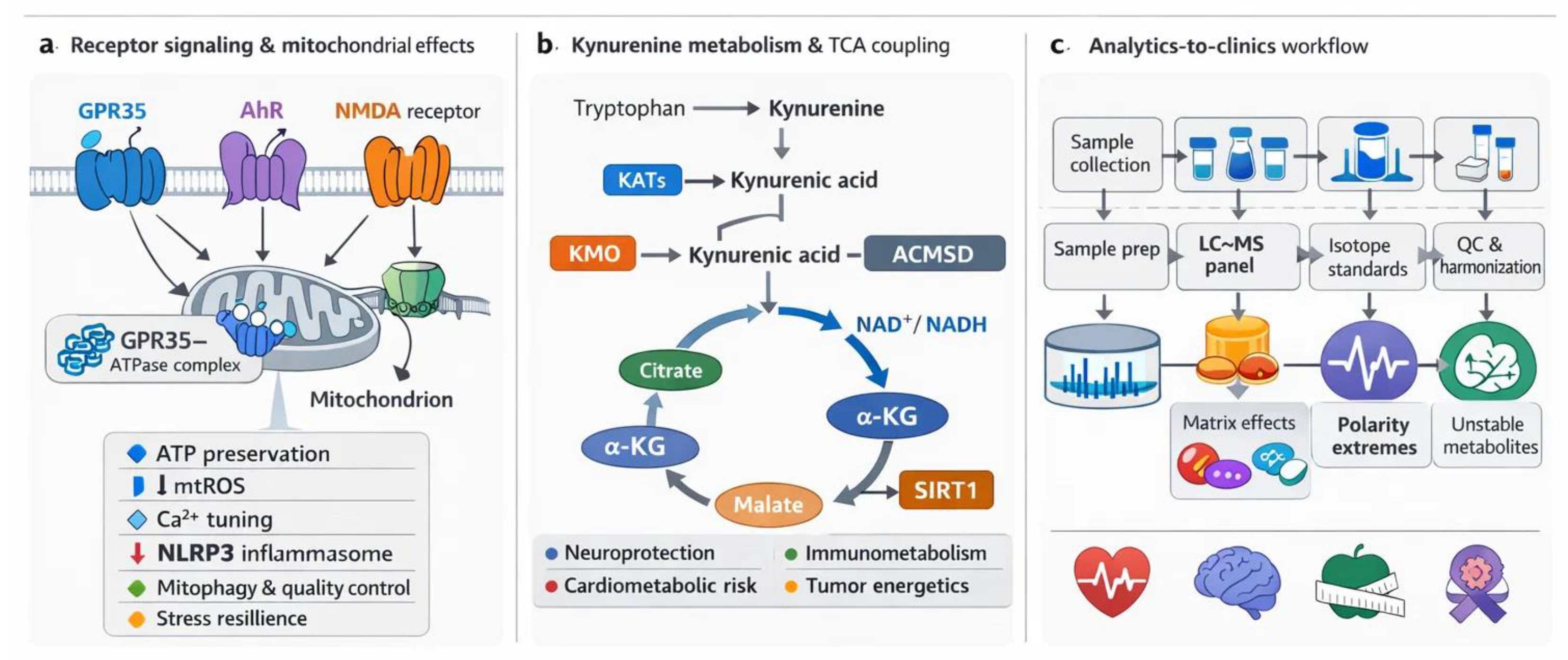
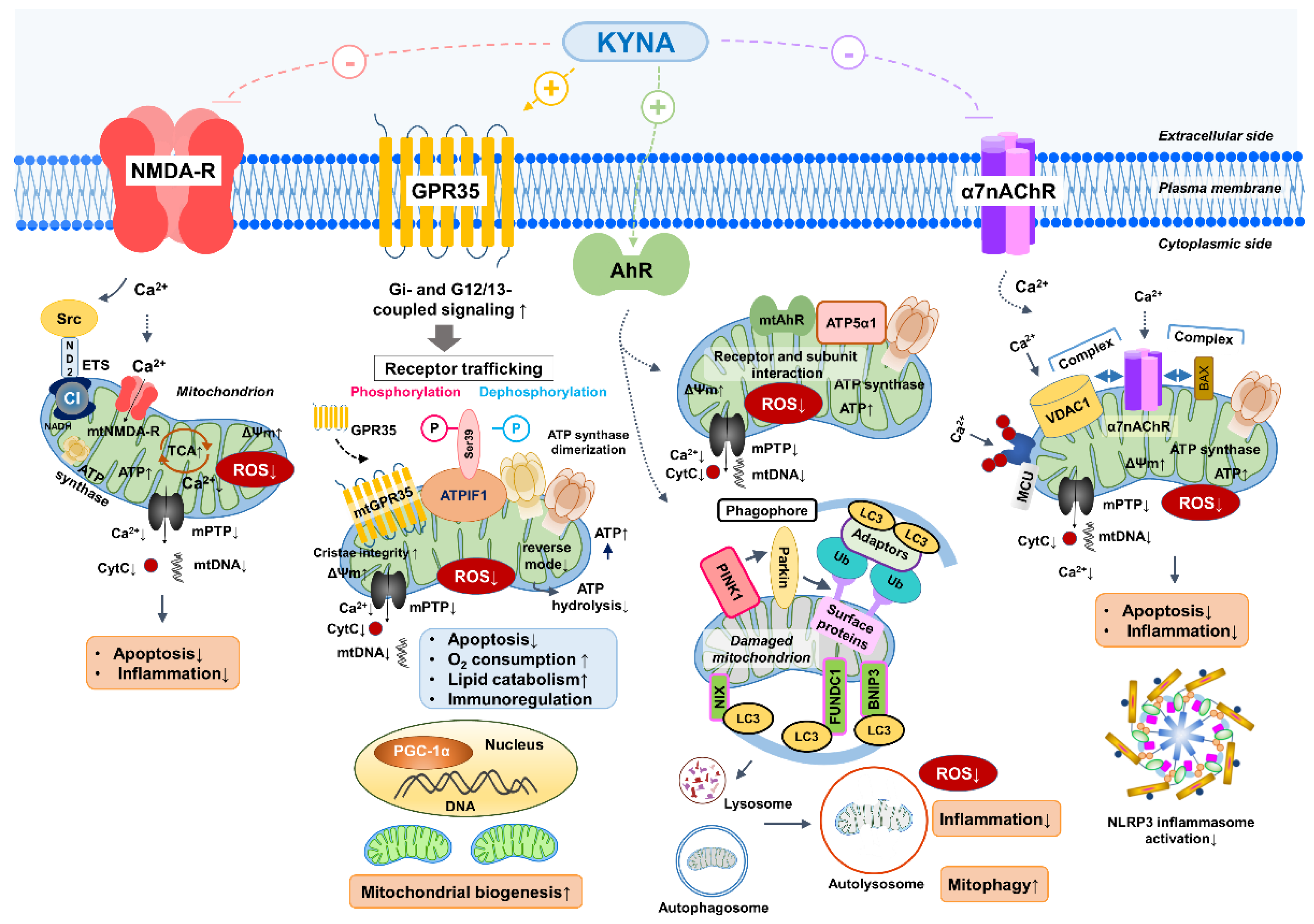
2. Receptor-Mediated Mitochondrial Regulation by Kynurenic Acid (KYNA)
2.1. KYNA and GPR35: Energy Homeostasis and Ischemic Protection
2.2. KYNA and Aryl Hydrocarbon Receptor (AhR): Mitophagy and Organelle Quality Control (QC)
2.3. Kynurenic Acid (KYNA) and N-methyl-D-aspartate (NMDA) Receptors: Calcium Regulation and Excitotoxicity
2.4. Nicotinic Acetylcholine Receptors (α7nAChR-like)
2.5. Other Potential KYNA-Sensitive Sites
| Target | Mitochondrial effects | Animal model/Cell type | Key findings | References |
|---|---|---|---|---|
| GPR35 | -ATP preservation -ATP turnover ↑ -Mitochondrial oxidative capacity ↑ -ROS production ↓ -mPTP opening inhibition -Calpain-1/2 activity ↓ |
Myocardial ischemia/reperfusion (rat, NMVMs) Myocardial ischemia/reperfusion (mouse, heart tissue) C57BL/6J mice, adipose tissue |
-KYNA–GPR35 activation stabilizes ATP synthase, maintains ΔΨm, prevents ATP hydrolysis, limits ROS and apoptosis -GPR35 blockade reduces calpain-mediated proteolysis, preserves mitochondrial integrity, and mitigates oxidative stress -KYNA–GPR35 axis enhances PGC-1α expression, promotes mitochondrial biogenesis, increases O2 consumption, and initiate anti inflammatory cytokine production |
[16,22,92,108,121,216] |
| mt GPR35 |
-ATP synthase dimerization -ATP preservation via the inhibition of ATP hydrolysis |
GPR35 knoclout mice and neonaatal cardiomyocytes | -Binds to ATPIF1 and associates with the mitochondrial outer membrane -Inhibits mitochondrial adenylate cyclase and thereby PKA -Allows ATPIF1 to promote ATP synthase dimerization and prevent ATP hydrolysis |
[22,121] |
| AhR | -Mitophagy (BNIP/PINK1–Parkin) ↑ -ROS production ↓ -ATP preservation -Oxidative metabolism ↑ |
Hepatocytes, AML12 cells, IPEC-J2 cells, AhR knockout mice, primary hepaocytes |
-KYNA and KYN activate AhR to induce PINK1 and BNIP3 expression, promoting mitophagy and preserving mitochondrial respiration under stress -Loss of AhR impairs mitochondrial quality control and increases ROS accumulation, disrupting energy metabolism |
[139,156,168,221,222] |
| mtAhR | -ATP synthase regulation -Fine-tuning ROS production |
Mitochondrial fraction, liver cells | -Mitochondrial AhR interacts with ATP5α1; its localization and activity depend on ligand status, possibly influencing ATP synthesis and redox balance | [221] |
| NMDA-R | -Ca2+ influx ↓ -mPTP opening ↓ -Cytochrome c release ↓ -Bcl-XL expression ↑ -Apoptosis ↓ -Complex I coupling |
Neurons, Microglia, neuronal cultures |
-KYNA blocks NMDA-R at the glycine site, limits Ca2+ overload, prevents mPTP opening, and protects against apoptosis -Potential crosstalk with complex I regulates bioenergetics |
[193,197] |
| mt NMDA-R | -Ca2+ flux modulation -Fine-tuning ROS production |
Rat heart mitochondria | -NR1/NR2B subunits detected in mitochondria; -Regulation of ROS production and Ca2+ level under hypoxia/ischemia |
[194] |
| α7nAChR | -Regulates Ca2+ flux, ROS production, and cytochrome c release via interaction with VDAC1 -Influences mPTP opening and OXPHOS activity through kinase signaling (PI3K/Akt, CaM, Src). -Limits apoptosis through the regulation of Bcl-2/Bcl-xL and caspases. |
Isolated mouse liver mitochondria; U373 human glioblastoma astrocytoma cells KAT II knockout (KAT II−/−) mice |
-α7nAChR–VDAC1 and α7nAChR–Bax complexes identified; receptor inhibition (methyllycaconitine) suppresses cytochrome c release; stimulation (PNU 282987) enhances it; acetylcholine reduces ROS. -Decreased KYNA levels increase α7nAChR activity; α7nAChR activation linked to neuroprotection and anti-apoptotic signaling; KYNA may physiologically regulate the receptor |
[37,211,213,223,224] |
| mitoKATP channels (?) | -Channel opening reduces mitochondrial Ca2+ overload, delays mPTP opening, and supports cellular survival during ischemic or oxidative stress; -Its function is modulated by GPR35 and ROS signaling |
In vivo ischemia–reperfusion models: cardiac and neuronal mitochondria |
-Diazoxide (mitoKATP opener) confers neuro- and cardioprotection; inhibition (5-hydroxydecanoate) abolishes protective effects; -ROS and GPR35 signaling may crosstalk to regulate mitoKATP and mPTP. |
[214,215,216] |
| Complex I / Complex III redox sites (?) | -Redox-sensitive cysteine residues regulate ROS generation (superoxide, H2O2); transient ROS acts as signaling for stress adaptation; -KYNAmay scavenge radicals and preserves electron transport. |
Isolated mitochondria; C. elegans | -KYNA may reduce ROS at Complex I and III independent of receptor mechanisms; mild Complex I ROS prolongs lifespan in C. elegans; antioxidant effects support mitochondrial stability. | [217,218,219,220] |
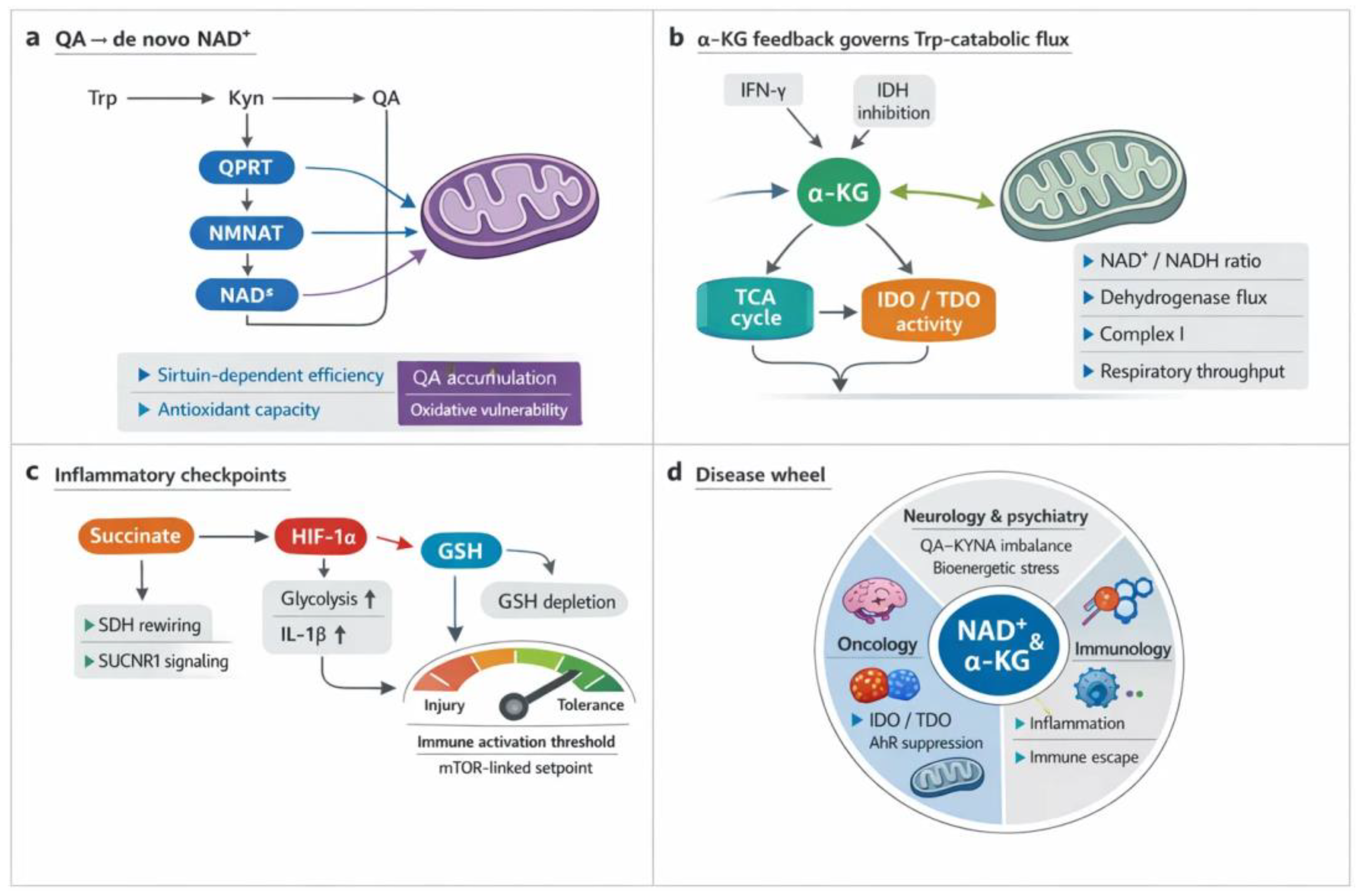
3. Crosstalk Between the Kynurenine (KYN) Metabolism and the TCA Cycle
3.1. Shared Metabolic Intermediates and Redox Balance
| Node (e.g., QA → NAD+) | Enzymes/regulators | Directionality | Impact on NAD+/NADH or anaplerosis | Immune/ROS consequence | Evidence class | References |
|---|---|---|---|---|---|---|
| QA → NAD+ | QPRT → NMNAT → NADS; modulation by aging/inflammation | KYN → NAD+ biogenesis → ETC | Increases cellular/matrix NAD(H); sustains complex I oxidation and O2 consumption | Supports macrophage respiration; QPRT loss ↓NAD+, ↑injury/ROS; neuroinflammation requires conversion for SIRT activity | Mechanistic (cells/animals), clinical/genetic | [14,42,225] |
| KYNA → GPR35 → mitochondrial nodes (incl. MAS) | KATs (KYNA production), GPR35; MAS components | KYNs ligands → receptor → mitochondria/TCA | Tunes ETC throughput and shuttle-coupled redox set-points | Sets immune tone; receptor-proximal control of inflammatory signaling | Mechanistic receptor signaling | [16,22,114] |
| IDO1/TDO2 rate-setting → α-KG availability | IDO1, TDO2; IFN, hypoxia, nutrient status | Immune cues → KYNs flux → TCA carbon | Pulls carbon from Trp; constrains or supports α-KG–linked anaplerosis | Biases tolerance vs activation; conditions efficacy of IDOs/TDO blockade | Multi-omic/tumor-inflammation frameworks | [26,226,227] |
| MCART1-mediated NAD+ import (cytosol → matrix) | MCART1 (SLC25 family) | Cytosol → mitochondria | Maintains matrix NAD+ for dehydrogenases; prevents collapse of OXPHOS | Preserves respiratory control; supports T-cell effector programs | Mechanistic (transport/respiration) | [228,229,230] |
| MDH2 → oxaloacetate restraint of Complex II | MDH2; OAA | TCA intermediate → ETC modulation | Re-routes electron flow; dynamically resets NAD+/NADH coupling | Shapes graded ROS signaling downstream of ratio | Mechanistic ETC control | [217,218,231] |
| NNT couples NADH ↔ NADPH demand | Nicotinamide nucleotide transhydrogenase (NNT) | Matrix redox coupling | Balances NADH oxidation with NADPH generation; stabilizes redox poise | Supports antioxidant defenses; buffers ROS during substrate shifts | Mechanistic redox coupling; | [232,233,234] |
| ME1 (malic enzyme-1): malate → pyruvate (NADPH) | ME1 | Cytosol anaplerosis ↔ redox | Raises NADPH; protects glutathione/NADPH buffering | Reinforces antioxidant capacity; feeds redox-encoded signaling | Mechanistic redox metabolism | [235,236] |
| Serine catabolism → NADH accumulation when respiration stalls | One-carbon/serine axis | Amino acid metabolism → redox | Builds cytosolic/mitochondrial NADH when ETC is limited; throttles biosynthesis | Constrains proliferative programs under low respiration | Mechanistic metabolic control | [2,9,237] |
| SDH lesion → alternative aspartate synthesis (matrix NAD+/NADH-dependent) | SDH/Complex II context; aspartate pathways | ETC defect → rerouted biosynthesis | Forces aspartate generation routes that depend on matrix NAD+/NADH | Salvages growth despite impaired cycling | Mechanistic pathology | [238,239,240] |
| De novo NAD+ from KYNs supports macrophage respiration | QPRT→NMNAT→NADS; macrophage programs | KYNs → NAD+ → OXPHOS | Expands NAD(H) pool; sustains respiratory control across tissues | Coordinates systemic redox communication; tunes inflammatory effectors | Mechanistic + systems | [14,42,241] |
| Type I IFN → IDH inhibition → citrate/α-KG ratio shift | IDH1/IDH2; Type I IFN | Immune signal → TCA wiring → KYNs context | Alters NADPH generation/redox milieu that licenses IDOs/TDO activity | Reprograms Trp catabolism vs defense state | Mechanistic immunometabolism | [26,219,231] |
| M1 macrophage “IDH break” → α-KG drop | IDH node; network integration | Polarization cue → TCA fragmentation | ↓Anaplerosis; altered NADPH; redox favoring effector programs and IDOs induction | Heightened inflammatory activation | Network/multi-omic | [231,242] |
| Sirtuin activity sustained by quinolinate-derived NAD+ | SIRTs; QPRT/NMNAT/NADS | KYNs → NAD+ → sirtuin deacylases | Preserves mitochondrial protein deacylation and efficiency | Supports neuronal viability; mitigates inflammatory stress | Mechanistic neuroinflammation | [14,25,243] |
| Ischemia–reperfusion diversion away from quinolinate | Pathway branch choice; NAD+ augmentation | Stress → KYN branch → NAD+ | NAD+ depletion when diverted; restoration rescues antioxidant capacity | Less oxidative injury with NAD+ repletion | Mechanistic/therapeutic modulation | [22,31,43] |
| UMPS bypass completing NAD+ synthesis when canonical steps fail | UMPS (bypass), salvage enzymes | Engineered/alternative route → NAD+ | Raises total NAD(H) when QPRT or steps are compromised | Enhances ETC throughput in designed systems | Engineering proof-of-principle | [244,245,246] |
| Quinolinate/NAM rise tracks mitochondrial work/biogenesis | Systemic KYNs NAD+ salvage | Workload/biogenesis → KYNs output | Correlated elevation of circulating quinolinate & nicotinamide with ETC demand | Links tissue respiratory programs to systemic KYNs tone | Integrative physiology; | [243,247,248] |
| Ubiquinol (CoQH2) oxidation requirement beyond NAD+ regeneration | ETC Complex III/CoQ cycle | ETC constraint → metabolic outcome | NAD+ repletion alone insufficient if CoQ oxidation is limited | Governs tumor growth constraints; bioenergetic bottleneck | Mechanistic tumor bioenergetics | [85,217,218] |
| LKB1 programs & thioredoxin circuits sculpt NADH turnover | LKB1, TRX/thioredoxin | Kinase/antioxidant systems → NADH flux | Adjusts NADH oxidation and chromatin-linked NAD+ usage | Sets T-cell effector capacity | Mechanistic immune control | [249,250,251] |
3.2. Immunometabolic Integration
3.3. Pathological Implications
| Indication (neurodegeneration/psychiatry/oncology) | Metabolite signature KYNA, QA, KYN, 3-hydroxykynurenine) | Mitochondrial phenotype | Clinical/functional readouts | Study type/size | References |
|---|---|---|---|---|---|
| Neurodegeneration | KYNA ↓, QA ↑; KYN context-dependent; 3-HK not primary in text | NMDA-driven ROS; depressed respiratory capacity; feed-forward bioenergetic failure and inflammation | Higher QA:KYNA ratios track tau/amyloid burden, neuronal dysfunction, faster progression | Clinical & biomarker studies; aging/disease cohorts; preclinical mechanistic work | [51,52,78] |
| Neurodegeneration (therapeutic angle) | Shift flux away from QA; raise KYNA (e.g., KMO modulation) | Mitochondrial stabilization; restored antioxidant defenses (incl. Nrf2 signaling) | Slower neurodegeneration trajectory; reduced excitotoxic stress | Preclinical + translational strategy proposals; early-phase targeting concepts | [3,8,205] |
| Psychiatry | Trp ↓, KYN ↓ (cohorts/meta-analyses); QA favored under immune activation; KYNA/KYN/QA show state-dependent oscillations; 3-HK not emphasized | Immune-bioenergetic coupling; mitochondrial/synaptic function shifts; serotonin depression when KYN metabolism is upshifted | Mood, psychosis, cognitive deficits; moderate blood–brain metabolite concordance indexing symptom burden/progression | Meta-analyses and multi-cohort studies; biomarker cohorts; mechanistic frameworks | [54,73,257] |
| Psychiatry (therapeutic angle) | Enzyme/flux control targeting; microbiome-sensitive modulators | Rebalancing KYNs–TCA redox and neurotransmission coupling | Symptom modulation and progression tracking via peripheral KYNs panels | Translational strategies; trial-readout integration (sizes vary) | [49,50,61] |
| Oncology (tumor immune escape) | KYN ↑ via IDO1/TDO2 → AhR activation; downstream KMO/KYNU shape invasive traits; KYNA/QA context-specific | Bioenergetic rewiring supporting growth; TME-conditioned redox | Treg expansion, CD8+ exhaustion; metastatic behavior, stromal crosstalk, chemoresistance | Clinical experience with IDOs/TDO blockade; preclinical tumor models; biomarker studies | [226,227,258] |
| Oncology (combination therapy) | KYN depletion (KYNU depots); AhR attenuation (e.g., small molecules) | Reprogrammed mitochondrial/immune metabolism; macrophage repolarization (e.g., GPX4–KYNU axis) | Synergy with PD-1 blockade; dismantling suppressive niches; overcoming resistance | Preclinical synergy studies; early translational combinations; emerging clinical strategies | [72,226,241] |
4. Analytical Strategies for Simultaneous Quantification
4.1. Rationale for Unified Measurement
4.2. Chromatographic and Mass Spectrometric Challenges
4.3. Practical Solutions
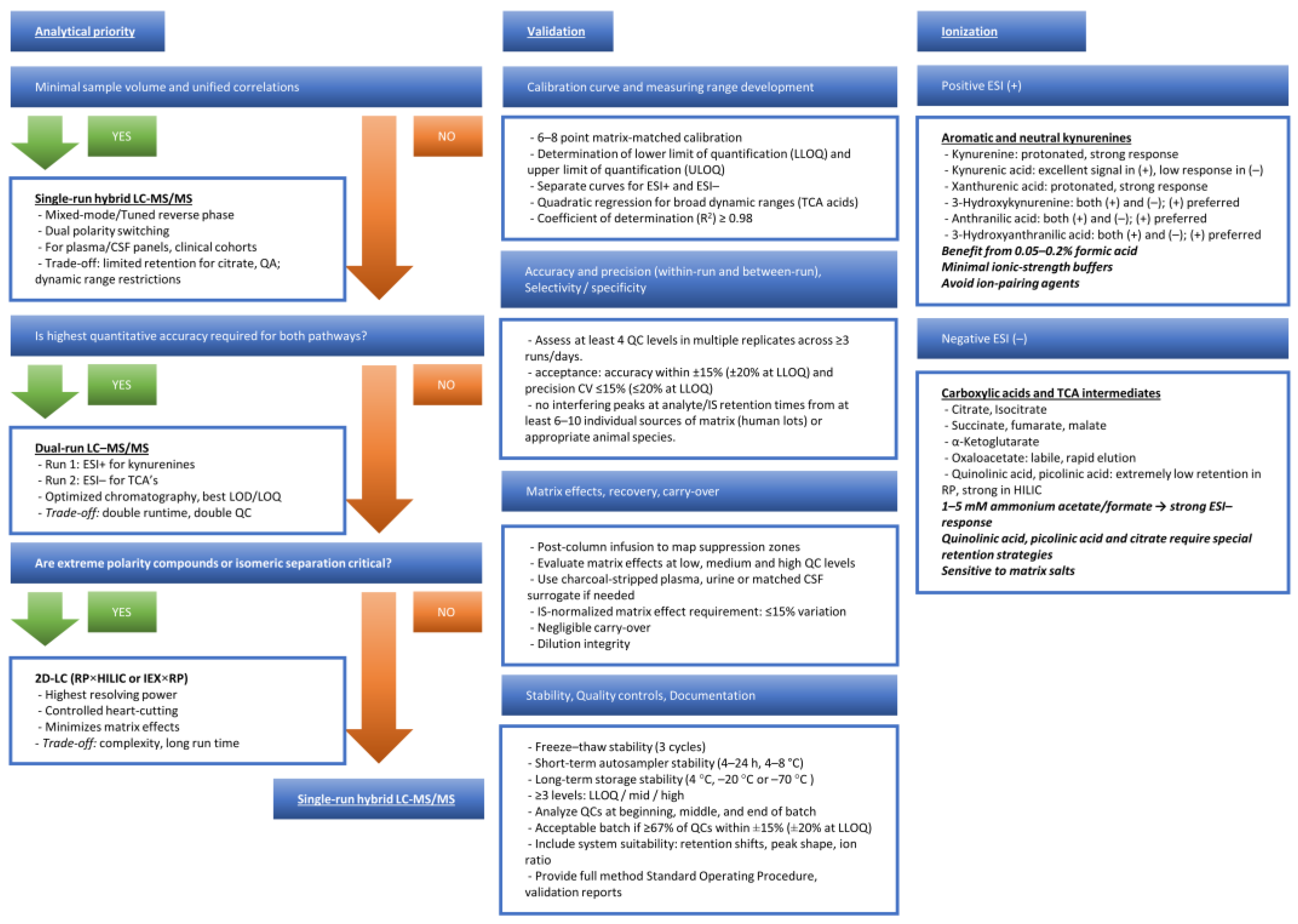
4.4. Validation and Clinical Feasibility
| Parameter | Acceptance Threshold | Notes / Mitigations | References |
|---|---|---|---|
| Calibration model | R2 ≥ 0.98; Back-calculated concentrations within ±15% (±20% at LLOQ) | Use matrix-matched standards; weighted (1/x or 1/x2) regression for wide dynamic ranges; verify linearity and curvature. | [477] |
| Linearity / Dynamic range | At least 6–8 calibration levels; deviation ≤15% (≤20% at LLOQ) | For TCA acids with steep response, consider quadratic fit; re-optimize injection volume to avoid saturation. | [478] |
| LLOQ / ULOQ | Signal ≥5× noise; precision ≤20%; accuracy 80–120% | Confirm LLOQ in actual matrix (plasma/CSF). Check for ion suppression at LLOQ. | [479] |
| Carryover | <20% of LLOQ signal and <5% of IS signal in blank after highest standard | Insert strong wash; consider needle wash with ACN/MeOH/water + 0.1% FA; extend gradient reequilibration. | [480] |
| Matrix effect (ME) | IS-normalized ME CV ≤15% across ≥6 donors | Perform post-column infusion; evaluate ME at low/mid/high QC; use isotopologues matched by polarity and retention. | [446,480,481] |
| Extraction recovery | 80–120% with CV ≤15% | Compare pre-spike vs post-spike; optimize precipitation solvent and pH; avoid ion-pairing agents in precipitant. | [446,482] |
| Precision—intra-day (repeatability) | CV ≤15% (≤20% at LLOQ) | Analyze ≥5 replicates each QC level; monitor retention shifts and ion ratio stability. | [479] |
| Precision—inter-day (reproducibility) | CV ≤15% | Include replicate QCs on ≥3 days; reinject archived QCs to detect long-term drift. | [478] |
| Accuracy | 85–115% (80–120% at LLOQ) | Compare to spiked reference material; evaluate both absolute and IS-normalized values. | [477] |
| Short-term autosampler stability | ≤15% change over 4–24 h at 4–8 °C | KYNs TCAs, may degrade—use immediate analysis or stabilizing additives. | [483] |
| Freeze–thaw stability | ≤15% change after 3 cycles | Avoid repeated thawing; aliquot samples; confirm TCA acids stability separately. | [480] |
| Long-term stability (–70 °C) | ≤15% deviation | Validate ≥1–3 months | [484] |
| Processed sample stability | ≤15% after 6–12 h in autosampler | For unstable analytes use rapid acquisition. | [485] |
| System suitability | Retention time shift <0.2–0.3 min; IS area CV ≤10%; ion ratio within ±20% | Verify before each batch; monitor column pressure, peak shape, and polarity switching efficiency. | [486] |
| QC frequency per batch | ≥3 levels (LQC/MQC/HQC); QCs at start, every 10–15 samples, and end | Large cohorts: insert pooled QC every 10–12 injections. | [479] |
| Batch acceptance criteria | ≥67% of all QCs and ≥50% at each level must be within ±15% (±20% at LLOQ) | If failure: investigate ME shifts, IS suppression, column fouling, or calibration instability. | [478] |
| Inter-batch comparability | QC CV ≤15% across batches | Use pooled QC and IS-normalized drift correction. | [487] |
5. Discussion: Strengths, Limitations, and Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α7nAChR | Nicotinic acetylcholine receptors containing α7 subunits |
| AhR | aryl hydrocarbon receptor |
| AKT | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ATP | adenosine triphosphate |
| ATPIF1 | ATP synthase inhibitory factor 1 |
| BNIP3 | BCL2 interacting protein 3 |
| cAMP | cyclic adenosine monophosphate |
| CD98 | cluster of differentiation 98 |
| CLSI | clinical and laboratory standards institute |
| COMETS | consortium of metabolomics studies |
| CSF | cerebrospinal fluid |
| ERK | extracellular signal-regulated kinase |
| FAIR | findable, accessible, interoperable, and reusable |
| GPR35 | G protein–coupled receptor 35 |
| GPX4 | glutathione peroxidase 4 |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| HILIC | hydrophilic interaction liquid chromatography |
| HRMS | high-resolution mass spectrometry |
| IDO | indoleamine 2,3-dioxygenase |
| IDO1 | indoleamine 2,3-dioxygenase 1 |
| IDO2 | indoleamine 2,3-dioxygenase 2 |
| KATs | kynurenine aminotransferases |
| KMO | kynurenine 3-monooxygenase |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| KYNU | kynureninase |
| LC–MS | liquid chromatography–mass spectrometry |
| LLOQ | lower limit of quantitation |
| MCART1 | mitochondrial carrier transporting NAD+ |
| MDH2 | malate dehydrogenase 2 |
| mPTP | mitochondrial permeability transition pore |
| NAD+ | nicotinamide adenine dinucleotide (oxidized form) |
| NADH | nicotinamide adenine dinucleotide (reduced form) |
| NADPH | nicotinamide adenine dinucleotide phosphate (reduced form) |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated b cells |
| NMDA | N-methyl-D-aspartate |
| NMD-R | N-methyl-D-aspartate receptor |
| NRF1 | nuclear respiratory factor 1 |
| NRF2 | nuclear factor erythroid 2–related factor 2 |
| PD-1 | programmed cell death protein 1 |
| PI3K | phosphoinositide 3-kinase |
| QC | quality control |
| QA | quinolinic acid |
| Rho | ras homolog family protein |
| ROS | reactive oxygen species |
| SAR | structure–activity relationship |
| SDH | succinate dehydrogenase |
| SOP | standard operating procedure |
| TCA | tricarboxylic acid |
| TDO | tryptophan 2,3-dioxygenase |
| TMCS | translational metabolic cohort study |
| Trp | tryptophan |
| UHPLC | ultra-high-performance liquid chromatography |
References
- Liu, B.H.; Xu, C.Z.; Liu, Y.; Lu, Z.L.; Fu, T.L.; Li, G.R.; Deng, Y.; Luo, G.Q.; Ding, S.; Li, N.; et al. Mitochondrial quality control in human health and disease. Mil Med Res 2024, 11, 32. [Google Scholar] [CrossRef]
- Chakrabarty, R.P.; Chandel, N.S. Mitochondria as Signaling Organelles Control Mammalian Stem Cell Fate. Cell Stem Cell 2021, 28, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Esteras, N.; Abramov, A.Y. Nrf2 as a regulator of mitochondrial function: Energy metabolism and beyond. Free Radic Biol Med 2022, 189, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Feng, J.; Wang, M.; Wufuer, R.; Liu, K.; Zhang, Z.; Zhang, Y. Nrf1 is an indispensable redox-determining factor for mitochondrial homeostasis by integrating multi-hierarchical regulatory networks. Redox Biol 2022, 57, 102470. [Google Scholar] [CrossRef]
- Ježek, P.; Holendová, B.; Garlid, K.D.; Jabůrek, M. Mitochondrial Uncoupling Proteins: Subtle Regulators of Cellular Redox Signaling. Antioxid Redox Signal 2018, 29, 667–714. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front Cell Dev Biol 2020, 8, 467. [Google Scholar] [CrossRef]
- Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals (Basel) 2025, 18. [Google Scholar] [CrossRef]
- Tan, D.Q.; Suda, T. Reactive Oxygen Species and Mitochondrial Homeostasis as Regulators of Stem Cell Fate and Function. Antioxid Redox Signal 2018, 29, 149–168. [Google Scholar] [CrossRef]
- Tanaka, M. Neurogenesis and Neuroinflammation in Dialogue: Mapping Gaps, Modulating Microglia, Rewiring Aging. Cells 2026, 15, 78. [Google Scholar] [CrossRef]
- Murakami, S.; Kusano, Y.; Okazaki, K.; Akaike, T.; Motohashi, H. NRF2 signalling in cytoprotection and metabolism. Br J Pharmacol 2023. [Google Scholar] [CrossRef]
- Ryoo, I.G.; Kwak, M.K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol Appl Pharmacol 2018, 359, 24–33. [Google Scholar] [CrossRef]
- Juhász, L.; Spisák, K.; Szolnoki, B.Z.; Nászai, A.; Szabó, Á.; Rutai, A.; Tallósy, S.P.; Szabó, A.; Toldi, J.; Tanaka, M.; et al. The Power Struggle: Kynurenine Pathway Enzyme Knockouts and Brain Mitochondrial Respiration. J Neurochem 2025, 169, e70075. [Google Scholar] [CrossRef]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp Gerontol 2020, 132, 110841. [Google Scholar] [CrossRef]
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab 2018, 27, 378–392.e375. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.; Liu, J.; Zhang, X.D.; Song, Z. Kynurenic Acid Acts as a Signaling Molecule Regulating Energy Expenditure and Is Closely Associated With Metabolic Diseases. Front Endocrinol (Lausanne) 2022, 13, 847611. [Google Scholar] [CrossRef]
- Sánchez Chapul, L.; Pérez de la Cruz, G.; Ramos Chávez, L.A.; Valencia León, J.F.; Torres Beltrán, J.; Estrada Camarena, E.; Carillo Mora, P.; Ramírez Ortega, D.; Baños Vázquez, J.U.; Martínez Nava, G.; et al. Characterization of Redox Environment and Tryptophan Catabolism through Kynurenine Pathway in Military Divers’ and Swimmers’ Serum Samples. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Guidetti, P.; Amori, L.; Sapko, M.T.; Okuno, E.; Schwarcz, R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem 2007, 102, 103–111. [Google Scholar] [CrossRef]
- Wyckelsma, V.L.; Lindkvist, W.; Venckunas, T.; Brazaitis, M.; Kamandulis, S.; Pääsuke, M.; Ereline, J.; Westerblad, H.; Andersson, D.C. Kynurenine aminotransferase isoforms display fiber-type specific expression in young and old human skeletal muscle. Exp Gerontol 2020, 134, 110880. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Á.G.Z.; Spekker, E.; Martos, D.; Szűcs, M.; Fejes-Szabó, A.; Fehér, Á.; Takeda, K.; Ozaki, K.; Inoue, H.; et al. Behavioral Balance in Tryptophan Turmoil: Regional Metabolic Rewiring in Kynurenine Aminotransferase II Knockout Mice. Cells 2025, 2025, 1711. [Google Scholar] [CrossRef]
- Wyant, G.A.; Yu, W.; Doulamis, I.P.; Nomoto, R.S.; Saeed, M.Y.; Duignan, T.; McCully, J.D.; Kaelin, W.G., Jr. Mitochondrial remodeling and ischemic protection by G protein-coupled receptor 35 agonists. Science 2022, 377, 621–629. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Lin, Q.; Li, S.; Jiang, N.; Shao, X.; Zhang, M.; Jin, H.; Zhang, Z.; Shen, J.; Zhou, Y.; Zhou, W.; et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol 2019, 26, 101254. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Liu, Y. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. Exp Gerontol 2020, 132, 110831. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S. Dualistic Dynamics in Neuropsychiatry: From Monoaminergic Modulators to Multiscale Biomarker Maps. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E.; et al. AdipoRon’s Impact on Alzheimer’s Disease-A Systematic Review and Meta-Analysis. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Redefining Roles: A Paradigm Shift in Tryptophan-Kynurenine Metabolism for Innovative Clinical Applications. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Gáspár, R.; Nógrádi-Halmi, D.; Demján, V.; Diószegi, P.; Igaz, N.; Vincze, A.; Pipicz, M.; Kiricsi, M.; Vécsei, L.; Csont, T. Kynurenic acid protects against ischemia/reperfusion injury by modulating apoptosis in cardiomyocytes. Apoptosis 2024, 29, 1483–1498. [Google Scholar] [CrossRef]
- Sun, T.; Xie, R.; He, H.; Xie, Q.; Zhao, X.; Kang, G.; Cheng, C.; Yin, W.; Cong, J.; Li, J.; et al. Kynurenic acid ameliorates NLRP3 inflammasome activation by blocking calcium mobilization via GPR35. Front Immunol 2022, 13, 1019365. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, M.; Zang, X.; Fan, Q.; Liu, Y.; Che, Y.; Guan, X.; Hou, Y.; Wang, G.; Hao, H. Kynurenic acid/GPR35 axis restricts NLRP3 inflammasome activation and exacerbates colitis in mice with social stress. Brain Behav Immun 2019, 79, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front Immunol 2017, 8, 1957. [Google Scholar] [CrossRef]
- Grishanova, A.Y.; Perepechaeva, M.L. Kynurenic Acid/AhR Signaling at the Junction of Inflammation and Cardiovascular Diseases. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci 2013, 34, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J Neurochem 2020, 152, 627–649. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Ota, R.; Shima, M.; Yamashita, A.; Sugiura, T. GPR35 is a novel lysophosphatidic acid receptor. Biochem Biophys Res Commun 2010, 395, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Hu, H.; Fang, Y. Multiple tyrosine metabolites are GPR35 agonists. Sci Rep 2012, 2, 373. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int J Tryptophan Res 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Minhas, P.S.; Liu, L.; Moon, P.K.; Joshi, A.U.; Dove, C.; Mhatre, S.; Contrepois, K.; Wang, Q.; Lee, B.A.; Coronado, M.; et al. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat Immunol 2019, 20, 50–63. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, P.; Tang, X.; Wang, S.; Shen, J.; Zheng, Y.; Gao, C.; Mi, P.; Zhang, C.; Qu, H.; et al. Metabolic Rewiring of Kynurenine Pathway during Hepatic Ischemia-Reperfusion Injury Exacerbates Liver Damage by Impairing NAD Homeostasis. Adv Sci (Weinh) 2022, 9, e2204697. [Google Scholar] [CrossRef]
- Yang, Y.; Borel, T.; de Azambuja, F.; Johnson, D.; Sorrentino, J.P.; Udokwu, C.; Davis, I.; Liu, A.; Altman, R.A. Diflunisal Derivatives as Modulators of ACMS Decarboxylase Targeting the Tryptophan-Kynurenine Pathway. J Med Chem 2021, 64, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Guillemin, G.J.; Grant, R. Effects of Kynurenine Pathway Inhibition on NAD Metabolism and Cell Viability in Human Primary Astrocytes and Neurons. Int J Tryptophan Res 2011, 4, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Stone, T.W.; Williams, R.O. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol Sci 2023, 44, 442–456. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, C.; Zhang, J.; Su, W.; Wang, G.; Wang, Z. The Kynurenine Pathway and Indole Pathway in Tryptophan Metabolism Influence Tumor Progression. Cancer Med 2025, 14, e70703. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. From Microbial Switches to Metabolic Sensors: Rewiring the Gut-Brain Kynurenine Circuit. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: a finger in every pie. Mol Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10. [Google Scholar] [CrossRef]
- Pathak, S.; Nadar, R.; Kim, S.; Liu, K.; Govindarajulu, M.; Cook, P.; Watts Alexander, C.S.; Dhanasekaran, M.; Moore, T. The Influence of Kynurenine Metabolites on Neurodegenerative Pathologies. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S. From Biomarkers to Behavior: Mapping the Neuroimmune Web of Pain, Mood, and Memory. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Strasser, B.; Becker, K.; Fuchs, D.; Gostner, J.M. Kynurenine pathway metabolism and immune activation: Peripheral measurements in psychiatric and co-morbid conditions. Neuropharmacology 2017, 112, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Special Issue “Translating Molecular Psychiatry: From Biomarkers to Personalized Therapies”. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Beyond the boundaries: Transitioning from categorical to dimensional paradigms in mental health diagnostics. Adv Clin Exp Med 2024, 33, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Liloia, D.; Zamfira, D.A.; Tanaka, M.; Manuello, J.; Crocetta, A.; Keller, R.; Cozzolino, M.; Duca, S.; Cauda, F.; Costa, T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci Biobehav Rev 2024, 164, 105791. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed) 2015, 20, 1116–1143. [Google Scholar] [CrossRef]
- Baumgartner, R.; Forteza, M.J.; Ketelhuth, D.F.J. The interplay between cytokines and the Kynurenine pathway in inflammation and atherosclerosis. Cytokine 2019, 122, 154148. [Google Scholar] [CrossRef]
- Kozieł, K.; Urbanska, E.M. Kynurenine Pathway in Diabetes Mellitus-Novel Pharmacological Target? Cells 2023, 12. [Google Scholar] [CrossRef]
- Pires, A.S.; Sundaram, G.; Heng, B.; Krishnamurthy, S.; Brew, B.J.; Guillemin, G.J. Recent advances in clinical trials targeting the kynurenine pathway. Pharmacol Ther 2022, 236, 108055. [Google Scholar] [CrossRef]
- Sforzini, L.; Nettis, M.A.; Mondelli, V.; Pariante, C.M. Inflammation in cancer and depression: a starring role for the kynurenine pathway. Psychopharmacology (Berl) 2019, 236, 2997–3011. [Google Scholar] [CrossRef]
- Eggertsen, P.P.; Hansen, J.; Andersen, M.L.; Nielsen, J.F.; Olsen, R.K.J.; Palmfeldt, J. Simultaneous measurement of kynurenine metabolites and explorative metabolomics using liquid chromatography-mass spectrometry: A novel accurate method applied to serum and plasma samples from a large healthy cohort. J Pharm Biomed Anal 2023, 227, 115304. [Google Scholar] [CrossRef]
- Hossen, M.M.; Fleiss, B.; Zakaria, R. The current state in liquid chromatography-mass spectrometry methods for quantifying kynurenine pathway metabolites in biological samples: a systematic review. Crit Rev Clin Lab Sci 2025, 62, 437–453. [Google Scholar] [CrossRef]
- Gomez-Gomez, A.; Olesti, E.; Montero-San-Martin, B.; Soldevila, A.; Deschamps, T.; Pizarro, N.; de la Torre, R.; Pozo, O.J. Determination of up to twenty carboxylic acid containing compounds in clinically relevant matrices by o-benzylhydroxylamine derivatization and liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 2022, 208, 114450. [Google Scholar] [CrossRef] [PubMed]
- Sadok, I.; Jędruchniewicz, K.; Rawicz-Pruszyński, K.; Staniszewska, M. UHPLC-ESI-MS/MS Quantification of Relevant Substrates and Metabolites of the Kynurenine Pathway Present in Serum and Peritoneal Fluid from Gastric Cancer Patients-Method Development and Validation. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Panuwet, P.; Hunter, R.E., Jr.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit Rev Anal Chem 2016, 46, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Whiley, L.; Nye, L.C.; Grant, I.; Andreas, N.; Chappell, K.E.; Sarafian, M.H.; Misra, R.; Plumb, R.S.; Lewis, M.R.; Nicholson, J.K.; et al. Ultrahigh-Performance Liquid Chromatography Tandem Mass Spectrometry with Electrospray Ionization Quantification of Tryptophan Metabolites and Markers of Gut Health in Serum and Plasma-Application to Clinical and Epidemiology Cohorts. Anal Chem 2019, 91, 5207–5216. [Google Scholar] [CrossRef] [PubMed]
- Chawdhury, A.; Shamsi, S.A.; Miller, A.; Liu, A. Capillary electrochromatography-mass spectrometry of kynurenine pathway metabolites. J Chromatogr A 2021, 1651, 462294. [Google Scholar] [CrossRef]
- Sadok, I.; Rachwał, K.; Staniszewska, M. Simultaneous Quantification of Selected Kynurenines Analyzed by Liquid Chromatography-Mass Spectrometry in Medium Collected from Cancer Cell Cultures. J Vis Exp 2020. [Google Scholar] [CrossRef]
- Khetarpal, V.; Herbst, T.; Akhtar, S.; LaFayette, A.; Miller, D.; Farnham, J.; Steege, T.; Miao, Z.; Marks, B.; Ledvina, A.; et al. Validation of LC-MS/MS methods for quantitative analysis of kynurenine pathway metabolites in human plasma and cerebrospinal fluid. Bioanalysis 2023, 15, 637–651. [Google Scholar] [CrossRef]
- Huang, N.; Winans, T.; Wyman, B.; Oaks, Z.; Faludi, T.; Choudhary, G.; Lai, Z.W.; Lewis, J.; Beckford, M.; Duarte, M.; et al. Rab4A-directed endosome traffic shapes pro-inflammatory mitochondrial metabolism in T cells via mitophagy, CD98 expression, and kynurenine-sensitive mTOR activation. Nat Commun 2024, 15, 2598. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Chen, Y.; Ju, Y.; Ma, M.; Qin, Y.; Bi, Y.; Liao, M.; Liu, B.; Liu, J.; Zhang, Y.; et al. The kynurenine pathway in major depressive disorder under different disease states: A systematic review and meta-analysis. J Affect Disord 2023, 339, 624–632. [Google Scholar] [CrossRef]
- Cristina, B.; Veronica, R.; Silvia, A.; Andrea, G.; Sara, C.; Luca, P.; Nicoletta, B.; C.B., M; Silvio, B.; Fabio, T. Identification and characterization of the kynurenine pathway in the pond snail Lymnaea stagnalis. Sci Rep 2022, 12, 15617. [Google Scholar] [CrossRef]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Sadok, I.; Staniszewska, M. Electrochemical Determination of Kynurenine Pathway Metabolites-Challenges and Perspectives. Sensors (Basel) 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Mrštná, K.; Krčmová, L.K.; Švec, F. Advances in kynurenine analysis. Clin Chim Acta 2023, 547, 117441. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S.; Liloia, D. Navigating Neurodegeneration: Integrating Biomarkers, Neuroinflammation, and Imaging in Parkinson’s, Alzheimer’s, and Motor Neuron Disorders. Biomedicines 2025, 13. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- da Rocha, A.L.; Pinto, A.P.; de Sousa Neto, I.V.; Muñoz, V.R.; Marafon, B.B.; da Silva, L.; Pauli, J.R.; Cintra, D.E.; Ropelle, E.R.; Simabuco, F.M.; et al. Exhaustive exercise abolishes REV-ERB-α circadian rhythm and shifts the kynurenine pathway to a neurotoxic profile in mice. J Physiol 2025, 603, 3923–3944. [Google Scholar] [CrossRef]
- O’Leary, K.M.; Slezak, T.; Kossiakoff, A.A. Conformation-specific synthetic intrabodies modulate mTOR signaling with subcellular spatial resolution. Proc Natl Acad Sci U S A 2025, 122, e2424679122. [Google Scholar] [CrossRef]
- Bader, J.M.; Albrecht, V.; Mann, M. MS-Based Proteomics of Body Fluids: The End of the Beginning. Mol Cell Proteomics 2023, 22, 100577. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Signal Transduct Target Ther 2023, 8, 333. [Google Scholar] [CrossRef]
- Rischke, S.; Hahnefeld, L.; Burla, B.; Behrens, F.; Gurke, R.; Garrett, T.J. Small molecule biomarker discovery: Proposed workflow for LC-MS-based clinical research projects. J Mass Spectrom Adv Clin Lab 2023, 28, 47–55. [Google Scholar] [CrossRef]
- Lee, J.; Yesilkanal, A.E.; Wynne, J.P.; Frankenberger, C.; Liu, J.; Yan, J.; Elbaz, M.; Rabe, D.C.; Rustandy, F.D.; Tiwari, P.; et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019, 568, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Schneditz, G.; Elias, J.E.; Pagano, E.; Zaeem Cader, M.; Saveljeva, S.; Long, K.; Mukhopadhyay, S.; Arasteh, M.; Lawley, T.D.; Dougan, G.; et al. GPR35 promotes glycolysis, proliferation, and oncogenic signaling by engaging with the sodium potassium pump. Sci Signal 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.E.; Debela, M.; Sewell, G.W.; Stopforth, R.J.; Partl, H.; Heissbauer, S.; Holland, L.M.; Karlsen, T.H.; Kaser, A.; Kaneider, N.C. GPR35 prevents osmotic stress induced cell damage. Commun Biol 2025, 8, 478. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, P.; Fan, H.; Zhang, C.; Yu, P.; Liang, X.; Chen, Y. GPR35 acts a dual role and therapeutic target in inflammation. Frontiers in Immunology 2023, 14, 1254446. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Quon, T.; Engberg, S.; Mackenzie, A.E.; Tobin, A.B.; Milligan, G. G Protein-Coupled Receptor GPR35 Suppresses Lipid Accumulation in Hepatocytes. ACS Pharmacol Transl Sci 2021, 4, 1835–1848. [Google Scholar] [CrossRef]
- Wei, X.; Yin, F.; Wu, M.; Xie, Q.; Zhao, X.; Zhu, C.; Xie, R.; Chen, C.; Liu, M.; Wang, X. G protein-coupled receptor 35 attenuates nonalcoholic steatohepatitis by reprogramming cholesterol homeostasis in hepatocytes. Acta pharmaceutica Sinica B 2023, 13, 1128–1144. [Google Scholar] [CrossRef]
- Nam, S.-Y.; Park, S.-J.; Im, D.-S. Protective effect of lodoxamide on hepatic steatosis through GPR35. Cellular Signalling 2019, 53, 190–200. [Google Scholar] [CrossRef]
- Nesci, S. GPR35, ally of the anti-ischemic ATPIF1-ATP synthase interaction. Trends Pharmacol Sci 2022, 43, 891–893. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, O.; Abir, A.H.; Potol, A.; Alam, M.; Banik, J.; Rahman, A.; Tarannum, N.; Wadud, R.; Habib, Z.F.; Rahman, M. Activation of GPR35 protects against cerebral ischemia by recruiting monocyte-derived macrophages. Sci Rep 2020, 10, 9400. [Google Scholar] [CrossRef]
- Boleij, A.; Fathi, P.; Dalton, W.; Park, B.; Wu, X.; Huso, D.; Allen, J.; Besharati, S.; Anders, R.A.; Housseau, F.; et al. G-protein coupled receptor 35 (GPR35) regulates the colonic epithelial cell response to enterotoxigenic Bacteroides fragilis. Commun Biol 2021, 4, 585. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; He, L.; Li, Y.; Li, X.; Qiu, C.; Pei, H.; Yang, D. Inhibition of GPR35 Preserves Mitochondrial Function After Myocardial Infarction by Targeting Calpain 1/2. J Cardiovasc Pharmacol 2020, 75, 556–563. [Google Scholar] [CrossRef]
- Milligan, G. GPR35: from enigma to therapeutic target. Trends Pharmacol Sci 2023, 44, 263–273. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, H.; Cai, X.; Zhang, Y.; Yu, S.; Hou, Y.; Yin, Z.; Yan, Q.; Wang, Q.; Sun, T.; et al. A Gpr35-tuned gut microbe-brain metabolic axis regulates depressive-like behavior. Cell Host Microbe 2024, 32, 227–243.e226. [Google Scholar] [CrossRef]
- Tanaka, M.; He, Z.; Han, S.; Battaglia, S. Editorial: Noninvasive brain stimulation: a promising approach to study and improve emotion regulation. Front Behav Neurosci 2025, 19, 1633936. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, P.; Fan, H.; Zhang, C.; Yu, P.; Liang, X.; Chen, Y. GPR35 acts a dual role and therapeutic target in inflammation. Front Immunol 2023, 14, 1254446. [Google Scholar] [CrossRef]
- Mackenzie, A.E.; Lappin, J.E.; Taylor, D.L.; Nicklin, S.A.; Milligan, G. GPR35 as a Novel Therapeutic Target. Front Endocrinol (Lausanne) 2011, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Darlington, L.G.; Badawy, A.A.-B.; Williams, R.O. The complex world of kynurenic acid: reflections on biological issues and therapeutic strategy. International Journal of Molecular Sciences 2024, 25, 9040. [Google Scholar] [CrossRef] [PubMed]
- Brust, T.F.; Conley, J.M.; Watts, V.J. Gα(i/o)-coupled receptor-mediated sensitization of adenylyl cyclase: 40 years later. Eur J Pharmacol 2015, 763, 223–232. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Pollock, C.; Steen, H.; Shaw, P.E.; Mischak, H.; Kolch, W. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol 2002, 22, 3237–3246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, G. Gq-Coupled Receptors in Autoimmunity. J Immunol Res 2016, 2016, 3969023. [Google Scholar] [CrossRef] [PubMed]
- Takkar, S.; Sharma, G.; Kaushal, J.B.; Abdullah, K.M.; Batra, S.K.; Siddiqui, J.A. From orphan to oncogene: The role of GPR35 in cancer and immune modulation. Cytokine Growth Factor Rev 2024, 77, 56–66. [Google Scholar] [CrossRef]
- Poźniak, O.; Sitarz, R.; Sitarz, M.Z.; Kowalczuk, D.; Słoń, E.; Dudzińska, E. Utilization of AhR and GPR35 Receptor Ligands as Superfoods in Cancer Prevention for Individuals with IBD. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Nógrádi-Halmi, D.; Erdélyi-Furka, B.; Csóré, D.; Plechl, É.; Igaz, N.; Juhász, L.; Poles, M.Z.; Nógrádi, B.; Patai, R.; Polgár, T.F.; et al. Kynurenic Acid Protects Against Myocardial Ischemia/Reperfusion Injury by Activating GPR35 Receptors and Preserving Mitochondrial Structure and Function. Biomolecules 2025, 15. [Google Scholar] [CrossRef]
- García-Trevijano, E.R.; Ortiz-Zapater, E.; Gimeno, A.; Viña, J.R.; Zaragozá, R. Calpains, the proteases of two faces controlling the epithelial homeostasis in mammary gland. Front Cell Dev Biol 2023, 11, 1249317. [Google Scholar] [CrossRef]
- Hernando, V.; Inserte, J.; Sartório, C.L.; Parra, V.M.; Poncelas-Nozal, M.; Garcia-Dorado, D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol 2010, 49, 271–279. [Google Scholar] [CrossRef]
- Chen, Q.; Lesnefsky, E.J. Heart mitochondria and calpain 1: Location, function, and targets. Biochim Biophys Acta 2015, 1852, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Won, D.J.; Krajewski, S.; Gottlieb, R.A. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 2002, 277, 29181–29186. [Google Scholar] [CrossRef]
- Zheng, D.; Cao, T.; Zhang, L.L.; Fan, G.C.; Qiu, J.; Peng, T.Q. Targeted inhibition of calpain in mitochondria alleviates oxidative stress-induced myocardial injury. Acta Pharmacol Sin 2021, 42, 909–920. [Google Scholar] [CrossRef]
- Ni, R.; Zheng, D.; Xiong, S.; Hill, D.J.; Sun, T.; Gardiner, R.B.; Fan, G.C.; Lu, Y.; Abel, E.D.; Greer, P.A.; et al. Mitochondrial Calpain-1 Disrupts ATP Synthase and Induces Superoxide Generation in Type 1 Diabetic Hearts: A Novel Mechanism Contributing to Diabetic Cardiomyopathy. Diabetes 2016, 65, 255–268. [Google Scholar] [CrossRef]
- Jenkins, L.; Alvarez-Curto, E.; Campbell, K.; de Munnik, S.; Canals, M.; Schlyer, S.; Milligan, G. Agonist activation of the G protein-coupled receptor GPR35 involves transmembrane domain III and is transduced via Gα13 and β-arrestin-2. Br J Pharmacol 2011, 162, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Zorita, S.; Romero-Carramiñana, I.; Santacatterina, F.; Esparza-Moltó, P.B.; Simó, C.; Del-Arco, A.; Núñez de Arenas, C.; Saiz, J.; Barbas, C.; Cuezva, J.M. IF1 ablation prevents ATP synthase oligomerization, enhances mitochondrial ATP turnover and promotes an adenosine-mediated pro-inflammatory phenotype. Cell Death Dis 2023, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Warnsmann, V.; Marschall, L.M.; Osiewacz, H.D. Impaired F(1)F(o)-ATP-Synthase Dimerization Leads to the Induction of Cyclophilin D-Mediated Autophagy-Dependent Cell Death and Accelerated Aging. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Zorita, S.; Romero-Carramiñana, I.; Cuezva, J.M.; Esparza-Moltó, P.B. The ATPase Inhibitory Factor 1 is a Tissue-Specific Physiological Regulator of the Structure and Function of Mitochondrial ATP Synthase: A Closer Look Into Neuronal Function. Front Physiol 2022, 13, 868820. [Google Scholar] [CrossRef]
- Nikolaou, P.E.; Lambrinidis, G.; Georgiou, M.; Karagiannis, D.; Efentakis, P.; Bessis-Lazarou, P.; Founta, K.; Kampoukos, S.; Konstantin, V.; Palmeira, C.M.; et al. Hydrolytic Activity of Mitochondrial F(1)F(O)-ATP Synthase as a Target for Myocardial Ischemia-Reperfusion Injury: Discovery and In Vitro and In Vivo Evaluation of Novel Inhibitors. J Med Chem 2023, 66, 15115–15140. [Google Scholar] [CrossRef]
- Mnatsakanyan, N.; Jonas, E.A. The new role of F(1)F(o) ATP synthase in mitochondria-mediated neurodegeneration and neuroprotection. Exp Neurol 2020, 332, 113400. [Google Scholar] [CrossRef]
- Acin-Perez, R.; Benincá, C.; Fernandez Del Rio, L.; Shu, C.; Baghdasarian, S.; Zanette, V.; Gerle, C.; Jiko, C.; Khairallah, R.; Khan, S.; et al. Inhibition of ATP synthase reverse activity restores energy homeostasis in mitochondrial pathologies. Embo j 2023, 42, e111699. [Google Scholar] [CrossRef]
- Pan, Y.; Ji, N.; Jiang, L.; Zhou, Y.; Feng, X.; Li, J.; Zeng, X.; Wang, J.; Shen, Y.Q.; Chen, Q. GPCRs identified on mitochondrial membranes: New therapeutic targets for diseases. J Pharm Anal 2025, 15, 101178. [Google Scholar] [CrossRef]
- García-Bermúdez, J.; Cuezva, J.M. The ATPase Inhibitory Factor 1 (IF1): A master regulator of energy metabolism and of cell survival. Biochim Biophys Acta 2016, 1857, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Kaim, G.; Dimroth, P. ATP synthesis by F-type ATP synthase is obligatorily dependent on the transmembrane voltage. Embo j 1999, 18, 4118–4127. [Google Scholar] [CrossRef]
- Dimroth, P.; Kaim, G.; Matthey, U. Crucial role of the membrane potential for ATP synthesis by F(1)F(o) ATP synthases. J Exp Biol 2000, 203, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal Biochem 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ 2003, 10, 709–717. [Google Scholar] [CrossRef]
- Zamzami, N.; Marchetti, P.; Castedo, M.; Zanin, C.; Vayssière, J.L.; Petit, P.X.; Kroemer, G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 1995, 181, 1661–1672. [Google Scholar] [CrossRef]
- Chen, K.; He, L.; Li, Y.; Li, X.; Qiu, C.; Pei, H.; Yang, D. Inhibition of GPR35 preserves mitochondrial function after myocardial infarction by targeting calpain 1/2. Journal of Cardiovascular Pharmacology 2020, 75, 556–563. [Google Scholar] [CrossRef]
- Dadvar, S.; Ferreira, D.M.S.; Cervenka, I.; Ruas, J.L. The weight of nutrients: kynurenine metabolites in obesity and exercise. J Intern Med 2018, 284, 519–533. [Google Scholar] [CrossRef]
- Jung, T.W.; Park, J.; Sun, J.L.; Ahn, S.H.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Kim, H.C.; Shim, J.H.; Shin, S.; Jeong, J.H. Administration of kynurenic acid reduces hyperlipidemia-induced inflammation and insulin resistance in skeletal muscle and adipocytes. Mol Cell Endocrinol 2020, 518, 110928. [Google Scholar] [CrossRef]
- Mándi, Y.; Endrész, V.; Mosolygó, T.; Burián, K.; Lantos, I.; Fülöp, F.; Szatmári, I.; Lőrinczi, B.; Balog, A.; Vécsei, L. The Opposite Effects of Kynurenic Acid and Different Kynurenic Acid Analogs on Tumor Necrosis Factor-α (TNF-α) Production and Tumor Necrosis Factor-Stimulated Gene-6 (TSG-6) Expression. Front Immunol 2019, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Fallarini, S.; Magliulo, L.; Paoletti, T.; de Lalla, C.; Lombardi, G. Expression of functional GPR35 in human iNKT cells. Biochem Biophys Res Commun 2010, 398, 420–425. [Google Scholar] [CrossRef]
- Richter, C.A.; Tillitt, D.E.; Hannink, M. Regulation of subcellular localization of the aryl hydrocarbon receptor (AhR). Arch Biochem Biophys 2001, 389, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kewley, R.J.; Whitelaw, M.L.; Chapman-Smith, A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol 2004, 36, 189–204. [Google Scholar] [CrossRef]
- Sorg, O. AhR signalling and dioxin toxicity. Toxicol Lett 2014, 230, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Corre, S.; Tardif, N.; Mouchet, N.; Leclair, H.M.; Boussemart, L.; Gautron, A.; Bachelot, L.; Perrot, A.; Soshilov, A.; Rogiers, A.; et al. Sustained activation of the Aryl hydrocarbon Receptor transcription factor promotes resistance to BRAF-inhibitors in melanoma. Nat Commun 2018, 9, 4775. [Google Scholar] [CrossRef]
- Dai, S.; Qu, L.; Li, J.; Zhang, Y.; Jiang, L.; Wei, H.; Guo, M.; Chen, X.; Chen, Y. Structural insight into the ligand binding mechanism of aryl hydrocarbon receptor. Nat Commun 2022, 13, 6234. [Google Scholar] [CrossRef]
- Polonio, C.M.; McHale, K.A.; Sherr, D.H.; Rubenstein, D.; Quintana, F.J. The aryl hydrocarbon receptor: a rehabilitated target for therapeutic immune modulation. Nat Rev Drug Discov 2025, 24, 610–630. [Google Scholar] [CrossRef]
- Heo, M.J.; Suh, J.H.; Lee, S.H.; Poulsen, K.L.; An, Y.A.; Moorthy, B.; Hartig, S.M.; Moore, D.D.; Kim, K.H. Aryl hydrocarbon receptor maintains hepatic mitochondrial homeostasis in mice. Mol Metab 2023, 72, 101717. [Google Scholar] [CrossRef]
- Bahman, F.; Choudhry, K.; Al-Rashed, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. Aryl hydrocarbon receptor: current perspectives on key signaling partners and immunoregulatory role in inflammatory diseases. Front Immunol 2024, 15, 1421346. [Google Scholar] [CrossRef]
- Nebert, D.W.; Karp, C.L. Endogenous functions of the aryl hydrocarbon receptor (AHR): intersection of cytochrome P450 1 (CYP1)-metabolized eicosanoids and AHR biology. J Biol Chem 2008, 283, 36061–36065. [Google Scholar] [CrossRef]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Nacarino-Palma, A.; González-Rico, F.J.; Rejano-Gordillo, C.M.; Ordiales-Talavero, A.; Merino, J.M.; Fernández-Salguero, P.M. The aryl hydrocarbon receptor promotes differentiation during mouse preimplantational embryo development. Stem Cell Reports 2021, 16, 2351–2363. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci 2010, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm (Vienna) 2012, 119, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wei, J.; Ran, W.; Liu, D.; Yi, Y.; Gong, M.; Liu, X.; Gong, Q.; Li, H.; Gao, J. The Gut Microbiota-Xanthurenic Acid-Aromatic Hydrocarbon Receptor Axis Mediates the Anticolitic Effects of Trilobatin. Adv Sci (Weinh) 2025, 12, e2412234. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 2013, 20, 31–42. [Google Scholar] [CrossRef]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct Target Ther 2023, 8, 304. [Google Scholar] [CrossRef]
- Palikaras, K.; Tavernarakis, N. Mitophagy in neurodegeneration and aging. Front Genet 2012, 3, 297. [Google Scholar] [CrossRef]
- Hamacher-Brady, A.; Brady, N.R. Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci 2016, 73, 775–795. [Google Scholar] [CrossRef]
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in human health, ageing and disease. Nat Metab 2023, 5, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, K.; Zhang, C.; Bao, J.P.; Vlf, C.; Gao, J.W.; Zhou, Z.M.; Wu, X.T. The mitochondrial antioxidant SS-31 attenuated lipopolysaccharide-induced apoptosis and pyroptosis of nucleus pulposus cells via scavenging mitochondrial ROS and maintaining the stability of mitochondrial dynamics. Free Radic Res 2021, 55, 1080–1093. [Google Scholar] [CrossRef]
- Cao, J.; Bao, Q.; Hao, H. Indole-3-Carboxaldehyde Alleviates LPS-Induced Intestinal Inflammation by Inhibiting ROS Production and NLRP3 Inflammasome Activation. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Guo, J.; Su, X.; Ma, B.; Wang, X.; Wangshao, M.; Zhong, K.; Wang, Y.; Yang, G.; Han, Y. Aryl hydrocarbon receptor (AhR) alleviates the LPS-induced inflammatory responses in IPEC-J2 cells by activating PINK1/Parkin-mediated mitophagy. Inflamm Res 2025, 74, 98. [Google Scholar] [CrossRef]
- Lanis, J.M.; Alexeev, E.E.; Curtis, V.F.; Kitzenberg, D.A.; Kao, D.J.; Battista, K.D.; Gerich, M.E.; Glover, L.E.; Kominsky, D.J.; Colgan, S.P. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol 2017, 10, 1133–1144. [Google Scholar] [CrossRef]
- Tanaka, M. Parkinson’s Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation. Cells 2025, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, W.; Lu, Y.; Zheng, Y.; Pan, L.; Wu, X.; Yuan, Y.; Shen, Z.; Ma, S.; Zhang, X.; et al. BNIP3L/NIX-mediated mitophagy: molecular mechanisms and implications for human disease. Cell Death Dis 2021, 13, 14. [Google Scholar] [CrossRef]
- Ding, W.X.; Yin, X.M. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 2012, 393, 547–564. [Google Scholar] [CrossRef]
- Antico, O.; Thompson, P.W.; Hertz, N.T.; Muqit, M.M.K.; Parton, L.E. Targeting mitophagy in neurodegenerative diseases. Nat Rev Drug Discov 2025, 24, 276–299. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. Embo j 2021, 40, e104705. [Google Scholar] [CrossRef]
- Birgisdottir Å, B.; Lamark, T.; Johansen, T. The LIR motif—crucial for selective autophagy. J Cell Sci 2013, 126, 3237–3247. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct Target Ther 2024, 9, 124. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov 2018, 17, 865–886. [Google Scholar] [CrossRef]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2014, 2, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Senft, A.P.; Dalton, T.P.; Nebert, D.W.; Genter, M.B.; Puga, A.; Hutchinson, R.J.; Kerzee, J.K.; Uno, S.; Shertzer, H.G. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic Biol Med 2002, 33, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Okamoto, K. Mitochondrial clearance: mechanisms and roles in cellular fitness. FEBS Lett 2021, 595, 1239–1263. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Redox homeostasis, oxidative stress and mitophagy. Mitochondrion 2020, 51, 105–117. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Tao, Y.; Ji, C.; Aniagu, S.; Jiang, Y.; Chen, T. AHR/ROS-mediated mitochondria apoptosis contributes to benzo[a]pyrene-induced heart defects and the protective effects of resveratrol. Toxicology 2021, 462, 152965. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Liu, L.; Jian, Z.; Cui, T.; Yang, Y.; Guo, S.; Yi, X.; Wang, G.; Li, C.; et al. Role of the aryl hydrocarbon receptor signaling pathway in promoting mitochondrial biogenesis against oxidative damage in human melanocytes. J Dermatol Sci 2019, 96, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, M.; Zou, H.; Aniagu, S.; Jiang, Y.; Chen, T. PM2.5 induces mitochondrial dysfunction via AHR-mediated cyp1a1 overexpression during zebrafish heart development. Toxicology 2023, 487, 153466. [Google Scholar] [CrossRef]
- Ren, R.; Fang, Y.; Sherchan, P.; Lu, Q.; Lenahan, C.; Zhang, J.H.; Zhang, J.; Tang, J. Kynurenine/Aryl Hydrocarbon Receptor Modulates Mitochondria-Mediated Oxidative Stress and Neuronal Apoptosis in Experimental Intracerebral Hemorrhage. Antioxid Redox Signal 2022, 37, 1111–1129. [Google Scholar] [CrossRef]
- Majláth, Z.; Török, N.; Toldi, J.; Vécsei, L. Memantine and Kynurenic Acid: Current Neuropharmacological Aspects. Curr Neuropharmacol 2016, 14, 200–209. [Google Scholar] [CrossRef]
- Oliver, M.W.; Kessler, M.; Larson, J.; Schottler, F.; Lynch, G. Glycine site associated with the NMDA receptor modulates long-term potentiation. Synapse 1990, 5, 265–270. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol 1988, 154, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Poles, M.Z.; Nászai, A.; Gulácsi, L.; Czakó, B.L.; Gál, K.G.; Glenz, R.J.; Dookhun, D.; Rutai, A.; Tallósy, S.P.; Szabó, A.; et al. Kynurenic Acid and Its Synthetic Derivatives Protect Against Sepsis-Associated Neutrophil Activation and Brain Mitochondrial Dysfunction in Rats. Front Immunol 2021, 12, 717157. [Google Scholar] [CrossRef] [PubMed]
- Kaszaki, J.; Erces, D.; Varga, G.; Szabó, A.; Vécsei, L.; Boros, M. Kynurenines and intestinal neurotransmission: the role of N-methyl-D-aspartate receptors. J Neural Transm (Vienna) 2012, 119, 211–223. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Menniti, F.S.; Traynelis, S.F. NMDA Receptors in the Central Nervous System. Methods Mol Biol 2017, 1677, 1–80. [Google Scholar] [CrossRef]
- Mony, L.; Paoletti, P. Mechanisms of NMDA receptor regulation. Curr Opin Neurobiol 2023, 83, 102815. [Google Scholar] [CrossRef]
- Hunter, D.R.; Haworth, R.A. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys 1979, 195, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Nászai, A.; Terhes, E.; Kaszaki, J.; Boros, M.; Juhász, L. Ca((2+))N It Be Measured? Detection of Extramitochondrial Calcium Movement With High-Resolution FluoRespirometry. Sci Rep 2019, 9, 19229. [Google Scholar] [CrossRef]
- Mira, R.G.; Cerpa, W. Building a Bridge Between NMDAR-Mediated Excitotoxicity and Mitochondrial Dysfunction in Chronic and Acute Diseases. Cell Mol Neurobiol 2021, 41, 1413–1430. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 2009, 46, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat Rev Mol Cell Biol 2022, 23, 266–285. [Google Scholar] [CrossRef]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The Mitochondrial Permeability Transition Pore-Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells 2023, 12. [Google Scholar] [CrossRef]
- Baev, A.Y.; Vinokurov, A.Y.; Potapova, E.V.; Dunaev, A.V.; Angelova, P.R.; Abramov, A.Y. Mitochondrial Permeability Transition, Cell Death and Neurodegeneration. Cells 2024, 13. [Google Scholar] [CrossRef]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep 2020, 21, e49799. [Google Scholar] [CrossRef]
- Patrushev, M.; Kasymov, V.; Patrusheva, V.; Ushakova, T.; Gogvadze, V.; Gaziev, A. Mitochondrial permeability transition triggers the release of mtDNA fragments. Cell Mol Life Sci 2004, 61, 3100–3103. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, H.; Zhao, A.; Yan, X.; Zhang, X.; Gan, J.; Guo, M.; Wang, J.; Zhang, F.; Jiang, Y.; et al. Mitochondrial DNA leakage: underlying mechanisms and therapeutic implications in neurological disorders. J Neuroinflammation 2025, 22, 34. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, K.S.; Lee, H.J.; Noh, Y.H.; Kim, D.H.; Lee, J.Y.; Cho, S.H.; Yoon, O.J.; Lee, W.B.; Kim, K.Y.; et al. Kynurenic acid attenuates MPP(+)-induced dopaminergic neuronal cell death via a Bax-mediated mitochondrial pathway. Eur J Cell Biol 2008, 87, 389–397. [Google Scholar] [CrossRef]
- Szabo, M.; Lajkó, N.; Dulka, K.; Szatmári, I.; Fülöp, F.; Mihály, A.; Vécsei, L.; Gulya, K. Kynurenic Acid and Its Analog SZR104 Exhibit Strong Antiinflammatory Effects and Alter the Intracellular Distribution and Methylation Patterns of H3 Histones in Immunochallenged Microglia-Enriched Cultures of Newborn Rat Brains. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Nesterov, S.V.; Skorobogatova, Y.A.; Panteleeva, A.A.; Pavlik, L.L.; Mikheeva, I.B.; Yaguzhinsky, L.S.; Nartsissov, Y.R. NMDA and GABA receptor presence in rat heart mitochondria. Chem Biol Interact 2018, 291, 40–46. [Google Scholar] [CrossRef]
- Korde, A.S.; Maragos, W.F. Identification of an N-methyl-D-aspartate receptor in isolated nervous system mitochondria. J Biol Chem 2012, 287, 35192–35200. [Google Scholar] [CrossRef]
- Korde, A.S.; Maragos, W.F. Mitochondrial N-methyl-d-aspartate receptor activation enhances bioenergetics by calcium-dependent and -Independent mechanisms. Mitochondrion 2021, 59, 76–82. [Google Scholar] [CrossRef]
- Gingrich, J.R.; Pelkey, K.A.; Fam, S.R.; Huang, Y.; Petralia, R.S.; Wenthold, R.J.; Salter, M.W. Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondrial protein NADH dehydrogenase subunit 2. Proc Natl Acad Sci U S A 2004, 101, 6237–6242. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.P.; Bah, A.; Krzeminski, M.; Zhang, W.; Leduc-Pessah, H.L.; Dong, Y.N.; Forman-Kay, J.D.; Salter, M.W. An evolutionary switch in ND2 enables Src kinase regulation of NMDA receptors. Nat Commun 2017, 8, 15220. [Google Scholar] [CrossRef]
- Salter, M.W.; Kalia, L.V. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 2004, 5, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J.; et al. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knockout Mice: A Novel Model for Despair-Based Depression and Post-Traumatic Stress Disorder. Front Biosci (Landmark Ed) 2025, 30, 25706. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals (Basel) 2025, 18. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J Neural Transm (Vienna) 2024, 131, 1367–1387. [Google Scholar] [CrossRef]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.T.; Tan, L. The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. J Neurol Sci 2012, 323, 1–8. [Google Scholar] [CrossRef]
- Németh, H.; Toldi, J.; Vécsei, L. Kynurenines, Parkinson’s disease and other neurodegenerative disorders: preclinical and clinical studies. J Neural Transm Suppl 2006, 285–304. [Google Scholar] [CrossRef]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci Ther 2022, 28, 19–35. [Google Scholar] [CrossRef]
- Gergalova, G.; Lykhmus, O.; Kalashnyk, O.; Koval, L.; Chernyshov, V.; Kryukova, E.; Tsetlin, V.; Komisarenko, S.; Skok, M. Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria. PLoS One 2012, 7, e31361. [Google Scholar] [CrossRef]
- Kalashnyk, O.; Lykhmus, O.; Uspenska, K.; Izmailov, M.; Komisarenko, S.; Skok, M. Mitochondrial α7 nicotinic acetylcholine receptors are displaced from complexes with VDAC1 to form complexes with Bax upon apoptosis induction. Int J Biochem Cell Biol 2020, 129, 105879. [Google Scholar] [CrossRef] [PubMed]
- Gergalova, G.; Lykhmus, O.; Komisarenko, S.; Skok, M. α7 nicotinic acetylcholine receptors control cytochrome c release from isolated mitochondria through kinase-mediated pathways. Int J Biochem Cell Biol 2014, 49, 26–31. [Google Scholar] [CrossRef]
- Abraham, S.M.; Suresh, S.; Komal, P. Targeting Neuronal Alpha7 Nicotinic Acetylcholine Receptor Upregulation in Age-Related Neurological Disorders. Cell Mol Neurobiol 2025, 45, 70. [Google Scholar] [CrossRef]
- Papke, R.L.; Horenstein, N.A. Therapeutic Targeting of α7 Nicotinic Acetylcholine Receptors. Pharmacol Rev 2021, 73, 1118–1149. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shen, F.; Lin, L.; Zhang, X.; Bruce, I.C.; Xia, Q. The neuroprotection conferred by activating the mitochondrial ATP-sensitive K+ channel is mediated by inhibiting the mitochondrial permeability transition pore. Neurosci Lett 2006, 402, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.E.; Qian, Y.Z.; Gross, G.J.; Kukreja, R.C. The ischemia-selective KATP channel antagonist, 5-hydroxydecanoate, blocks ischemic preconditioning in the rat heart. J Mol Cell Cardiol 1997, 29, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiao, Y.; Zhang, Y.; Wang, Q.; Wu, W.; Zheng, J.; Li, J. G-Protein Coupled Receptor 35 Induces Intervertebral Disc Degeneration by Mediating the Influx of Calcium Ions and Upregulating Reactive Oxygen Species. Oxid Med Cell Longev 2022, 2022, 5469220. [Google Scholar] [CrossRef]
- Hirst, J. Mitochondrial complex I. Annu Rev Biochem 2013, 82, 551–575. [Google Scholar] [CrossRef]
- Bleier, L.; Dröse, S. Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim Biophys Acta 2013, 1827, 1320–1331. [Google Scholar] [CrossRef]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol 2023, 67, 102926. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol Teratol 2011, 33, 538–547. [Google Scholar] [CrossRef]
- Tappenden, D.M.; Lynn, S.G.; Crawford, R.B.; Lee, K.; Vengellur, A.; Kaminski, N.E.; Thomas, R.S.; LaPres, J.J. The aryl hydrocarbon receptor interacts with ATP5α1, a subunit of the ATP synthase complex, and modulates mitochondrial function. Toxicol Appl Pharmacol 2011, 254, 299–310. [Google Scholar] [CrossRef]
- Brinkmann, V.; Ale-Agha, N.; Haendeler, J.; Ventura, N. The Aryl Hydrocarbon Receptor (AhR) in the Aging Process: Another Puzzling Role for This Highly Conserved Transcription Factor. Front Physiol 2019, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Alkondon, M.; Pereira, E.F.; Yu, P.; Arruda, E.Z.; Almeida, L.E.; Guidetti, P.; Fawcett, W.P.; Sapko, M.T.; Randall, W.R.; Schwarcz, R.; et al. Targeted deletion of the kynurenine aminotransferase ii gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci 2004, 24, 4635–4648. [Google Scholar] [CrossRef]
- Mugayar, A.A.; da Silva Guimarães, G.; de Oliveira, P.H.T.; Miranda, R.L.; Dos Santos, A.A. Apoptosis in the neuroprotective effect of α7 nicotinic receptor in neurodegenerative models. J Neurosci Res 2023, 101, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Grant, R.; Brew, B.J.; Adams, S.; Jayasena, T.; Guillemin, G.J. Effects of Kynurenine Pathway Metabolites on Intracellular NAD Synthesis and Cell Death in Human Primary Astrocytes and Neurons. Int J Tryptophan Res 2009, 2, 61–69. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy—Challenges and Opportunities. Trends Pharmacol Sci 2018, 39, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Gouasmi, R.; Ferraro-Peyret, C.; Nancey, S.; Coste, I.; Renno, T.; Chaveroux, C.; Aznar, N.; Ansieau, S. The Kynurenine Pathway and Cancer: Why Keep It Simple When You Can Make It Complicated. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Luongo, T.S.; Eller, J.M.; Lu, M.J.; Niere, M.; Raith, F.; Perry, C.; Bornstein, M.R.; Oliphint, P.; Wang, L.; McReynolds, M.R.; et al. SLC25A51 is a mammalian mitochondrial NAD(+) transporter. Nature 2020, 588, 174–179. [Google Scholar] [CrossRef]
- Kory, N.; Uit de Bos, J.; van der Rijt, S.; Jankovic, N.; Güra, M.; Arp, N.; Pena, I.A.; Prakash, G.; Chan, S.H.; Kunchok, T.; et al. MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci Adv 2020, 6. [Google Scholar] [CrossRef]
- Girardi, E.; Agrimi, G.; Goldmann, U.; Fiume, G.; Lindinger, S.; Sedlyarov, V.; Srndic, I.; Gürtl, B.; Agerer, B.; Kartnig, F.; et al. Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat Commun 2020, 11, 6145. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, J.A.; Francisco, A.; Passos, L.A.; Figueira, T.R.; Castilho, R.F. The Contribution of Nicotinamide Nucleotide Transhydrogenase to Peroxide Detoxification Is Dependent on the Respiratory State and Counterbalanced by Other Sources of NADPH in Liver Mitochondria. J Biol Chem 2016, 291, 20173–20187. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, J.A.; Figueira, T.R.; Ravagnani, F.G.; Oliveira, H.C.; Vercesi, A.E.; Castilho, R.F. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic Biol Med 2013, 63, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.H.; Lin, C.T.; Ryan, T.E.; Reese, L.R.; Gilliam, L.A.; Cathey, B.L.; Lark, D.S.; Smith, C.D.; Muoio, D.M.; Neufer, P.D. Pyruvate dehydrogenase complex and nicotinamide nucleotide transhydrogenase constitute an energy-consuming redox circuit. Biochem J 2015, 467, 271–280. [Google Scholar] [CrossRef]
- Simmen, F.A.; Alhallak, I.; Simmen, R.C.M. Malic enzyme 1 (ME1) in the biology of cancer: it is not just intermediary metabolism. J Mol Endocrinol 2020, 65, R77–r90. [Google Scholar] [CrossRef]
- Ying, M.; You, D.; Zhu, X.; Cai, L.; Zeng, S.; Hu, X. Lactate and glutamine support NADPH generation in cancer cells under glucose deprived conditions. Redox Biol 2021, 46, 102065. [Google Scholar] [CrossRef]
- Hernandez-Baixauli, J.; Abasolo, N.; Palacios-Jordan, H.; Foguet-Romero, E.; Suñol, D.; Galofré, M.; Caimari, A.; Baselga-Escudero, L.; Del Bas, J.M.; Mulero, M. Imbalances in TCA, Short Fatty Acids and One-Carbon Metabolisms as Important Features of Homeostatic Disruption Evidenced by a Multi-Omics Integrative Approach of LPS-Induced Chronic Inflammation in Male Wistar Rats. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Hart, M.L.; Quon, E.; Vigil, A.B.G.; Engstrom, I.A.; Newsom, O.J.; Davidsen, K.; Hoellerbauer, P.; Carlisle, S.M.; Sullivan, L.B. Mitochondrial redox adaptations enable alternative aspartate synthesis in SDH-deficient cells. Elife 2023, 12. [Google Scholar] [CrossRef]
- Lussey-Lepoutre, C.; Hollinshead, K.E.; Ludwig, C.; Menara, M.; Morin, A.; Castro-Vega, L.J.; Parker, S.J.; Janin, M.; Martinelli, C.; Ottolenghi, C.; et al. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat Commun 2015, 6, 8784. [Google Scholar] [CrossRef]
- Esteban-Amo, M.J.; Jiménez-Cuadrado, P.; Serrano-Lorenzo, P.; de la Fuente, M.; Simarro, M. Succinate Dehydrogenase and Human Disease: Novel Insights into a Well-Known Enzyme. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Wan, J.; Cheng, C.; Hu, J.; Huang, H.; Han, Q.; Jie, Z.; Zou, Q.; Shi, J.H.; Yu, X. De novo NAD(+) synthesis contributes to CD8(+) T cell metabolic fitness and antitumor function. Cell Rep 2023, 42, 113518. [Google Scholar] [CrossRef]
- Sadeghdoust, M.; Das, A.; Kaushik, D.K. Fueling neurodegeneration: metabolic insights into microglia functions. J Neuroinflammation 2024, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.R.; Arun, P.; Puthillathu, N.; Vengilote, R.; Ives, J.A.; Badawy, A.A.; Namboodiri, A.M. Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD(+) Synthesis During Inflammation and Infection. Front Immunol 2020, 11, 31. [Google Scholar] [CrossRef]
- McReynolds, M.R.; Wang, W.; Holleran, L.M.; Hanna-Rose, W. Uridine monophosphate synthetase enables eukaryotic de novo NAD(+) biosynthesis from quinolinic acid. J Biol Chem 2017, 292, 11147–11153. [Google Scholar] [CrossRef]
- Ding, Y.; Li, X.; Horsman, G.P.; Li, P.; Wang, M.; Li, J.; Zhang, Z.; Liu, W.; Wu, B.; Tao, Y.; et al. Construction of an Alternative NAD(+) De Novo Biosynthesis Pathway. Adv Sci (Weinh) 2021, 8, 2004632. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, R.; Yao, L.; Lin, Z.; Li, Y.; Li, S.; Wu, L. De novo NAD(+) synthesis is ineffective for NAD(+) supply in axenically cultured Caenorhabditis elegans. Commun Biol 2025, 8, 545. [Google Scholar] [CrossRef]
- Trepci, A.; Imbeault, S.; Wyckelsma, V.L.; Westerblad, H.; Hermansson, S.; Andersson, D.C.; Piehl, F.; Venckunas, T.; Brazaitis, M.; Kamandulis, S.; et al. Quantification of Plasma Kynurenine Metabolites Following One Bout of Sprint Interval Exercise. Int J Tryptophan Res 2020, 13, 1178646920978241. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, W.; Huang, C.; Sun, L.; Wu, X.; Xu, L.; Xiao, P. A rapid and reliable targeted LC-MS/MS method for quantitative analysis of the Tryptophan-NAD metabolic network disturbances in tissues and blood of sleep deprivation mice. Anal Chim Acta 2024, 1328, 343125. [Google Scholar] [CrossRef]
- Baixauli, F.; Piletic, K.; Puleston, D.J.; Villa, M.; Field, C.S.; Flachsmann, L.J.; Quintana, A.; Rana, N.; Edwards-Hicks, J.; Matsushita, M.; et al. An LKB1-mitochondria axis controls T(H)17 effector function. Nature 2022, 610, 555–561. [Google Scholar] [CrossRef]
- Muri, J.; Heer, S.; Matsushita, M.; Pohlmeier, L.; Tortola, L.; Fuhrer, T.; Conrad, M.; Zamboni, N.; Kisielow, J.; Kopf, M. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. Nat Commun 2018, 9, 1851. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.M.; Lee, R.T. Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxid Redox Signal 2013, 18, 1165–1207. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15. [Google Scholar] [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. Decoupling Behavioral Domains via Kynurenic Acid Analog Optimization: Implications for Schizophrenia and Parkinson’s Disease Therapeutics. Cells 2025, 14. [Google Scholar] [CrossRef]
- Tanaka, M. From Monoamines to Systems Psychiatry: Rewiring Depression Science and Care (1960s–2025). Biomedicines 2026, 14, 35. [Google Scholar] [CrossRef]
- Chehadi, A.C.P.d.; L., E.; Detregiachi, C.R.P.; Santos de Argollo Haber, R.; Catharin, V.M.C.S.; Fornari Laurindo, L.; Engracia Valenti, V.; Machado Galhardi, C.; Tanaka, M.; Maria Barbalho, S. Harnessing Dietary Tryptophan: Bridging the Gap Between Neurobiology and Psychiatry in Depression Management. International Journal of Molecular Sciences 2026, 27, 465. [Google Scholar] [CrossRef] [PubMed]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci Biobehav Rev 2018, 90, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.H.; Ma, Z.X.; Feltenberger, J.B.; Chen, H.; Chen, H.; Scarlett, C.; Lin, Z.; Satyshur, K.A.; Cortopassi, M.; Jefcoate, C.R.; et al. Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR). J Biol Chem 2018, 293, 1994–2005. [Google Scholar] [CrossRef]
- Palzkill, V.R.; Thome, T.; Murillo, A.L.; Khattri, R.B.; Ryan, T.E. Increasing plasma L-kynurenine impairs mitochondrial oxidative phosphorylation prior to the development of atrophy in murine skeletal muscle: A pilot study. Front Physiol 2022, 13, 992413. [Google Scholar] [CrossRef] [PubMed]
- Galla, Z.; Rajda, C.; Rácz, G.; Grecsó, N.; Baráth, Á.; Vécsei, L.; Bereczki, C.; Monostori, P. Simultaneous determination of 30 neurologically and metabolically important molecules: A sensitive and selective way to measure tyrosine and tryptophan pathway metabolites and other biomarkers in human serum and cerebrospinal fluid. Journal of Chromatography A 2021, 1635, 461775. [Google Scholar] [CrossRef]
- Galla, Z.; Rácz, G.; Grecsó, N.; Baráth, Á.; Kósa, M.; Bereczki, C.; Monostori, P. Improved LC-MS/MS method for the determination of 42 neurologically and metabolically important molecules in urine. J Chromatogr B Analyt Technol Biomed Life Sci 2021, 1179, 122846. [Google Scholar] [CrossRef]
- Al Kadhi, O.; Melchini, A.; Mithen, R.; Saha, S. Development of a LC-MS/MS Method for the Simultaneous Detection of Tricarboxylic Acid Cycle Intermediates in a Range of Biological Matrices. J Anal Methods Chem 2017, 2017, 5391832. [Google Scholar] [CrossRef]
- Le, A.; Mak, J.; Cowan, T.M. Metabolic profiling by reversed-phase/ion-exchange mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2020, 1143, 122072. [Google Scholar] [CrossRef]
- Cajka, T.; Hricko, J.; Rudl Kulhava, L.; Paucova, M.; Novakova, M.; Kuda, O. Optimization of Mobile Phase Modifiers for Fast LC-MS-Based Untargeted Metabolomics and Lipidomics. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Carlsson, H.; Vaivade, A.; Emami Khoonsari, P.; Burman, J.; Kultima, K. Evaluation of polarity switching for untargeted lipidomics using liquid chromatography coupled to high resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2022, 1195, 123200. [Google Scholar] [CrossRef]
- Ren, J.L.; Zhang, A.H.; Kong, L.; Wang, X.J. Advances in mass spectrometry-based metabolomics for investigation of metabolites. RSC Adv 2018, 8, 22335–22350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, J.; Johnson, M.; Mandal, R.; Cruz, M.; Martínez-Huélamo, M.; Andres-Lacueva, C.; Wishart, D.S. A Comprehensive LC-MS Metabolomics Assay for Quantitative Analysis of Serum and Plasma. Metabolites 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kremer, D.M.; Sajjakulnukit, P.; Zhang, L.; Lyssiotis, C.A. A large-scale analysis of targeted metabolomics data from heterogeneous biological samples provides insights into metabolite dynamics. Metabolomics 2019, 15, 103. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Duggan, J.X.; Aubry, A.F.; Zeng, J.; Lee, J.W.; Cojocaru, L.; Dufield, D.; Garofolo, F.; Kaur, S.; Schultz, G.A.; et al. Recommendations for validation of LC-MS/MS bioanalytical methods for protein biotherapeutics. Aaps j 2015, 17, 1–16. [Google Scholar] [CrossRef]
- . [PubMed]
- Thomas, S.N.; French, D.; Jannetto, P.J.; Rappold, B.A.; Clarke, W.A. Liquid chromatography-tandem mass spectrometry for clinical diagnostics. Nat Rev Methods Primers 2022, 2, 96. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, J.H.; Kemper, G.E.; Hummon, A.B. Quantitative mass spectrometry imaging: therapeutics & biomolecules. Chem Commun (Camb) 2024, 60, 2137–2151. [Google Scholar] [CrossRef]
- Thorsteinsdóttir, U.A.; Thorsteinsdóttir, M. Design of experiments for development and optimization of a liquid chromatography coupled to tandem mass spectrometry bioanalytical assay. J Mass Spectrom 2021, 56, e4727. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Schwarcz, R.; Erhardt, S. Neuroactive Kynurenines as Pharmacological Targets: New Experimental Tools and Exciting Therapeutic Opportunities. Pharmacol Rev 2024, 76, 978–1008. [Google Scholar] [CrossRef]
- Kurhaluk, N. Tricarboxylic Acid Cycle Intermediates and Individual Ageing. Biomolecules 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N. Tricarboxylic acid cycle intermediates and individual ageing. Biomolecules 2024, 14, 260. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, S.; Zhang, W.; Yang, R.; Yu, X.; Wang, X.; Mu, H.; Li, H.; Ji, F.; Chen, W. A single-run, rapid polarity switching method for simultaneous quantification of cardiovascular disease-related metabolites using liquid chromatography–tandem mass spectrometry. International Journal of Mass Spectrometry 2021, 461, 116500. [Google Scholar] [CrossRef]
- Nakatani, K.; Izumi, Y.; Takahashi, M.; Bamba, T. Unified-Hydrophilic-Interaction/Anion-Exchange Liquid Chromatography Mass Spectrometry (Unified-HILIC/AEX/MS): A Single-Run Method for Comprehensive and Simultaneous Analysis of Polar Metabolome. Anal Chem 2022, 94, 16877–16886. [Google Scholar] [CrossRef]
- Quan, S.; Zhao, S.; Li, L. Study of Metabolite Detectability in Simultaneous Profiling of Amine/Phenol and Hydroxyl Submetabolomes by Analyzing a Mixture of Two Separately Dansyl-Labeled Samples. Metabolites 2025, 15. [Google Scholar] [CrossRef]
- Huan, T.; Xian, J.W.; Leung, W.N.; Li, L.; Chan, C.W. Cerebrospinal Fluid Metabolomics After Natural Product Treatment in an Experimental Model of Cerebral Ischemia. Omics 2016, 20, 670–680. [Google Scholar] [CrossRef]
- Yan, J.; Kuzhiumparambil, U.; Bandodkar, S.; Dale, R.C.; Fu, S. Cerebrospinal fluid metabolomics: detection of neuroinflammation in human central nervous system disease. Clin Transl Immunology 2021, 10, e1318. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, H.; Abujrais, S.; Herman, S.; Khoonsari, P.E.; Åkerfeldt, T.; Svenningsson, A.; Burman, J.; Kultima, K. Targeted metabolomics of CSF in healthy individuals and patients with secondary progressive multiple sclerosis using high-resolution mass spectrometry. Metabolomics 2020, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Saoi, M.; Britz-McKibbin, P. New Advances in Tissue Metabolomics: A Review. Metabolites 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Unsihuay, D.; Phipps, W.S.; Paulovich, A.G.; Chapman, J.R.; Ducret, A.; Eberlin, L.S.; Spraggins, J.M.; Goodwin, R.J.A. Tissue Mass Spectrometry: How Solid Is Our Future? Clin Chem 2023, 69, 676–683. [Google Scholar] [CrossRef]
- Harstad, E.; Andaya, R.; Couch, J.; Ding, X.; Liang, X.; Liederer, B.M.; Messick, K.; Nguyen, T.; Schweiger, M.; Tarrant, J.; et al. Balancing Blood Sample Volume with 3Rs: Implementation and Best Practices for Small Molecule Toxicokinetic Assessments in Rats. Ilar j 2016, 57, 157–165. [Google Scholar] [CrossRef]
- Jeppesen, M.J.; Powers, R. Multiplatform untargeted metabolomics. Magn Reson Chem 2023, 61, 628–653. [Google Scholar] [CrossRef]
- Patel, V.D.; Shamsi, S.A.; Miller, A.; Liu, A.; Powell, M. Simultaneous separation and detection of nine kynurenine pathway metabolites by reversed-phase liquid chromatography-mass spectrometry: Quantitation of inflammation in human cerebrospinal fluid and plasma. Anal Chim Acta 2023, 1278, 341659. [Google Scholar] [CrossRef]
- Abusoglu, S.; Eryavuz Onmaz, D.; Abusoglu, G.; Humeyra Yerlikaya, F.; Unlu, A. Measurement of kynurenine pathway metabolites by tandem mass spectrometry. J Mass Spectrom Adv Clin Lab 2023, 28, 114–121. [Google Scholar] [CrossRef]
- Rathod, R.; Gajera, B.; Nazir, K.; Wallenius, J.; Velagapudi, V. Simultaneous Measurement of Tricarboxylic Acid Cycle Intermediates in Different Biological Matrices Using Liquid Chromatography-Tandem Mass Spectrometry; Quantitation and Comparison of TCA Cycle Intermediates in Human Serum, Plasma, Kasumi-1 Cell and Murine Liver Tissue. Metabolites 2020, 10. [Google Scholar] [CrossRef]
- Ivanisevic, J.; Want, E.J. From Samples to Insights into Metabolism: Uncovering Biologically Relevant Information in LC-HRMS Metabolomics Data. Metabolites 2019, 9. [Google Scholar] [CrossRef]
- Wei, R.; Li, G.; Seymour, A.B. Multiplexed, quantitative, and targeted metabolite profiling by LC-MS/MRM. Methods Mol Biol 2014, 1198, 171–199. [Google Scholar] [CrossRef]
- Pukoli, D.; Vécsei, L. Kynurenines and Mitochondrial Disturbances in Multiple Sclerosis. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Gomez-Gomez, A.; Aguilera, P.; Langohr, K.; Casals, G.; Pavon, C.; Marcos, J.; To-Figueras, J.; Pozo, O.J. Evaluation of Metabolic Changes in Acute Intermittent Porphyria Patients by Targeted Metabolomics. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- Myint, A.M.; Halaris, A. Imbalances in Kynurenines as Potential Biomarkers in the Diagnosis and Treatment of Psychiatric Disorders. Front Psychiatry 2022, 13, 913303. [Google Scholar] [CrossRef]
- Boyd, A.; Boccara, F.; Meynard, J.L.; Ichou, F.; Bastard, J.P.; Fellahi, S.; Samri, A.; Sauce, D.; Haddour, N.; Autran, B.; et al. Serum Tryptophan-Derived Quinolinate and Indole-3-Acetate Are Associated With Carotid Intima-Media Thickness and its Evolution in HIV-Infected Treated Adults. Open Forum Infect Dis 2019, 6, ofz516. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Qi, Y.B.; Gao, Y.N.; Chen, W.G.; Zhou, T.; Zang, Y.; Li, J. Astrocyte metabolism and signaling pathways in the CNS. Front Neurosci 2023, 17, 1217451. [Google Scholar] [CrossRef] [PubMed]
- Harrieder, E.M.; Kretschmer, F.; Böcker, S.; Witting, M. Current state-of-the-art of separation methods used in LC-MS based metabolomics and lipidomics. J Chromatogr B Analyt Technol Biomed Life Sci 2022, 1188, 123069. [Google Scholar] [CrossRef] [PubMed]
- Fuertig, R.; Ceci, A.; Camus, S.M.; Bezard, E.; Luippold, A.H.; Hengerer, B. LC-MS/MS-based quantification of kynurenine metabolites, tryptophan, monoamines and neopterin in plasma, cerebrospinal fluid and brain. Bioanalysis 2016, 8, 1903–1917. [Google Scholar] [CrossRef]
- Walvekar, A.; Rashida, Z.; Maddali, H.; Laxman, S. A versatile LC-MS/MS approach for comprehensive, quantitative analysis of central metabolic pathways. Wellcome Open Res 2018, 3, 122. [Google Scholar] [CrossRef]
- Iqbal, K.; Intemann, T.; Börnhorst, C.; Zhang, J.; Aleksandrova, K. Approaches for harmonization of biomarker data from multiple studies: a narrative methodological review. AJE Advances: Research in Epidemiology 2025, 1, uuaf005. [Google Scholar] [CrossRef]
- Ivanovová, E.; Piskláková, B.; Dobešová, D.; Janečková, H.; Foltenová, H.; Kvasnička, A.; Prídavok, M.; Bouchalová, K.; de Sousa, J.; Friedecký, D. Wide metabolite coverage LC-MS/MS assay for the diagnosis of inherited metabolic disorders in urine. Talanta 2024, 271, 125699. [Google Scholar] [CrossRef]
- Roth, H.E.; Powers, R. Meta-Analysis Reveals Both the Promises and the Challenges of Clinical Metabolomics. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Sloan, A.; Cheng, C.; Rosner, B.; Ziegler, R.G.; Smith-Warner, S.A.; Wang, M. A repeated measures approach to pooled and calibrated biomarker data. Biometrics 2023, 79, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Waikar, S.S.; Rebholz, C.M.; Zheng, Z.; Perichon, R.; Clish, C.B.; Evans, A.M.; Avila, J.; Denburg, M.R.; Anderson, A.H.; et al. Variability of Two Metabolomic Platforms in CKD. Clin J Am Soc Nephrol 2019, 14, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Debnath, N.; Mosoyan, G.; Chauhan, K.; Vasquez-Rios, G.; Soudant, C.; Menez, S.; Parikh, C.R.; Coca, S.G. Systematic Review and Meta-Analysis of Plasma and Urine Biomarkers for CKD Outcomes. J Am Soc Nephrol 2022, 33, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Lippa, K.A.; Aristizabal-Henao, J.J.; Beger, R.D.; Bowden, J.A.; Broeckling, C.; Beecher, C.; Clay Davis, W.; Dunn, W.B.; Flores, R.; Goodacre, R.; et al. Reference materials for MS-based untargeted metabolomics and lipidomics: a review by the metabolomics quality assurance and quality control consortium (mQACC). Metabolomics 2022, 18, 24. [Google Scholar] [CrossRef]
- Rehder, C.; Bean, L.J.H.; Bick, D.; Chao, E.; Chung, W.; Das, S.; O’Daniel, J.; Rehm, H.; Shashi, V.; Vincent, L.M. Next-generation sequencing for constitutional variants in the clinical laboratory, 2021 revision: a technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2021, 23, 1399–1415. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Beger, R.D.; Cheng, L.L.; Cumeras, R.; Cuthbertson, D.J.; Dasari, S.; Davis, W.C.; Dunn, W.B.; Evans, A.M.; Fernández-Ochoa, A.; et al. Current Practices in LC-MS Untargeted Metabolomics: A Scoping Review on the Use of Pooled Quality Control Samples. Anal Chem 2023, 95, 18645–18654. [Google Scholar] [CrossRef]
- Ou, F.S.; Michiels, S.; Shyr, Y.; Adjei, A.A.; Oberg, A.L. Biomarker Discovery and Validation: Statistical Considerations. J Thorac Oncol 2021, 16, 537–545. [Google Scholar] [CrossRef]
- Mattsson-Carlgren, N.; Palmqvist, S.; Blennow, K.; Hansson, O. Publisher Correction: Increasing the reproducibility of fluid biomarker studies in neurodegenerative studies. Nat Commun 2021, 12, 196. [Google Scholar] [CrossRef]
- Chau, C.H.; Rixe, O.; McLeod, H.; Figg, W.D. Validation of analytic methods for biomarkers used in drug development. Clin Cancer Res 2008, 14, 5967–5976. [Google Scholar] [CrossRef]
- Saito, K.; Goda, R.; Arai, K.; Asahina, K.; Kawabata, M.; Uchiyama, H.; Andou, T.; Shimizu, H.; Takahara, K.; Kakehi, M.; et al. Interlaboratory evaluation of LC-MS-based biomarker assays. Bioanalysis 2024, 16, 389–402. [Google Scholar] [CrossRef]
- Jia, Z.; Qiu, Q.; He, R.; Zhou, T.; Chen, L. Identification of Metabolite Interference Is Necessary for Accurate LC-MS Targeted Metabolomics Analysis. Anal Chem 2023, 95, 7985–7992. [Google Scholar] [CrossRef] [PubMed]
- Addona, T.A.; Abbatiello, S.E.; Schilling, B.; Skates, S.J.; Mani, D.R.; Bunk, D.M.; Spiegelman, C.H.; Zimmerman, L.J.; Ham, A.J.; Keshishian, H.; et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol 2009, 27, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.W.; Adams, K.J.; Adamski, J.; Asad, Y.; Borts, D.; Bowden, J.A.; Byram, G.; Dang, V.; Dunn, W.B.; Fernandez, F.; et al. International Ring Trial of a High Resolution Targeted Metabolomics and Lipidomics Platform for Serum and Plasma Analysis. Anal Chem 2019, 91, 14407–14416. [Google Scholar] [CrossRef]
- Fernández-Metzler, C.; Ackermann, B.; Garofolo, F.; Arnold, M.E.; DeSilva, B.; Gu, H.; Laterza, O.; Mao, Y.; Rose, M.; Vazvaei-Smith, F.; et al. Biomarker Assay Validation by Mass Spectrometry. Aaps j 2022, 24, 66. [Google Scholar] [CrossRef]
- Brunius, C.; Shi, L.; Landberg, R. Large-scale untargeted LC-MS metabolomics data correction using between-batch feature alignment and cluster-based within-batch signal intensity drift correction. Metabolomics 2016, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, R.; Hageman, J.A.; van Eeuwijk, F.; Kooke, R.; Flood, P.J.; Wijnker, E.; Keurentjes, J.J.; Lommen, A.; van Eekelen, H.D.; Hall, R.D.; et al. Improved batch correction in untargeted MS-based metabolomics. Metabolomics 2016, 12, 88. [Google Scholar] [CrossRef]
- Malinka, F.; Zareie, A.; Prochazka, J.; Sedlacek, R.; Novosadova, V. Batch alignment via retention orders for preprocessing large-scale multi-batch LC-MS experiments. Bioinformatics 2022, 38, 3759–3767. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Tu, L.; Yu, T.; Chen, B.; Huang, P.; Hu, Y.; Luan, T. Normalization Approach by a Reference Material to Improve LC-MS-Based Metabolomic Data Comparability of Multibatch Samples. Anal Chem 2023, 95, 1309–1317. [Google Scholar] [CrossRef]
- Siegel, D.; Permentier, H.; Reijngoud, D.J.; Bischoff, R. Chemical and technical challenges in the analysis of central carbon metabolites by liquid-chromatography mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2014, 966, 21–33. [Google Scholar] [CrossRef]
- Jankech, T.; Gerhardtova, I.; Majerova, P.; Piestansky, J.; Jampilek, J.; Kovac, A. Derivatization of carboxylic groups prior to their LC analysis—A review. Anal Chim Acta 2024, 1300, 342435. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal Chem 2010, 82, 3212–3221. [Google Scholar] [CrossRef]
- Vazquez, S.; Truscott, R.J.; O’Hair, R.A.; Weimann, A.; Sheil, M.M. A study of kynurenine fragmentation using electrospray tandem mass spectrometry. J Am Soc Mass Spectrom 2001, 12, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Kiontke, A.; Oliveira-Birkmeier, A.; Opitz, A.; Birkemeyer, C. Electrospray Ionization Efficiency Is Dependent on Different Molecular Descriptors with Respect to Solvent pH and Instrumental Configuration. PLoS One 2016, 11, e0167502. [Google Scholar] [CrossRef] [PubMed]
- Steckel, A.; Schlosser, G. An Organic Chemist’s Guide to Electrospray Mass Spectrometric Structure Elucidation. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Liigand, J.; Laaniste, A.; Kruve, A. pH Effects on Electrospray Ionization Efficiency. J Am Soc Mass Spectrom 2017, 28, 461–469. [Google Scholar] [CrossRef]
- Hermans, J.; Ongay, S.; Markov, V.; Bischoff, R. Physicochemical Parameters Affecting the Electrospray Ionization Efficiency of Amino Acids after Acylation. Anal Chem 2017, 89, 9159–9166. [Google Scholar] [CrossRef]
- Ngere, J.B.; Ebrahimi, K.H.; Williams, R.; Pires, E.; Walsby-Tickle, J.; McCullagh, J.S.O. Ion-Exchange Chromatography Coupled to Mass Spectrometry in Life Science, Environmental, and Medical Research. Anal Chem 2023, 95, 152–166. [Google Scholar] [CrossRef]
- Jang, C.; Chen, L.; Rabinowitz, J.D. Metabolomics and Isotope Tracing. Cell 2018, 173, 822–837. [Google Scholar] [CrossRef]
- Wang, G.; Kakumanu, R.; Amer, B. Reversed phase LC-MS analysis of organic acids involved in the tricarboxylic acid cycle. 2025. [Google Scholar]
- Ovbude, S.T.; Sharmeen, S.; Kyei, I.; Olupathage, H.; Jones, J.; Bell, R.J.; Powers, R.; Hage, D.S. Applications of chromatographic methods in metabolomics: A review. J Chromatogr B Analyt Technol Biomed Life Sci 2024, 1239, 124124. [Google Scholar] [CrossRef] [PubMed]
- Keefer, J.F.; Schuster, S.M. Separation of citric acid cycle intermediates by high-performance liquid chromatography with ion pairing. J Chromatogr 1986, 383, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Q.; Zou, L.; Yin, X.X.; Ong, C.N. HILIC-MS for metabolomics: An attractive and complementary approach to RPLC-MS. Mass spectrometry reviews 2016, 35, 574–600. [Google Scholar] [CrossRef]
- Schwaiger, M.; Schoeny, H.; El Abiead, Y.; Hermann, G.; Rampler, E.; Koellensperger, G. Merging metabolomics and lipidomics into one analytical run. Analyst 2018, 144, 220–229. [Google Scholar] [CrossRef]
- Hiefner, J.; Rische, J.; Bunders, M.J.; Worthmann, A. A liquid chromatography-tandem mass spectrometry based method for the quantification of adenosine nucleotides and NAD precursors and products in various biological samples. Front Immunol 2023, 14, 1250762. [Google Scholar] [CrossRef] [PubMed]
- Meinitzer, A.; Tomaschitz, A.; Pilz, S.; Truber, M.; Zechner, G.; Gaksch, M.; Prietl, B.; Treiber, G.; Schwarz, M.; Baranyi, A. Development of a liquid chromatography-mass spectrometry method for the determination of the neurotoxic quinolinic acid in human serum. Clin Chim Acta 2014, 436, 268–272. [Google Scholar] [CrossRef]
- Tömösi, F.; Kecskeméti, G.; Cseh, E.K.; Szabó, E.; Rajda, C.; Kormány, R.; Szabó, Z.; Vécsei, L.; Janáky, T. A validated UHPLC-MS method for tryptophan metabolites: Application in the diagnosis of multiple sclerosis. J Pharm Biomed Anal 2020, 185, 113246. [Google Scholar] [CrossRef]
- Brookhart, A.; Arora, M.; McCullagh, M.; Wilson, I.D.; Plumb, R.S.; Vissers, J.P.; Tanna, N. Understanding mobile phase buffer composition and chemical structure effects on electrospray ionization mass spectrometry response. J Chromatogr A 2023, 1696, 463966. [Google Scholar] [CrossRef]
- Nshanian, M.; Lakshmanan, R.; Chen, H.; Ogorzalek Loo, R.R.; Loo, J.A. Enhancing Sensitivity of Liquid Chromatography-Mass Spectrometry of Peptides and Proteins Using Supercharging Agents. Int J Mass Spectrom 2018, 427, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.H.; Blaze, M.T.M.; Boissel, C. Electrospray ionization mass spectrometry ion suppression/enhancement caused by column bleed for three mixed-mode reversed-phase/anion-exchange high-performance liquid chromatography columns. Rapid Commun Mass Spectrom 2021, 35, e9098. [Google Scholar] [CrossRef]
- Sterling, H.J.; Batchelor, J.D.; Wemmer, D.E.; Williams, E.R. Effects of buffer loading for electrospray ionization mass spectrometry of a noncovalent protein complex that requires high concentrations of essential salts. J Am Soc Mass Spectrom 2010, 21, 1045–1049. [Google Scholar] [CrossRef]
- Zheng, J.J.; Lynch, E.D.; Unger, S.E. Comparison of SPE and fast LC to eliminate mass spectrometric matrix effects from microsomal incubation products. J Pharm Biomed Anal 2002, 28, 279–285. [Google Scholar] [CrossRef]
- Rakusanova, S.; Cajka, T. Tips and tricks for LC–MS-based metabolomics and lipidomics analysis. TrAC Trends in Analytical Chemistry 2024, 180, 117940. [Google Scholar] [CrossRef]
- Silvester, S. Mobile phase pH and organic modifier in reversed-phase LC-ESI-MS bioanalytical methods: assessment of sensitivity, chromatography and correlation of retention time with in silico logD predictions. Bioanalysis 2013, 5, 2753–2770. [Google Scholar] [CrossRef]
- Liigand, J.; de Vries, R.; Cuyckens, F. Optimization of flow splitting and make-up flow conditions in liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 2019, 33, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, S.; Rocca, J. Effect of mobile phase composition, pH and buffer type on the retention of ionizable compounds in reversed-phase liquid chromatography: application to method development. Journal of Chromatography A 2004, 1048, 183–193. [Google Scholar] [CrossRef]
- Liigand, P.; Kaupmees, K.; Haav, K.; Liigand, J.; Leito, I.; Girod, M.; Antoine, R.; Kruve, A. Think Negative: Finding the Best Electrospray Ionization/MS Mode for Your Analyte. Anal Chem 2017, 89, 5665–5668. [Google Scholar] [CrossRef]
- Henriksen, T.; Juhler, R.K.; Svensmark, B.; Cech, N.B. The relative influences of acidity and polarity on responsiveness of small organic molecules to analysis with negative ion electrospray ionization mass spectrometry (ESI-MS). J Am Soc Mass Spectrom 2005, 16, 446–455. [Google Scholar] [CrossRef]
- Venter, P. The Effects of Solvation Enthalpy, Surface Tension, and Conductivity of Common Additives on Positive Electrospray Ionization in Selected Pharmaceuticals. Molecules 2025, 30. [Google Scholar] [CrossRef]
- Han, J.; Gagnon, S.; Eckle, T.; Borchers, C.H. Metabolomic analysis of key central carbon metabolism carboxylic acids as their 3-nitrophenylhydrazones by UPLC/ESI-MS. Electrophoresis 2013, 34, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Louie, A.; Yang, Q.; Massenkoff, N.; Xu, C.; Hunt, P.W.; Gee, W. A simple LC-MS/MS method for determination of kynurenine and tryptophan concentrations in human plasma from HIV-infected patients. Bioanalysis 2013, 5, 1397–1407. [Google Scholar] [CrossRef]
- Breitkopf, S.B.; Ricoult, S.J.H.; Yuan, M.; Xu, Y.; Peake, D.A.; Manning, B.D.; Asara, J.M. A relative quantitative positive/negative ion switching method for untargeted lipidomics via high resolution LC-MS/MS from any biological source. Metabolomics 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Mikkilä, J.; Bengs, A.; Koirala, M.; Mikkilä, J.; Holm, S.; Juuti, P.; Meder, M.; Partovi, F.; Shcherbinin, A.; et al. Extending the Range of Detectable Trace Species with the Fast Polarity Switching of Chemical Ionization Orbitrap Mass Spectrometry. Anal Chem 2024, 96, 8604–8612. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc 2012, 7, 872–881. [Google Scholar] [CrossRef]
- Kluger, B.; Bueschl, C.; Neumann, N.; Stückler, R.; Doppler, M.; Chassy, A.W.; Waterhouse, A.L.; Rechthaler, J.; Kampleitner, N.; Thallinger, G.G.; et al. Untargeted profiling of tracer-derived metabolites using stable isotopic labeling and fast polarity-switching LC-ESI-HRMS. Anal Chem 2014, 86, 11533–11537. [Google Scholar] [CrossRef]
- Zherebker, A.; Kostyukevich, Y.; Kononikhin, A.; Roznyatovsky, V.A.; Popov, I.; Grishin, Y.K.; Perminova, I.V.; Nikolaev, E. High desolvation temperature facilitates the ESI-source H/D exchange at non-labile sites of hydroxybenzoic acids and aromatic amino acids. Analyst 2016, 141, 2426–2434. [Google Scholar] [CrossRef]
- Paíga, P.; Silva, L.M.; Delerue-Matos, C. Optimization of the Ion Source-Mass Spectrometry Parameters in Non-Steroidal Anti-Inflammatory and Analgesic Pharmaceuticals Analysis by a Design of Experiments Approach. J Am Soc Mass Spectrom 2016, 27, 1703–1714. [Google Scholar] [CrossRef]
- Pedro, L.; Van Voorhis, W.C.; Quinn, R.J. Optimization of Electrospray Ionization by Statistical Design of Experiments and Response Surface Methodology: Protein-Ligand Equilibrium Dissociation Constant Determinations. J Am Soc Mass Spectrom 2016, 27, 1520–1530. [Google Scholar] [CrossRef]
- Prothmann, J.; Molins-Delgado, D.; Braune, A.; Sandahl, M.; Turner, C.; Spégel, P. Examining functional group-dependent effects on the ionization of lignin monomers using supercritical fluid chromatography/electrospray ionization mass spectrometry. Anal Bioanal Chem 2024, 416, 4007–4014. [Google Scholar] [CrossRef]
- Kruve, A.; Kaupmees, K. Adduct Formation in ESI/MS by Mobile Phase Additives. J Am Soc Mass Spectrom 2017, 28, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Flick, T.G.; Merenbloom, S.I.; Williams, E.R. Anion effects on sodium ion and acid molecule adduction to protein ions in electrospray ionization mass spectrometry. J Am Soc Mass Spectrom 2011, 22, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Carnevale Neto, F.; Pascua, V.; Raftery, D. Formation of sodium cluster ions complicates liquid chromatography-mass spectrometry metabolomics analyses. Rapid Commun Mass Spectrom 2021, 35, e9175. [Google Scholar] [CrossRef] [PubMed]
- Mortier, K.A.; Zhang, G.F.; van Peteghem, C.H.; Lambert, W.E. Adduct formation in quantitative bioanalysis: effect of ionization conditions on paclitaxel. J Am Soc Mass Spectrom 2004, 15, 585–592. [Google Scholar] [CrossRef]
- Stricker, T.; Bonner, R.; Lisacek, F.; Hopfgartner, G. Adduct annotation in liquid chromatography/high-resolution mass spectrometry to enhance compound identification. Anal Bioanal Chem 2021, 413, 503–517. [Google Scholar] [CrossRef]
- Birdsall, R.E.; Gilar, M.; Shion, H.; Yu, Y.Q.; Chen, W. Reduction of metal adducts in oligonucleotide mass spectra in ion-pair reversed-phase chromatography/mass spectrometry analysis. Rapid Commun Mass Spectrom 2016, 30, 1667–1679. [Google Scholar] [CrossRef]
- Erngren, I.; Nestor, M.; Pettersson, C.; Hedeland, M. Improved Sensitivity in Hydrophilic Interaction Liquid Chromatography-Electrospray-Mass Spectrometry after Removal of Sodium and Potassium Ions from Biological Samples. Metabolites 2021, 11. [Google Scholar] [CrossRef]
- Nash, W.J.; Ngere, J.B.; Najdekr, L.; Dunn, W.B. Characterization of Electrospray Ionization Complexity in Untargeted Metabolomic Studies. Anal Chem 2024, 96, 10935–10942. [Google Scholar] [CrossRef]
- Erngren, I.; Haglöf, J.; Engskog, M.K.R.; Nestor, M.; Hedeland, M.; Arvidsson, T.; Pettersson, C. Adduct formation in electrospray ionisation-mass spectrometry with hydrophilic interaction liquid chromatography is strongly affected by the inorganic ion concentration of the samples. J Chromatogr A 2019, 1600, 174–182. [Google Scholar] [CrossRef]
- Stokvis, E.; Rosing, H.; Beijnen, J.H. Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not? Rapid Commun Mass Spectrom 2005, 19, 401–407. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Vaidyanathan, S. Towards quantitative mass spectrometry-based metabolomics in microbial and mammalian systems. Philos Trans A Math Phys Eng Sci 2016, 374. [Google Scholar] [CrossRef]
- Liigand, P.; Liigand, J.; Kaupmees, K.; Kruve, A. 30 Years of research on ESI/MS response: Trends, contradictions and applications. Anal Chim Acta 2021, 1152, 238117. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Willenbockel, H.F.; Cordes, T. Mapping the Metabolic Niche of Citrate Metabolism and SLC13A5. Metabolites 2023, 13. [Google Scholar] [CrossRef]
- Dankel, S.N.; Kalleklev, T.L.; Tungland, S.L.; Stafsnes, M.H.; Bruheim, P.; Aloysius, T.A.; Lindquist, C.; Skorve, J.; Nygård, O.K.; Madsen, L.; et al. Changes in Plasma Pyruvate and TCA Cycle Metabolites upon Increased Hepatic Fatty Acid Oxidation and Ketogenesis in Male Wistar Rats. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Darlington, L.G.; Badawy, A.A.; Williams, R.O. The Complex World of Kynurenic Acid: Reflections on Biological Issues and Therapeutic Strategy. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Tang, K.; Page, J.S.; Smith, R.D. Charge competition and the linear dynamic range of detection in electrospray ionization mass spectrometry. J Am Soc Mass Spectrom 2004, 15, 1416–1423. [Google Scholar] [CrossRef]
- Bruin, M.A.C.; Rosing, H.; Lucas, L.; Wang, J.; Huitema, A.D.R.; Schinkel, A.H.; Beijnen, J.H. Development and validation of an LC-MS/MS method with a broad linear dynamic range for the quantification of tivozanib in human and mouse plasma, mouse tissue homogenates, and culture medium. J Chromatogr B Analyt Technol Biomed Life Sci 2019, 1125, 121723. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ser, Z.; Locasale, J.W. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal Chem 2014, 86, 2175–2184. [Google Scholar] [CrossRef]
- Sands, C.J.; Gómez-Romero, M.; Correia, G.; Chekmeneva, E.; Camuzeaux, S.; Izzi-Engbeaya, C.; Dhillo, W.S.; Takats, Z.; Lewis, M.R. Representing the Metabolome with High Fidelity: Range and Response as Quality Control Factors in LC-MS-Based Global Profiling. Anal Chem 2021, 93, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Annesley, T.M. Ion suppression in mass spectrometry. Clin Chem 2003, 49, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Cech, N.B.; Enke, C.G. Practical implications of some recent studies in electrospray ionization fundamentals. Mass Spectrom Rev 2001, 20, 362–387. [Google Scholar] [CrossRef]
- King, R.; Bonfiglio, R.; Fernandez-Metzler, C.; Miller-Stein, C.; Olah, T. Mechanistic investigation of ionization suppression in electrospray ionization. J Am Soc Mass Spectrom 2000, 11, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gao, W.; Phelps, M.A.; Wu, D.; Miller, D.D.; Dalton, J.T. Favorable effects of weak acids on negative-ion electrospray ionization mass spectrometry. Anal Chem 2004, 76, 839–847. [Google Scholar] [CrossRef]
- Cheng, W.L.; Markus, C.; Lim, C.Y.; Tan, R.Z.; Sethi, S.K.; Loh, T.P. Calibration Practices in Clinical Mass Spectrometry: Review and Recommendations. Ann Lab Med 2023, 43, 5–18. [Google Scholar] [CrossRef]
- Jeanne Dit Fouque, D.; Maroto, A.; Memboeuf, A. Internal Standard Quantification Using Tandem Mass Spectrometry of a Tryptic Peptide in the Presence of an Isobaric Interference. Anal Chem 2018, 90, 14126–14130. [Google Scholar] [CrossRef]
- Tkalec, Ž.; Koudelka, Š.; Klánová, J.; Price, E.J. Dual LC column characterization for mass spectrometry-based small molecule profiling of human plasma and serum. Anal Chim Acta 2025, 1356, 343942. [Google Scholar] [CrossRef]
- Wuli, W.; Tsai, S.T.; Chiou, T.W.; Harn, H.J. Human-Induced Pluripotent Stem Cells and Herbal Small-Molecule Drugs for Treatment of Alzheimer’s Disease. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Zhang, T.; Noll, S.E.; Peng, J.T.; Klair, A.; Tripka, A.; Stutzman, N.; Cheng, C.; Zare, R.N.; Dickinson, A.J. Chemical imaging reveals diverse functions of tricarboxylic acid metabolites in root growth and development. Nat Commun 2023, 14, 2567. [Google Scholar] [CrossRef]
- Vass, A.; Robles-Molina, J.; Pérez-Ortega, P.; Gilbert-López, B.; Dernovics, M.; Molina-Díaz, A.; García-Reyes, J.F. Study of different HILIC, mixed-mode, and other aqueous normal-phase approaches for the liquid chromatography/mass spectrometry-based determination of challenging polar pesticides. Anal Bioanal Chem 2016, 408, 4857–4869. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)--a powerful separation technique. Anal Bioanal Chem 2012, 402, 231–247. [Google Scholar] [CrossRef]
- Kohler, I.; Verhoeven, M.; Haselberg, R.; Gargano, A.F. Hydrophilic interaction chromatography–mass spectrometry for metabolomics and proteomics: state-of-the-art and current trends. Microchemical Journal 2022, 175, 106986. [Google Scholar]
- Zhang, K.; Liu, X. Mixed-mode chromatography in pharmaceutical and biopharmaceutical applications. J Pharm Biomed Anal 2016, 128, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Sawada, T.; Hatayama, K.; Ohyagi, A.; Tsukuda, Y.; Namekawa, K.; Ito, R.; Saito, K.; Nakazawa, H. Separation technique for the determination of highly polar metabolites in biological samples. Metabolites 2012, 2, 496–515. [Google Scholar] [CrossRef]
- Chapel, S.; Rouvière, F.; Guillarme, D.; Heinisch, S. Reversed HILIC Gradient: A Powerful Strategy for On-Line Comprehensive 2D-LC. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Grübner, M.; Dunkel, A.; Steiner, F.; Hofmann, T. Comparative evaluation of comprehensive offline 2D-LC strategies coupled to MS for untargeted metabolomic studies of human urine. Anal Bioanal Chem 2025. [Google Scholar] [CrossRef] [PubMed]
- Papatheocharidou, C.; Samanidou, V. Two-Dimensional High-Performance Liquid Chromatography as a Powerful Tool for Bioanalysis: The Paradigm of Antibiotics. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Aly, A.A.; Górecki, T. Two-dimensional liquid chromatography with reversed phase in both dimensions: A review. J Chromatogr A 2024, 1721, 464824. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, Q.; Pan, J.; Deng, S.; Wang, S.; Li, Q.; Cao, J. Advanced applications of two-dimensional liquid chromatography in quantitative analysis of natural products. J Chromatogr A 2025, 1743, 465662. [Google Scholar] [CrossRef]
- Ortmayr, K.; Hann, S.; Koellensperger, G. Complementing reversed-phase selectivity with porous graphitized carbon to increase the metabolome coverage in an on-line two-dimensional LC-MS setup for metabolomics. Analyst 2015, 140, 3465–3473. [Google Scholar] [CrossRef]
- Willemse, C.M.; Stander, M.A.; Tredoux, A.G.; de Villiers, A. Comprehensive two-dimensional liquid chromatographic analysis of anthocyanins. J Chromatogr A 2014, 1359, 189–201. [Google Scholar] [CrossRef]
- Nell, E.H.J.; Muller, M.; de Villiers, A. Advances in Online Comprehensive Two-Dimensional Liquid Chromatography Method Development. Annu Rev Anal Chem (Palo Alto Calif) 2025, 18, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Pardon, M.; Reis, R.; de Witte, P.; Chapel, S.; Cabooter, D. Detailed comparison of in-house developed and commercially available heart-cutting and selective comprehensive two-dimensional liquid chromatography systems. J Chromatogr A 2024, 1713, 464565. [Google Scholar] [CrossRef] [PubMed]
- Malerod, H.; Lundanes, E.; Greibrokk, T. Recent advances in on-line multidimensional liquid chromatography. Analytical Methods 2010, 2, 110–122. [Google Scholar] [CrossRef]
- Wernisch, S.; Pennathur, S. Evaluation of coverage, retention patterns, and selectivity of seven liquid chromatographic methods for metabolomics. Anal Bioanal Chem 2016, 408, 6079–6091. [Google Scholar] [CrossRef] [PubMed]
- Aarika, K.; Rajyalakshmi, R.; Nalla, L.V.; Gajula, S.N.R. From complexity to clarity: expanding metabolome coverage with innovative analytical strategies. Journal of Separation Science 2025, 48, e70099. [Google Scholar] [CrossRef]
- Xu, K.; Berthiller, F.; Metzler-Zebeli, B.U.; Schwartz-Zimmermann, H.E. Development and Validation of Targeted Metabolomics Methods Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) for the Quantification of 235 Plasma Metabolites. Molecules 2025, 30. [Google Scholar] [CrossRef]
- Basov, N.V.; Rogachev, A.D.; Aleshkova, M.A.; Gaisler, E.V.; Sotnikova, Y.S.; Patrushev, Y.V.; Tolstikova, T.G.; Yarovaya, O.I.; Pokrovsky, A.G.; Salakhutdinov, N.F. Global LC-MS/MS targeted metabolomics using a combination of HILIC and RP LC separation modes on an organic monolithic column based on 1-vinyl-1,2,4-triazole. Talanta 2024, 267, 125168. [Google Scholar] [CrossRef]
- Lu, W.; Bennett, B.D.; Rabinowitz, J.D. Analytical strategies for LC-MS-based targeted metabolomics. J Chromatogr B Analyt Technol Biomed Life Sci 2008, 871, 236–242. [Google Scholar] [CrossRef]
- Gamoh, K.; Saitoh, H.; Wada, H. Improved liquid chromatography/mass spectrometric analysis of low molecular weight carboxylic acids by ion exclusion separation with electrospray ionization. Rapid communications in mass spectrometry 2003, 17, 685–689. [Google Scholar] [CrossRef]
- Zeki Ö, C.; Eylem, C.C.; Nemutlu, E. Optimization of GC-MS run time for untargeted metabolomics: Trade-offs between speed, coverage, and repeatability. J Pharm Biomed Anal 2025, 266, 117068. [Google Scholar] [CrossRef]
- Pinto, F.G.; Giddey, A.D.; Mohamed, N.; Almarri, R.S.B.; Murtaza, M.; Nassir, N.; Alkhnbashi, O.S.; Uddin, M.J.; Soares, N.C. Repurposing proteomic nanoLC-MS platforms for untargeted metabolomics: evaluating DIA and polarity switching performance in human plasma. Expert Rev Proteomics 2025, 22, 307–314. [Google Scholar] [CrossRef]
- Skogvold, H.B.; Sandås, E.M.; Østeby, A.; Løkken, C.; Rootwelt, H.; Rønning, P.O.; Wilson, S.R.; Elgstøen, K.B.P. Bridging the Polar and Hydrophobic Metabolome in Single-Run Untargeted Liquid Chromatography-Mass Spectrometry Dried Blood Spot Metabolomics for Clinical Purposes. J Proteome Res 2021, 20, 4010–4021. [Google Scholar] [CrossRef]
- Gika, H.G.; Theodoridis, G.A.; Vrhovsek, U.; Mattivi, F. Quantitative profiling of polar primary metabolites using hydrophilic interaction ultrahigh performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 2012, 1259, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Violi, J.P.; Phillips, C.R.; Gertner, D.S.; Westerhausen, M.T.; Padula, M.P.; Bishop, D.P.; Rodgers, K.J. Comprehensive untargeted polar metabolite analysis using solvent switching liquid chromatography tandem mass spectrometry. Talanta 2025, 287, 127610. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, F.; Zhu, J. Bridging Targeted and Untargeted Mass Spectrometry-Based Metabolomics via Hybrid Approaches. Metabolites 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.; NourEldein, M.; Bezdekova, D.; Schram, A.; Howard, R.; Powers, R. A Reproducibility Crisis for Clinical Metabolomics Studies. Trends Analyt Chem 2024, 180. [Google Scholar] [CrossRef] [PubMed]
- Ivanisevic, J.; Zhu, Z.J.; Plate, L.; Tautenhahn, R.; Chen, S.; O’Brien, P.J.; Johnson, C.H.; Marletta, M.A.; Patti, G.J.; Siuzdak, G. Toward ‘omic scale metabolite profiling: a dual separation-mass spectrometry approach for coverage of lipid and central carbon metabolism. Anal Chem 2013, 85, 6876–6884. [Google Scholar] [CrossRef]
- Arrivault, S.; Guenther, M.; Fry, S.C.; Fuenfgeld, M.M.; Veyel, D.; Mettler-Altmann, T.; Stitt, M.; Lunn, J.E. Synthesis and Use of Stable-Isotope-Labeled Internal Standards for Quantification of Phosphorylated Metabolites by LC-MS/MS. Anal Chem 2015, 87, 6896–6904. [Google Scholar] [CrossRef]
- Ulvik, A.; McCann, A.; Midttun, Ø.; Meyer, K.; Godfrey, K.M.; Ueland, P.M. Quantifying Precision Loss in Targeted Metabolomics Based on Mass Spectrometry and Nonmatching Internal Standards. Anal Chem 2021, 93, 7616–7624. [Google Scholar] [CrossRef]
- Mahmud, I.; Wei, B.; Veillon, L.; Tan, L.; Martinez, S.; Tran, B.; Raskind, A.; de Jong, F.; Liu, Y.; Ding, J.; et al. Ion suppression correction and normalization for non-targeted metabolomics. Nat Commun 2025, 16, 1347. [Google Scholar] [CrossRef]
- Ćeranić, A.; Bueschl, C.; Doppler, M.; Parich, A.; Xu, K.; Lemmens, M.; Buerstmayr, H.; Schuhmacher, R. Enhanced Metabolome Coverage and Evaluation of Matrix Effects by the Use of Experimental-Condition-Matched (13)C-Labeled Biological Samples in Isotope-Assisted LC-HRMS Metabolomics. Metabolites 2020, 10. [Google Scholar] [CrossRef]
- Bueschl, C.; Krska, R.; Kluger, B.; Schuhmacher, R. Isotopic labeling-assisted metabolomics using LC-MS. Anal Bioanal Chem 2013, 405, 27–33. [Google Scholar] [CrossRef]
- Bruheim, P.; Kvitvang, H.F.; Villas-Boas, S.G. Stable isotope coded derivatizing reagents as internal standards in metabolite profiling. J Chromatogr A 2013, 1296, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S. Development and Application of Chemical Isotope Labeling Methods and Metabolite Identification Solution for Liquid Chromatography-Mass Spectrometry-Based Metabolomics. 2018. [Google Scholar]
- Mathon, C.; Bovard, D.; Dutertre, Q.; Sendyk, S.; Bentley, M.; Hoeng, J.; Knorr, A. Impact of sample preparation upon intracellular metabolite measurements in 3D cell culture systems. Metabolomics 2019, 15, 92. [Google Scholar] [CrossRef]
- Sysi-Aho, M.; Katajamaa, M.; Yetukuri, L.; Oresic, M. Normalization method for metabolomics data using optimal selection of multiple internal standards. BMC Bioinformatics 2007, 8, 93. [Google Scholar] [CrossRef]
- Boysen, A.K.; Heal, K.R.; Carlson, L.T.; Ingalls, A.E. Best-Matched Internal Standard Normalization in Liquid Chromatography-Mass Spectrometry Metabolomics Applied to Environmental Samples. Anal Chem 2018, 90, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L. Sample normalization methods in quantitative metabolomics. J Chromatogr A 2016, 1430, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhao, Y.; DeMichele, M.; Zheng, N.; Zhang, Y.J.; Pillutla, R.; Zeng, J. In-Sample Calibration Curve Using Multiple Isotopologue Reaction Monitoring of a Stable Isotopically Labeled Analyte for Instant LC-MS/MS Bioanalysis and Quantitative Proteomics. Anal Chem 2019, 91, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.R.; Abbatiello, S.E.; Carr, S.A. Statistical characterization of multiple-reaction monitoring mass spectrometry (MRM-MS) assays for quantitative proteomics. BMC Bioinformatics 2012, 13 Suppl 16, S9. [Google Scholar] [CrossRef] [PubMed]
- Beger, R.D.; Goodacre, R.; Jones, C.M.; Lippa, K.A.; Mayboroda, O.A.; O’Neill, D.; Najdekr, L.; Ntai, I.; Wilson, I.D.; Dunn, W.B. Analysis types and quantification methods applied in UHPLC-MS metabolomics research: a tutorial. Metabolomics 2024, 20, 95. [Google Scholar] [CrossRef]
- Verhaeghe, T. Systematic internal standard variability and issue resolution: two case studies. Bioanalysis 2019, 11, 1685–1692. [Google Scholar] [CrossRef]
- Liao, H.W.; Cheng, Y.W.; Tang, S.C.; Kuo, C.H. Bias caused by incomplete metabolite extraction and matrix effect: Evaluation of critical factors for plasma sample preparation prior to metabolomics. J Pharm Biomed Anal 2022, 219, 114930. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Huang, J.; Zhu, Y.; Zhang, R.; He, J.; Abliz, Z. Development and validation of a sensitive and reliable targeted metabolomics method for the quantification of cardiovascular disease-related biomarkers in plasma using ultrahigh-performance liquid chromatography-tandem mass spectrometry. Rapid Commun Mass Spectrom 2022, 36, e9292. [Google Scholar] [CrossRef]
- Begou, O.; Gika, H.G.; Theodoridis, G.A.; Wilson, I.D. Quality Control and Validation Issues in LC-MS Metabolomics. Methods Mol Biol 2018, 1738, 15–26. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Estanyol-Torres, N.; Brunius, C.; Landberg, R.; González-Domínguez, R. QComics: Recommendations and Guidelines for Robust, Easily Implementable and Reportable Quality Control of Metabolomics Data. Anal Chem 2024, 96, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Looby, N.; Chandran, V.; Kulasingam, V. Challenges in the Metabolomics-Based Biomarker Validation Pipeline. Metabolites 2024, 14. [Google Scholar] [CrossRef]
- Furlanello, T.; Bertolini, F.M.; Zoia, A.; Sanchez Del Pulgar, J.; Masti, R. Establishment and Validation of Sensitive Liquid Chromatography-Tandem Mass Spectrometry Method for Aldosterone Quantification in Feline Serum with Reference Interval Determination. Animals (Basel) 2025, 15. [Google Scholar] [CrossRef]
- Brêtas, J.M.; César, I.C.; Brêtas, C.M.; Teixeira Lde, S.; Bellorio, K.B.; Mundim, I.M.; Pianetti, G.A. Development and validation of an LC-ESI-MS/MS method for the simultaneous quantification of naproxen and sumatriptan in human plasma: application to a pharmacokinetic study. Anal Bioanal Chem 2016, 408, 3981–3992. [Google Scholar] [CrossRef]
- Prakash, K.; Adiki, S.K.; Kalakuntla, R.R. Development and validation of a liquid chromatography-mass spectrometry method for the determination of zileuton in human plasma. Sci Pharm 2014, 82, 571–583. [Google Scholar] [CrossRef]
- Nandania, J.; Peddinti, G.; Pessia, A.; Kokkonen, M.; Velagapudi, V. Validation and Automation of a High-Throughput Multitargeted Method for Semiquantification of Endogenous Metabolites from Different Biological Matrices Using Tandem Mass Spectrometry. Metabolites 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.T.; Eberlin, M.N.; Simionato, A.V.C. Targeted metabolomics: Liquid chromatography coupled to mass spectrometry method development and validation for the identification and quantitation of modified nucleosides as putative cancer biomarkers. Talanta 2020, 210, 120640. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Groenke, K.; Takors, R.; Wandrey, C.; Oldiges, M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. J Chromatogr A 2007, 1147, 153–164. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Steiner, D.; Krska, R.; Malachová, A.; Taschl, I.; Sulyok, M. Evaluation of Matrix Effects and Extraction Efficiencies of LC-MS/MS Methods as the Essential Part for Proper Validation of Multiclass Contaminants in Complex Feed. J Agric Food Chem 2020, 68, 3868–3880. [Google Scholar] [CrossRef]
- Cortese, M.; Gigliobianco, M.R.; Magnoni, F.; Censi, R.; Di Martino, P.D. Compensate for or Minimize Matrix Effects? Strategies for Overcoming Matrix Effects in Liquid Chromatography-Mass Spectrometry Technique: A Tutorial Review. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Taylor, P.J. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin Biochem 2005, 38, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Gika, H.G.; Theodoridis, G.A.; Wilson, I.D. Liquid chromatography and ultra-performance liquid chromatography-mass spectrometry fingerprinting of human urine: sample stability under different handling and storage conditions for metabonomics studies. J Chromatogr A 2008, 1189, 314–322. [Google Scholar] [CrossRef]
- van de Merbel, N.; Savoie, N.; Yadav, M.; Ohtsu, Y.; White, J.; Riccio, M.F.; Dong, K.; de Vries, R.; Diancin, J. Stability: recommendation for best practices and harmonization from the Global Bioanalysis Consortium Harmonization Team. Aaps j 2014, 16, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C. Assessing stability in bioanalysis: reflections on the last 10 years. Bioanalysis 2019, 11, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Eilertsen, H.C.; Huseby, S.; Degerlund, M.; Eriksen, G.K.; Ingebrigtsen, R.A.; Hansen, E. The effect of freeze/thaw cycles on reproducibility of metabolic profiling of marine microalgal extracts using direct infusion high-resolution mass spectrometry (HR-MS). Molecules 2014, 19, 16373–16380. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, S.Y.; Shin, S.Y. Effect of Repeated Freezing and Thawing on Biomarker Stability in Plasma and Serum Samples. Osong Public Health Res Perspect 2015, 6, 357–362. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R. Bioanalytical method validation: An updated review. Pharm Methods 2010, 1, 25–38. [Google Scholar] [CrossRef]
- Theodorsson, E.; Magnusson, B. Full method validation in clinical chemistry. Accreditation and Quality Assurance 2017, 22, 235–246. [Google Scholar] [CrossRef]
- Gika, H.G.; Theodoridis, G.A.; Earll, M.; Wilson, I.D. A QC approach to the determination of day-to-day reproducibility and robustness of LC-MS methods for global metabolite profiling in metabonomics/metabolomics. Bioanalysis 2012, 4, 2239–2247. [Google Scholar] [CrossRef]
- Hoffman, D. Statistical considerations for assessment of bioanalytical incurred sample reproducibility. Aaps j 2009, 11, 570–580. [Google Scholar] [CrossRef]
- Ermer, J. Validation in pharmaceutical analysis. Part I: an integrated approach. J Pharm Biomed Anal 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Verch, T.; Campa, C.; Chéry, C.C.; Frenkel, R.; Graul, T.; Jaya, N.; Nakhle, B.; Springall, J.; Starkey, J.; Wypych, J.; et al. Analytical Quality by Design, Life Cycle Management, and Method Control. Aaps j 2022, 24, 34. [Google Scholar] [CrossRef]
- Paglia, G.; Astarita, G. A High-Throughput HILIC-MS-Based Metabolomic Assay for the Analysis of Polar Metabolites. Methods Mol Biol 2022, 2396, 137–159. [Google Scholar] [CrossRef]
- Spagou, K.; Tsoukali, H.; Raikos, N.; Gika, H.; Wilson, I.D.; Theodoridis, G. Hydrophilic interaction chromatography coupled to MS for metabonomic/metabolomic studies. J Sep Sci 2010, 33, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Kopaciewicz, W.; Regnier, F.E. Mobile phase selection for the high-performance ion-exchange chromatography of proteins. Anal Biochem 1983, 133, 251–259. [Google Scholar] [CrossRef]
- Choy, D.Y.; Creagh, A.L.; Haynes, C. Improved isoelectric focusing chromatography on strong anion exchange media via a new model that custom designs mobile phases using simple buffers. Biotechnol Bioeng 2014, 111, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Jaćkowska, M.; Bocian, S.; Kosobucki, P.; Buszewski, B. Polymeric Functionalized Stationary Phase for Separation of Ionic Compounds by IC. Chromatographia 2010, 72, 611–616. [Google Scholar] [CrossRef]
- Studzińska, S.; Bocian, S.; Kilanowska, A.; Buszewski, B. Dendrimer Anion-Exchange Stationary Phase for Separation of Oligonucleotides. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Law, B.; Hussain, M.A. Re-evaluation of anion-exchange HPLC for the analysis of acidic compounds. J Pharm Biomed Anal 2000, 22, 149–154. [Google Scholar] [CrossRef]
- Ikeda, K.; Takahashi, M.; Bamba, T.; Izumi, Y. Comparison of Amine-Modified Polymeric Stationary Phases for Polar Metabolomic Analysis Based on Unified-Hydrophilic Interaction/Anion Exchange Liquid Chromatography/High-Resolution Mass Spectrometry (Unified-HILIC/AEX/HRMS). Mass Spectrom (Tokyo) 2024, 13, A0143. [Google Scholar] [CrossRef]
- Tang, D.Q.; Zou, L.; Yin, X.X.; Ong, C.N. HILIC-MS for metabolomics: An attractive and complementary approach to RPLC-MS. Mass Spectrom Rev 2016, 35, 574–600. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, A.H.; Miao, J.H.; Sun, H.; Han, Y.; Yan, G.L.; Wu, F.F.; Wang, X.J. Metabolomics biotechnology, applications, and future trends: a systematic review. RSC Adv 2019, 9, 37245–37257. [Google Scholar] [CrossRef]
- Castelli, F.A.; Rosati, G.; Moguet, C.; Fuentes, C.; Marrugo-Ramírez, J.; Lefebvre, T.; Volland, H.; Merkoçi, A.; Simon, S.; Fenaille, F.; et al. Metabolomics for personalized medicine: the input of analytical chemistry from biomarker discovery to point-of-care tests. Anal Bioanal Chem 2022, 414, 759–789. [Google Scholar] [CrossRef]
- Peng, B.; Li, H.; Peng, X.X. Functional metabolomics: from biomarker discovery to metabolome reprogramming. Protein Cell 2015, 6, 628–637. [Google Scholar] [CrossRef]
- Li, K.; Naviaux, J.C.; Bright, A.T.; Wang, L.; Naviaux, R.K. A robust, single-injection method for targeted, broad-spectrum plasma metabolomics. Metabolomics 2017, 13, 122. [Google Scholar] [CrossRef]
- Hosseinkhani, F.; Huang, L.; Dubbelman, A.C.; Guled, F.; Harms, A.C.; Hankemeier, T. Systematic Evaluation of HILIC Stationary Phases for Global Metabolomics of Human Plasma. Metabolites 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.H.; Davidson, M.T.; Narowski, T.M.; Lin, C.T.; Koves, T.R.; Muoio, D.M. Mitochondrial Diagnostics: A Multiplexed Assay Platform for Comprehensive Assessment of Mitochondrial Energy Fluxes. Cell Rep 2018, 24, 3593–3606.e3510. [Google Scholar] [CrossRef] [PubMed]
- Mordaunt, D.; Cox, D.; Fuller, M. Metabolomics to Improve the Diagnostic Efficiency of Inborn Errors of Metabolism. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of new methods. Forensic Sci Int 2007, 165, 216–224. [Google Scholar] [CrossRef]
- Viswanathan, C.T.; Bansal, S.; Booth, B.; DeStefano, A.J.; Rose, M.J.; Sailstad, J.; Shah, V.P.; Skelly, J.P.; Swann, P.G.; Weiner, R. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm Res 2007, 24, 1962–1973. [Google Scholar] [CrossRef]
- Shah, V.P.; Midha, K.K.; Findlay, J.W.; Hill, H.M.; Hulse, J.D.; McGilveray, I.J.; McKay, G.; Miller, K.J.; Patnaik, R.N.; Powell, M.L.; et al. Bioanalytical method validation--a revisit with a decade of progress. Pharm Res 2000, 17, 1551–1557. [Google Scholar] [CrossRef]
- Van Eeckhaut, A.; Lanckmans, K.; Sarre, S.; Smolders, I.; Michotte, Y. Validation of bioanalytical LC-MS/MS assays: evaluation of matrix effects. J Chromatogr B Analyt Technol Biomed Life Sci 2009, 877, 2198–2207. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom Rev 2011, 30, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.T.; Remane, D. Aspects of matrix effects in applications of liquid chromatography-mass spectrometry to forensic and clinical toxicology--a review. Anal Bioanal Chem 2012, 403, 2155–2172. [Google Scholar] [CrossRef] [PubMed]
- Kostiainen, R.; Kauppila, T.J. Effect of eluent on the ionization process in liquid chromatography-mass spectrometry. J Chromatogr A 2009, 1216, 685–699. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Francis, L. Handbook of LC-MS bioanalysis: best practices, experimental protocols, and regulations; 2013. [Google Scholar]
- Jemal, M.; Schuster, A.; Whigan, D.B. Liquid chromatography/tandem mass spectrometry methods for quantitation of mevalonic acid in human plasma and urine: method validation, demonstration of using a surrogate analyte, and demonstration of unacceptable matrix effect in spite of use of a stable isotope analog internal standard. Rapid Commun Mass Spectrom 2003, 17, 1723–1734. [Google Scholar] [CrossRef]
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat Protoc 2010, 5, 1005–1018. [Google Scholar] [CrossRef]
- Dunn, W.B.; Erban, A.; Weber, R.J.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J. Mass appeal: metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 2013, 9, 44–66. [Google Scholar]
- Liu, H.; Ding, L.; Zhang, H.; Mellor, D.; Wu, H.; Zhao, D.; Wu, C.; Lin, Z.; Yuan, J.; Peng, D. The Metabolic Factor Kynurenic Acid of Kynurenine Pathway Predicts Major Depressive Disorder. Front Psychiatry 2018, 9, 552. [Google Scholar] [CrossRef]
- Pan, J.X.; Xia, J.J.; Deng, F.L.; Liang, W.W.; Wu, J.; Yin, B.M.; Dong, M.X.; Chen, J.J.; Ye, F.; Wang, H.Y.; et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: a targeted metabolomics study. Transl Psychiatry 2018, 8, 130. [Google Scholar] [CrossRef]
- Bi, Q.; Zhao, J.; Nie, J.; Huang, F. Metabolic pathway analysis of tumors using stable isotopes. Semin Cancer Biol 2025, 113, 9–24. [Google Scholar] [CrossRef]
- Emwas, A.H.; Szczepski, K.; Al-Younis, I.; Lachowicz, J.I.; Jaremko, M. Fluxomics—New Metabolomics Approaches to Monitor Metabolic Pathways. Front Pharmacol 2022, 13, 805782. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.W.; Lorkiewicz, P.K.; Sellers, K.; Moseley, H.N.; Higashi, R.M.; Lane, A.N. Stable isotope-resolved metabolomics and applications for drug development. Pharmacol Ther 2012, 133, 366–391. [Google Scholar] [CrossRef]
- Liu, K.H.; Nellis, M.; Uppal, K.; Ma, C.; Tran, V.; Liang, Y.; Walker, D.I.; Jones, D.P. Reference Standardization for Quantification and Harmonization of Large-Scale Metabolomics. Anal Chem 2020, 92, 8836–8844. [Google Scholar] [CrossRef]
- Gáspár, R.; Halmi, D.; Demján, V.; Berkecz, R.; Pipicz, M.; Csont, T. Kynurenine Pathway Metabolites as Potential Clinical Biomarkers in Coronary Artery Disease. Front Immunol 2021, 12, 768560. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Wang, Y.N.; Feng, H.Y.; Guo, Z.Y.; Li, X.; Nie, X.L.; Zhao, Y.Y. Host/microbiota interactions-derived tryptophan metabolites modulate oxidative stress and inflammation via aryl hydrocarbon receptor signaling. Free Radic Biol Med 2022, 184, 30–41. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenate and FG9041 have both competitive and non-competitive antagonist actions at excitatory amino acid receptors. Eur J Pharmacol 1988, 151, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Sawa-Wejksza, K.; Parada-Turska, J.; Turski, W. The Pharmacological Evidences for the Involvement of AhR and GPR35 Receptors in Kynurenic Acid-Mediated Cytokine and Chemokine Secretion by THP-1-Derived Macrophages. Molecules 2025, 30. [Google Scholar] [CrossRef] [PubMed]
- Villani, S.; Fallarini, S.; Rezzi, S.J.; Di Martino, R.M.C.; Aprile, S.; Del Grosso, E. Selective inhibition of indoleamine and tryptophan 2,3-dioxygenases: Comparative study on kynurenine pathway in cell lines via LC-MS/MS-based targeted metabolomics. J Pharm Biomed Anal 2024, 237, 115750. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, X.; Li, L. Chemical Isotope Labeling LC-MS for High Coverage and Quantitative Profiling of the Hydroxyl Submetabolome in Metabolomics. Anal Chem 2016, 88, 10617–10623. [Google Scholar] [CrossRef]
- Schönberger, K.; Mitterer, M.; Glaser, K.; Stecher, M.; Hobitz, S.; Schain-Zota, D.; Schuldes, K.; Lämmermann, T.; Rambold, A.S.; Cabezas-Wallscheid, N.; et al. LC-MS-Based Targeted Metabolomics for FACS-Purified Rare Cells. Anal Chem 2023, 95, 4325–4334. [Google Scholar] [CrossRef] [PubMed]
- Nadour, Z.; Simian, C.; Laprévote, O.; Loriot, M.A.; Larabi, I.A.; Pallet, N. Validation of a liquid chromatography coupled to tandem mass spectrometry method for simultaneous quantification of tryptophan and 10 key metabolites of the kynurenine pathway in plasma and urine: Application to a cohort of acute kidney injury patients. Clin Chim Acta 2022, 534, 115–127. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Z.; Jin, L.; Nizhamuding, X.; Zeng, J.; Zhang, T.; Zhang, J.; Wang, J.; Zhao, H.; Zhou, W.; et al. Altered neopterin and IDO in kynurenine metabolism based on LC-MS/MS metabolomics study: Novel therapeutic checkpoints for type 2 diabetes mellitus. Clin Chim Acta 2024, 557, 117859. [Google Scholar] [CrossRef]
- Pang, Z.; Xu, L.; Viau, C.; Lu, Y.; Salavati, R.; Basu, N.; Xia, J. MetaboAnalystR 4.0: a unified LC-MS workflow for global metabolomics. Nat Commun 2024, 15, 3675. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, P.; Qin, S.; Tan, B.; Li, S.; Tang, S.; Liao, C.; Zhang, Y.; Zhang, Z.; Xu, F. Stepwise solid phase extraction integrated with chemical derivatization for all-in-one injection LC-MS/MS analysis of metabolome and lipidome. Anal Chim Acta 2023, 1241, 340807. [Google Scholar] [CrossRef]
- Saigusa, D.; Okamura, Y.; Motoike, I.N.; Katoh, Y.; Kurosawa, Y.; Saijyo, R.; Koshiba, S.; Yasuda, J.; Motohashi, H.; Sugawara, J.; et al. Establishment of Protocols for Global Metabolomics by LC-MS for Biomarker Discovery. PLoS One 2016, 11, e0160555. [Google Scholar] [CrossRef]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: a compensatory mechanism or a driving force? Trends Mol Med 2021, 27, 946–954. [Google Scholar] [CrossRef]
- Braidy, N.; Berg, J.; Clement, J.; Khorshidi, F.; Poljak, A.; Jayasena, T.; Grant, R.; Sachdev, P. Role of Nicotinamide Adenine Dinucleotide and Related Precursors as Therapeutic Targets for Age-Related Degenerative Diseases: Rationale, Biochemistry, Pharmacokinetics, and Outcomes. Antioxid Redox Signal 2019, 30, 251–294. [Google Scholar] [CrossRef]
- Wee, H.N.; Liu, J.J.; Ching, J.; Kovalik, J.P.; Lim, S.C. The Kynurenine Pathway in Acute Kidney Injury and Chronic Kidney Disease. Am J Nephrol 2021, 52, 771–787. [Google Scholar] [CrossRef]
- Zhai, Y.; Chavez, J.A.; D’Aquino, K.E.; Meng, R.; Nawrocki, A.R.; Pocai, A.; Wang, L.; Ma, L.J. Kynurenine 3-monooxygenase limits de novo NAD(+) synthesis through dietary tryptophan in renal proximal tubule epithelial cell models. Am J Physiol Cell Physiol 2024, 326, C1423–c1436. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, Tryptophan Metabolism, and Kynurenine Pathway: A Complex Interconnected Loop Influencing Human Health Status. Int J Tryptophan Res 2019, 12, 1178646919852996. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Raftery, D. Five Easy Metrics of Data Quality for LC-MS-Based Global Metabolomics. Anal Chem 2020, 92, 12925–12933. [Google Scholar] [CrossRef] [PubMed]
- Konakchieva, R.; Mladenov, M.; Konaktchieva, M.; Sazdova, I.; Gagov, H.; Nikolaev, G. Circadian Clock Deregulation and Metabolic Reprogramming: A System Biology Approach to Tissue-Specific Redox Signaling and Disease Development. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Froy, O.; Weintraub, Y. The circadian clock, metabolism, and inflammation-the holy trinity of inflammatory bowel diseases. Clin Sci (Lond) 2025, 139, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Moaddel, R.; Candia, J.; Ubaida-Mohien, C.; Tanaka, T.; Moore, A.Z.; Zhu, M.; Fantoni, G.; Church, S.; D’Agostino, J.; Fan, J.; et al. Healthy Aging Metabolomic and Proteomic Signatures Across Multiple Physiological Compartments. Aging Cell 2025, 24, e70014. [Google Scholar] [CrossRef]
- Overgaard, N.H.; Fan, T.M.; Schachtschneider, K.M.; Principe, D.R.; Schook, L.B.; Jungersen, G. Of Mice, Dogs, Pigs, and Men: Choosing the Appropriate Model for Immuno-Oncology Research. Ilar j 2018, 59, 247–262. [Google Scholar] [CrossRef]
- Guerrero-López, P.; Martín-Pardillos, A.; Bonet-Aleta, J.; Mosseri, A.; Hueso, J.L.; Santamaria, J.; Garcia-Aznar, J.M. 2D versus 3D tumor-on-chip models to study the impact of tumor organization on metabolic patterns in vitro. Sci Rep 2025, 15, 19506. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Ji, F.; Chen, Y.; Cai, Z. statTarget: A streamlined tool for signal drift correction and interpretations of quantitative mass spectrometry-based omics data. Anal Chim Acta 2018, 1036, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pedraz-Petrozzi, B.; Marszalek-Grabska, M.; Kozub, A.; Szalaj, K.; Trzpil, A.; Stachniuk, A.; Lamadé, E.K.; Gilles, M.; Deuschle, M.; Turski, W.A.; et al. LC-MS/MS-based quantification of tryptophan, kynurenine, and kynurenic acid in human placental, fetal membranes, and umbilical cord samples. Sci Rep 2023, 13, 12554. [Google Scholar] [CrossRef]
- Wang, G.; Heijs, B.; Kostidis, S.; Mahfouz, A.; Rietjens, R.G.J.; Bijkerk, R.; Koudijs, A.; van der Pluijm, L.A.K.; van den Berg, C.W.; Dumas, S.J.; et al. Analyzing cell-type-specific dynamics of metabolism in kidney repair. Nat Metab 2022, 4, 1109–1118. [Google Scholar] [CrossRef]
- Buergel, T.; Steinfeldt, J.; Ruyoga, G.; Pietzner, M.; Bizzarri, D.; Vojinovic, D.; Upmeier Zu Belzen, J.; Loock, L.; Kittner, P.; Christmann, L.; et al. Metabolomic profiles predict individual multidisease outcomes. Nat Med 2022, 28, 2309–2320. [Google Scholar] [CrossRef]
- Golubova, A.; Lanekoff, I. Quantitative approaches for spatial metabolomics with isomer differentiation using surface sampling capillary electrophoresis mass spectrometry. Talanta 2026, 296, 128482. [Google Scholar] [CrossRef]
- van der Walt, G.; Louw, R. Novel mitochondrial and cytosolic purification pipeline for compartment-specific metabolomics in mammalian disease model tissues. Metabolomics 2020, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fu, W.; Huo, M.; He, B.; Liu, Y.; Tian, L.; Li, W.; Zhou, Z.; Wang, B.; Xia, J.; et al. Spatial-resolved metabolomics reveals tissue-specific metabolic reprogramming in diabetic nephropathy by using mass spectrometry imaging. Acta Pharm Sin B 2021, 11, 3665–3677. [Google Scholar] [CrossRef]
- Knitsch, R.; AlWahsh, M.; Raschke, H.; Lambert, J.; Hergenröder, R. In Vitro Spatio-Temporal NMR Metabolomics of Living 3D Cell Models. Anal Chem 2021, 93, 13485–13494. [Google Scholar] [CrossRef]
- van der Walt, G.; Lindeque, J.Z.; Mason, S.; Louw, R. Sub-Cellular Metabolomics Contributes Mitochondria-Specific Metabolic Insights to a Mouse Model of Leigh Syndrome. Metabolites 2021, 11. [Google Scholar] [CrossRef]
- Qin, S.; Gao, M.; Zhang, Q.; Xiao, Q.; Fu, J.; Tian, Y.; Jiao, Y.; Zhang, Z.; Zhang, P.; Xu, F. High-Coverage Strategy for Multi-Subcellular Metabolome Analysis Using Dansyl-Labeling-Based LC-MS/MS. Anal Chem 2023, 95, 10034–10043. [Google Scholar] [CrossRef] [PubMed]
- More, T.H.; Hiller, K. Complexity of subcellular metabolism: strategies for compartment-specific profiling. Curr Opin Biotechnol 2022, 75, 102711. [Google Scholar] [CrossRef]
- Talmor-Barkan, Y.; Bar, N.; Shaul, A.A.; Shahaf, N.; Godneva, A.; Bussi, Y.; Lotan-Pompan, M.; Weinberger, A.; Shechter, A.; Chezar-Azerrad, C.; et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat Med 2022, 28, 295–302. [Google Scholar] [CrossRef]
- Geier, B.; Sogin, E.M.; Michellod, D.; Janda, M.; Kompauer, M.; Spengler, B.; Dubilier, N.; Liebeke, M. Spatial metabolomics of in situ host-microbe interactions at the micrometre scale. Nat Microbiol 2020, 5, 498–510. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, N.; Mai, Y.; Ren, L.; Chen, Q.; Cao, Z.; Chen, Q.; Liu, Y.; Hou, W.; Yang, J.; et al. Correcting batch effects in large-scale multiomics studies using a reference-material-based ratio method. Genome Biol 2023, 24, 201. [Google Scholar] [CrossRef]
- Viant, M.R.; Ebbels, T.M.D.; Beger, R.D.; Ekman, D.R.; Epps, D.J.T.; Kamp, H.; Leonards, P.E.G.; Loizou, G.D.; MacRae, J.I.; van Ravenzwaay, B.; et al. Use cases, best practice and reporting standards for metabolomics in regulatory toxicology. Nat Commun 2019, 10, 3041. [Google Scholar] [CrossRef] [PubMed]
- Nian, M.; Chen, X.; Fang, M. Addressing Pitfalls of Metabolomics for Toxicology: A Call for Standardization, Reproducibility and Data Sharing. Chem Res Toxicol 2025, 38, 1150–1156. [Google Scholar] [CrossRef]
- Rampler, E.; Abiead, Y.E.; Schoeny, H.; Rusz, M.; Hildebrand, F.; Fitz, V.; Koellensperger, G. Recurrent Topics in Mass Spectrometry-Based Metabolomics and Lipidomics-Standardization, Coverage, and Throughput. Anal Chem 2021, 93, 519–545. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.; Ptolemy, A.S.; Pickett, S.; Kellogg, M.D.; Peake, R.W.A. Untargeted Metabolomics for Inborn Errors of Metabolism: Development and Evaluation of a Sustainable Reference Material for Correcting Inter-Batch Variability. Clin Chem 2024, 70, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Long, N.P.; Nghi, T.D.; Kang, Y.P.; Anh, N.H.; Kim, H.M.; Park, S.K.; Kwon, S.W. Toward a Standardized Strategy of Clinical Metabolomics for the Advancement of Precision Medicine. Metabolites 2020, 10. [Google Scholar] [CrossRef]
- Malinowska, J.M.; Whelan, M. The important role of standards for the uptake of transcriptomics and metabolomics based in vitro methods in regulatory toxicology. Arch Toxicol 2025, 99, 3865–3875. [Google Scholar] [CrossRef]
- Liu, J. Computational aspects of psychometric methods with R by Patricia Martinková and Adéla Hladká ISBN: 9781003054313, https://doi.org/10.1201/9781003054313. In Biometrics; Chapman & Hall/CRC, 2023. [Google Scholar] [CrossRef]
- Harada, S.; Iida, M.; Miyagawa, N.; Hirata, A.; Kuwabara, K.; Matsumoto, M.; Okamura, T.; Edagawa, S.; Kawada, Y.; Miyake, A.; et al. Study Profile of the Tsuruoka Metabolomics Cohort Study (TMCS). J Epidemiol 2024, 34, 393–401. [Google Scholar] [CrossRef]
- Sturm, G.; Monzel, A.S.; Karan, K.R.; Michelson, J.; Ware, S.A.; Cardenas, A.; Lin, J.; Bris, C.; Santhanam, B.; Murphy, M.P.; et al. A multi-omics longitudinal aging dataset in primary human fibroblasts with mitochondrial perturbations. Sci Data 2022, 9, 751. [Google Scholar] [CrossRef]
- Dukovski, I.; Bajić, D.; Chacón, J.M.; Quintin, M.; Vila, J.C.C.; Sulheim, S.; Pacheco, A.R.; Bernstein, D.B.; Riehl, W.J.; Korolev, K.S.; et al. A metabolic modeling platform for the computation of microbial ecosystems in time and space (COMETS). Nat Protoc 2021, 16, 5030–5082. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jin, J.; Xue, Z.; Zhao, J.; Cai, W.; Zhang, W. Integrated multi-omics analysis and machine learning developed a prognostic model based on mitochondrial function in a large multicenter cohort for Gastric Cancer. J Transl Med 2024, 22, 381. [Google Scholar] [CrossRef]
- Weiss, E.; de la Peña-Ramirez, C.; Aguilar, F.; Lozano, J.J.; Sánchez-Garrido, C.; Sierra, P.; Martin, P.I.; Diaz, J.M.; Fenaille, F.; Castelli, F.A.; et al. Sympathetic nervous activation, mitochondrial dysfunction and outcome in acutely decompensated cirrhosis: the metabolomic prognostic models (CLIF-C MET). Gut 2023, 72, 1581–1591. [Google Scholar] [CrossRef]
- Xu, Y.; Ritchie, S.C.; Liang, Y.; Timmers, P.; Pietzner, M.; Lannelongue, L.; Lambert, S.A.; Tahir, U.A.; May-Wilson, S.; Foguet, C.; et al. An atlas of genetic scores to predict multi-omic traits. Nature 2023, 616, 123–131. [Google Scholar] [CrossRef]
- Alston, C.L.; Stenton, S.L.; Hudson, G.; Prokisch, H.; Taylor, R.W. The genetics of mitochondrial disease: dissecting mitochondrial pathology using multi-omic pipelines. J Pathol 2021, 254, 430–442. [Google Scholar] [CrossRef]
- Lane, A.N.; Tan, J.; Wang, Y.; Yan, J.; Higashi, R.M.; Fan, T.W. Probing the metabolic phenotype of breast cancer cells by multiple tracer stable isotope resolved metabolomics. Metab Eng 2017, 43, 125–136. [Google Scholar] [CrossRef]
- Roci, I.; Gallart-Ayala, H.; Schmidt, A.; Watrous, J.; Jain, M.; Wheelock, C.E.; Nilsson, R. Metabolite Profiling and Stable Isotope Tracing in Sorted Subpopulations of Mammalian Cells. Anal Chem 2016, 88, 2707–2713. [Google Scholar] [CrossRef]
- Timón-Gómez, A.; Doerrier, C.; Sumbalová, Z.; Garcia-Souza, L.F.; Baglivo, E.; Cardoso, L.H.D.; Gnaiger, E. Bioenergetic profiles and respiratory control in mitochondrial physiology: Precision analysis of oxidative phosphorylation. Exp Physiol 2025. [Google Scholar] [CrossRef]
- Seifert, J.; Taubert, M.; Jehmlich, N.; Schmidt, F.; Völker, U.; Vogt, C.; Richnow, H.H.; von Bergen, M. Protein-based stable isotope probing (protein-SIP) in functional metaproteomics. Mass Spectrom Rev 2012, 31, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.; Laskowski, L.J.; Pallais, J.P.; Bock, H.A.; Cavalco, N.G.; Anderson, E.I.; Calkins, M.M.; Razzoli, M.; Sham, Y.Y.; McCorvy, J.D.; et al. Functional profiling of the G protein-coupled receptor C3aR1 reveals ligand-mediated biased agonism. J Biol Chem 2024, 300, 105549. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 2001, 21, 7463–7473. [Google Scholar] [CrossRef] [PubMed]
- Sapko, M.T.; Guidetti, P.; Yu, P.; Tagle, D.A.; Pellicciari, R.; Schwarcz, R. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: Implications for Huntington’s disease. Exp Neurol 2006, 197, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bock, A.; Bermudez, M. Allosteric coupling and biased agonism in G protein-coupled receptors. 2021, 288, 2513–2528. [Google Scholar] [CrossRef]
- Cadenas, S. Mitochondria rescue cells from ischemic injury. Science 2022, 377, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem Rev 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Faccenda, D.; Campanella, M. Pharmacological advances in mitochondrial therapy. EBioMedicine 2021, 65, 103244. [Google Scholar] [CrossRef]
- Stone, T.W.; Williams, R.O. Tryptophan metabolism as a ‘reflex’ feature of neuroimmune communication: Sensor and effector functions for the indoleamine-2, 3-dioxygenase kynurenine pathway. J Neurochem 2024, 168, 3333–3357. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





