1. Introduction
Cellulite, clinically defined as edematous fibrosclerotic panniculopathy, is characterized by dimpling and irregularities of the skin surface resembling an “orange peel.” It predominantly affects women, with prevalence estimates of 80–98% among postpubertal females, reflecting its substantial aesthetic and psychological burden [
1]. Beyond cosmetic implications, cellulite is associated with diminished self-esteem and reduced quality of life [
2,
3].
Its pathophysiology is multifactorial, encompassing microcirculatory alterations, adipocyte hypertrophy, structural remodeling of fibrous septa, and changes in adipose tissue architecture [
4,
5,
6]. Hormonal factors further contribute to subcutaneous tissue remodeling and fibrosis [
1,
6]. Additional findings include increased skin thickness, dermal thinning, reduced dermal density [
4], local fat accumulation, and adipocyte hypertrophy [
5,
7]. These insights have guided the development of therapeutic strategies targeting microcirculatory dysfunction, fibrous septal tension, and localized adiposity [
8,
9,
10].
Traditionally, cellulite assessment relies on subjective visual scales and photographic analyses, which are limited by diagnostic imprecision and considerable inter-observer variability [
11,
12]. Concordance among evaluators is often poor [
13], underscoring the need for objective and reproducible diagnostic tools [
14,
15]. Anthropometric indices such as BMI or limb circumference show weak correlations with cellulite severity [
16]. Advances in imaging now enable detailed visualization of subcutaneous structures and provide quantitative, reproducible parameters that support diagnostic accuracy and individualized treatment planning, while reducing a operator-dependent assessment variability [
11,
17,
18].
Over the years, ultrasound has emerged as a non-invasive and accessible imaging modality capable of providing real-time visualization of adipose tissue, fibrous septa, edema, and dermal alterations [
3,
4,
19,
20,
21] Its ability to characterize these structures allows meaningful correlations between echogenicity patterns and cellulite severity [
16], positioning ultrasound as a more objective and reproducible alternative to traditional visual grading scales [
22]. Beyond enhancing diagnostic accuracy, ultrasound also facilitates the classification of morphological subtypes and enriches the information provided by established grading systems such as the Nürnberger–Müller scale [
15,
17]. This alignment supports more individualized therapeutic planning [
3] and enables consistent, reliable monitoring of treatment responses over time [
23]. Building on these strengths, ultrasound is increasingly employed to evaluate outcomes across diverse therapeutic modalities, including radiofrequency [
10,
24,
25], collagenase injections [
24], shockwave therapy [
26,
27], and combined treatment approaches [
28]. Its capacity to quantitatively assess changes in adipose thickness, fibrous septal architecture, and tissue edema provides clinicians with objective, reproducible metrics for evaluating therapeutic effects and guiding longitudinal management.
Despite its potential and its growing use in clinical research, the literature on the broad clinical application of ultrasound in cellulite remains heterogeneous. The primary aim of this systematic review was to evaluate the role of ultrasound, particularly ultrasound, in the diagnosis and treatment monitoring of cellulite. Specifically, we sought to: (1) summarize the diagnostic accuracy and reliability of ultrasound for grading and characterizing cellulite; (2) describe the contribution of quantitative ultrasound parameters to the assessment and monitoring of treatment effects; (3) examine the relationships between ultrasound findings and established clinical or photographic grading scales; and (4) identify methodological limitations and gaps in the evidence to inform future research and support the standardization of ultrasound use in aesthetic and dermatologic practice.
2. Materials and Methods
This systematic review was conducted according to PRISMA statement 2020 [
29] and registered in the database PROSPERO (CRD420251185486).
The components of the PICO question were: (population) adult individuals (≥18 years old) with clinically diagnosed cellulite of any severity and anatomical location; (intervention) ultrasound-based assessment using any modality, including B-mode, high-frequency ultrasound, Doppler, or elastography, applied for diagnostic evaluation, morphological characterization, severity grading, or treatment monitoring; (comparator) clinical grading scales, photographic assessment, alternative imaging techniques such as MRI, or anthropometric measures when available; (outcome) diagnostic performance measures, quantitative ultrasound parameters or inter- and intra-observer agreement.
2.1. Inclusion and Exclusion Criteria
We included studies enrolling adults (≥18 years) with clinically diagnosed cellulite, irrespective of anatomical location or severity grade. Eligible studies assessed ultrasound-based techniques for diagnostic evaluation, characterization of tissue structure, grading of cellulite severity, or monitoring of treatment response. Studies in which ultrasound was used exclusively to measure changes following a therapeutic intervention were included only when the ultrasound parameters were relevant to the structural characterization of cellulite (e.g., dermal thickness, subcutaneous architecture, adipose protrusions). Studies evaluating only circumferential or surface-level cosmetic outcomes without structural ultrasound assessment were excluded. Both randomized and non-randomized designs were eligible, including randomized controlled trials, cohort studies, case–control studies, and cross-sectional studies. Exclusion criteria encompassed studies involving minors, animal models, or dermatologic conditions unrelated to cellulite. Case reports, editorials, narrative reviews, and conference abstracts were also excluded.
2.2. Information Sources
The following electronic databases were searched without language or date restrictions: PubMed (last searched: 14 November 2025), Scopus (last searched: 14 November 2025), and CENTRAL – Cochrane Central Register of Controlled Trials (last searched: 14 November 2025). Additional sources included backward and forward citation searching of all included studies, manual screening of reference lists, and consultation of study registries for potentially relevant trials. Only published peer-reviewed studies were considered.
2.3. Search Strategy
A systematic search for relevant studies was conducted in all cited databases from September to November 2025 without time or language restrictions using the following search strategy: (cellulite OR panniculopathy OR (edematous AND fibrosclerotic AND panniculopathy) OR gynoid lipodystrophy) AND (ultrasound OR ultrasonography OR high-frequency ultrasound OR B-mode OR Doppler OR elastography). No filters or limits were applied other than the exclusion of non-human studies.
2.4. Selection Process
The full list of articles retrieved from the systematic search was uploaded to Rayyan (
https://www.rayyan.ai) and screened independently by two reviewers. After removal of duplicates, titles and abstracts were assessed using predefined eligibility criteria. Potentially relevant studies were subsequently evaluated in full text by the same two independent reviewers. Any discrepancies arising during either the title/abstract screening or full-text assessment were resolved through discussion, and consultation with a third external reviewer was planned if consensus could not be reached. No automation tools beyond Rayyan’s organizational interface were used.
2.5. Data Collection Process
Data extraction was performed independently by the two reviewers using standardized and piloted extraction forms to ensure consistency and accuracy. Prior to full extraction, the reviewers completed a calibration exercise on a subset of studies to harmonize data interpretation and reduce discrepancies. Extracted information included study characteristics (authors, year, country, design, sample size), participant demographics (age, sex, eligibility criteria), cellulite severity, and detailed descriptions of the ultrasound methodology (device type, probe frequency, imaging settings, acquisition protocol, and measurement procedures). Outcomes collected encompassed diagnostic performance measures, type of quantitative ultrasound parameters such as dermal and subcutaneous thickness, echogenicity patterns, configuration of fibrous septa, and presence of edema, as well as inter- and intra-observer reliability metrics. No study authors were contacted for additional information because all included studies reported sufficient methodological and outcome information to enable qualitative synthesis. Any discrepancies between reviewers were resolved through discussion until consensus was reached.
2.6. Outcomes and Additional Data
The following outcomes were sought from each study: (1) Diagnostic performance of ultrasound; (2) Type of quantitative ultrasound parameters (dermal thickness, subcutaneous fat thickness, echogenicity, septa visualization, tissue homogeneity); (3) Reproducibility outcomes (inter- and intra-observer agreement). All available results compatible with these domains were extracted, regardless of measurement scale, time point, or analytic approach.
2.7. Risk of Bias Assessment
The risk of bias was assessed independently by two reviewers using Newcastle–Ottawa Scale (NOS) for observational studies and ROBIN-I for interventistic studies. Any disagreements were resolved by consensus. No automation tools were used.
2.8. Synthesis Methods
The included studies were presented in chronological order to illustrate the historical progression of ultrasound applications in cellulite assessment and treatment monitoring. Subsequently, key findings were synthesized across diagnostic and treatment-monitoring domains to provide an integrated overview of the evidence. Since this review was conducted as a qualitative systematic synthesis without performing a meta-analysis, no statistical harmonization or quantitative pooling was required. Findings were therefore integrated through a structured narrative approach, supported by detailed tables describing study characteristics, ultrasound parameters, and treatment-related tissue changes. Heterogeneity across studies was examined qualitatively. Particular attention was given to variations in ultrasound methodology, cellulite severity, participant characteristics, and methodological rigor.
3. Results
3.1. Study Selection
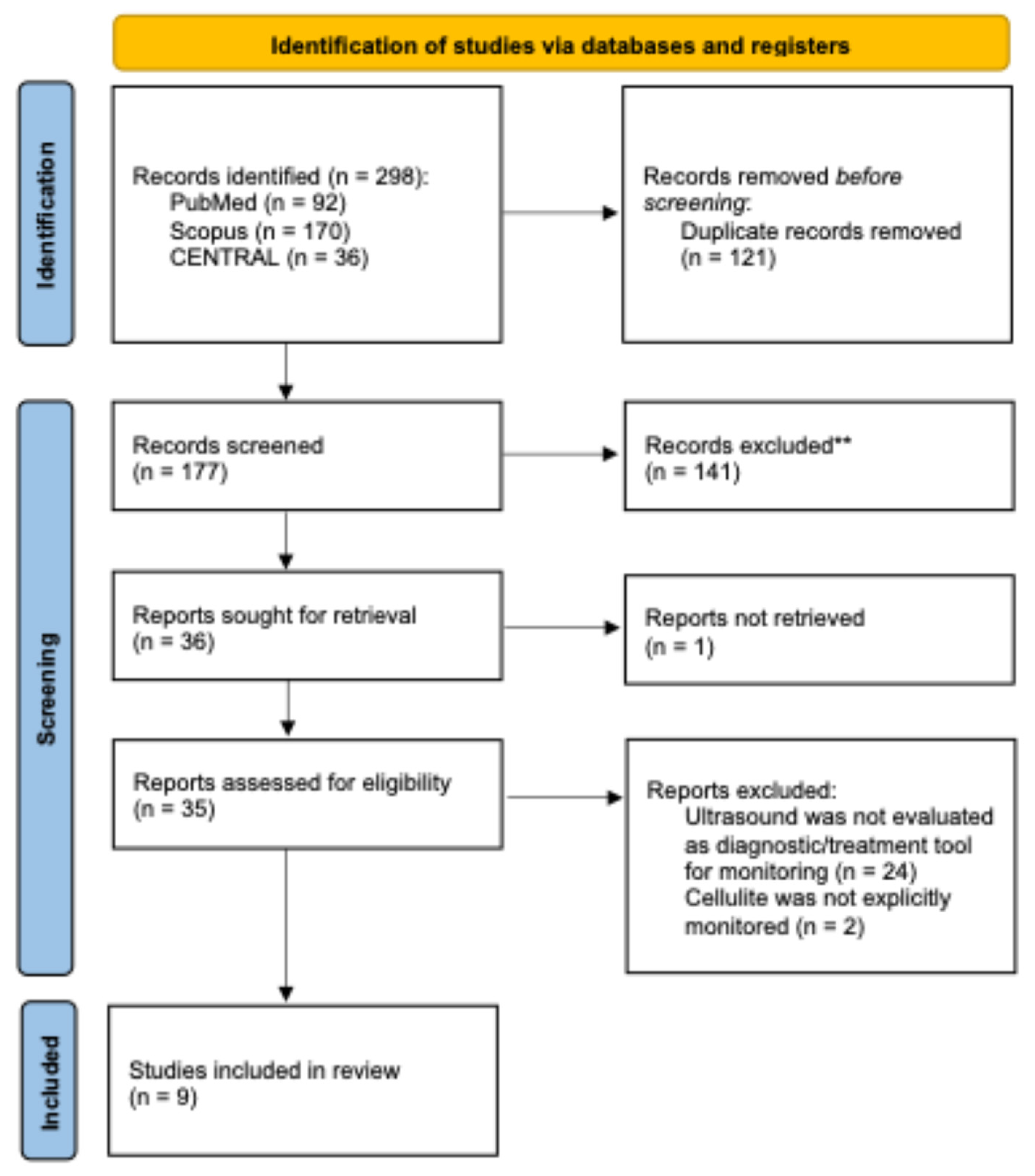
A total of 298 records were identified through database searching, including 92 from PubMed, 170 from Scopus, and 36 from CENTRAL, no additional reports were identified through grey literature and citation chain. After removal of 121 duplicate records, 177 unique records underwent title and abstract screening. Of these, 141 records were excluded as they did not meet the inclusion criteria. A total of 36 full-text reports were sought for retrieval, of which one could not be obtained. The remaining 35 full-text articles were assessed for eligibility. Following full-text evaluation, 26 reports were excluded, primarily because ultrasound was not evaluated as a diagnostic or monitoring tool (n = 24) or because cellulite was not explicitly investigated (n = 2). Ultimately, nine studies met all predefined inclusion criteria and were included in the qualitative synthesis. The complete study selection workflow is illustrated in the
Figure 1.
3.2. Study Characteristics
The nine studies included in this review were conducted between 2008 and 2025 (
Table 1). Early investigations focused primarily on the ability of ultrasound to delineate dermal–subcutaneous interface irregularities, while subsequent studies expanded the application of ultrasound to the monitoring of tissue changes following cosmetic or pharmacological interventions. More recent contributions further refined the use of ultrasound by integrating elastography, Doppler assessment, and more sophisticated morphological classifications.
Sample sizes ranged from 26 participants [
12] to 150 [
17]. All studies enrolled adult women with clinically confirmed cellulite, typically located on the buttocks, posterior thighs, or lateral thighs. Four studies evaluated the effects of topical agents [
18,
30,
31], mechanical treatments or injectable therapies [
32]. Five studies used observational or diagnostic designs [
12,
15,
17,
33,
34], with the most recent developing a structured ultrasound-based classification for stage III cellulite.
Across all studies, high-frequency ultrasound was used to quantify structural skin and subcutaneous alterations associated with cellulite severity or treatment response. Frequencies ranged from 12 MHz to 35 MHz, with several investigations employing 20 MHz HFUS systems or 18 MHz linear radiologic probes. Three studies incorporated 3D ultrasound or elastography to enhance structural characterization. Standardization of the acquisition process varied across studies, although only two investigations provided detailed methodological controls.
Clinical comparators were similarly heterogeneous. The Nürnberger–Müller scale was used in several studies, whereas photonumeric clinical grading scales were adopted in two studies. Additional comparators included thigh circumference measurements, bioimpedance analysis, dermatologist-rated photographic scales, and elasticity assessments.
3.3. Risk of Bias
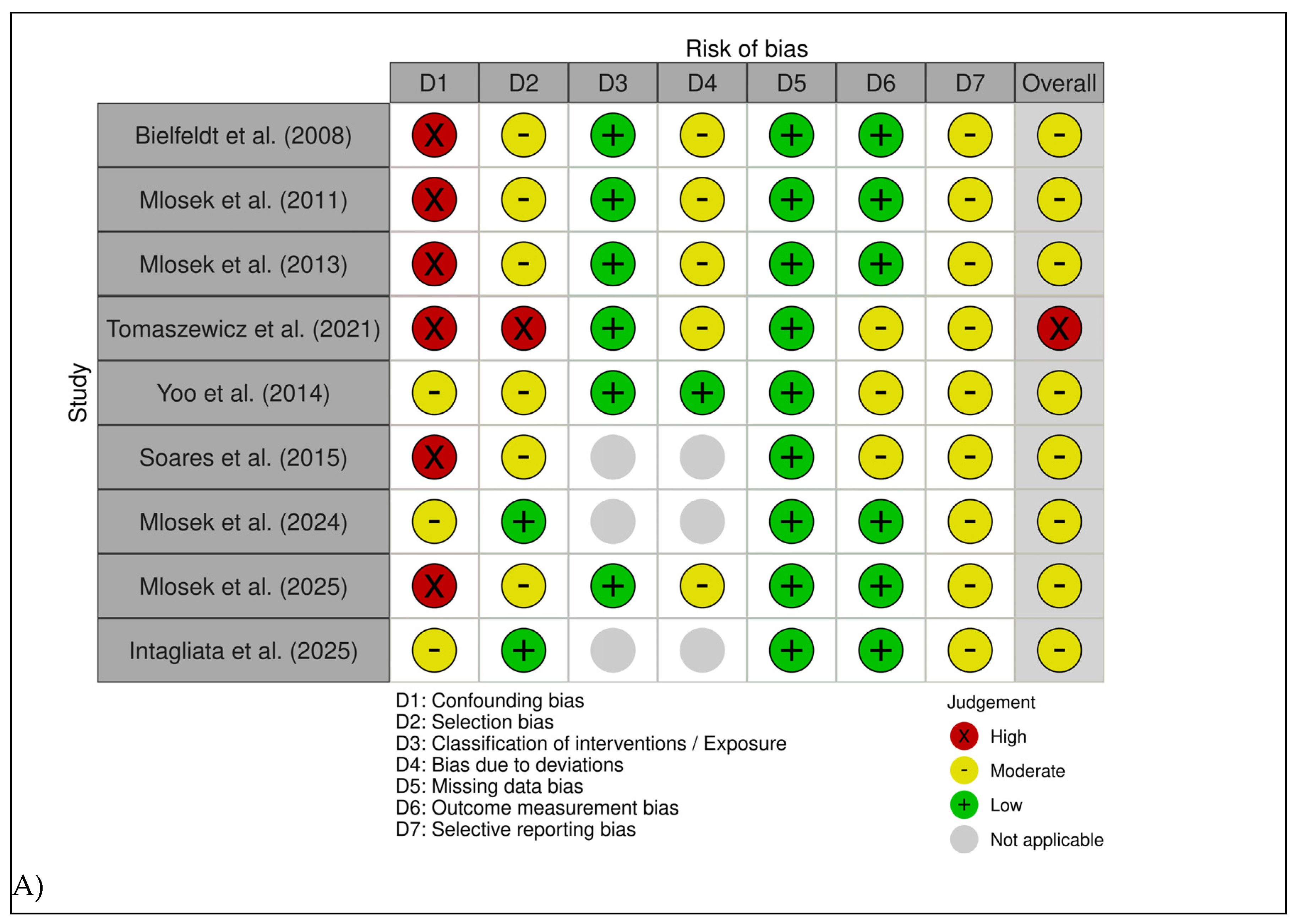
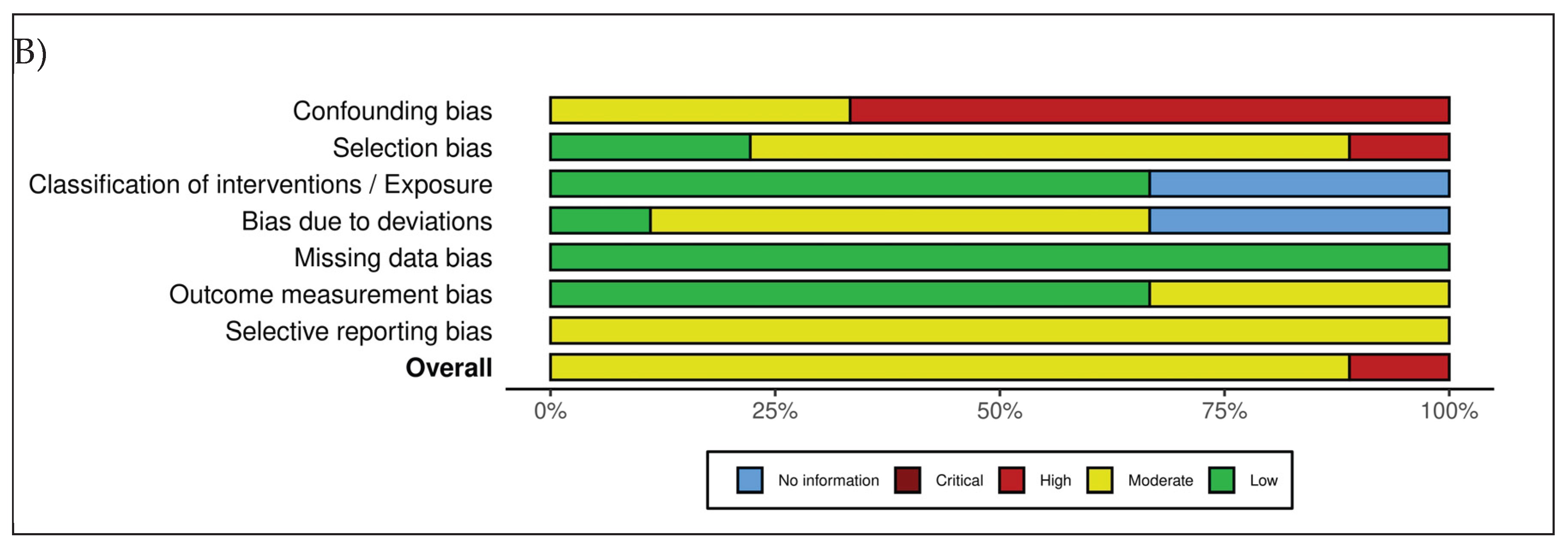
As summarized in
Figure 2, the overall methodological quality of the included studies was heterogeneous, with all investigations judged as having at least a moderate risk of bias, and several presenting moderate risk of bias in specific domains. The primary sources of bias were related to the insufficient control of confounding variables and limited blinding of outcome assessors.
Six studies [
18,
30,
31,
32,
33,
34] were assessed using the ROBINS-I tool, yielding overall judgments ranging from moderate to high risk of bias. Confounding emerged as the main methodological limitation: none of the studies implemented statistical adjustment for key covariates such as age, BMI, hormonal status, baseline cellulite severity, or lifestyle factors. Selection bias was rated as moderate to serious in most cases, primarily due to the recruitment of highly motivated volunteers from aesthetic or dermatologic settings.
Across the included studies, classification of interventions was consistently judged at low risk of bias, supported by the clear and standardized nature of the treatment procedures. Deviations from intended interventions generally presented a moderate risk, largely attributable to limited monitoring of adherence to topical regimens and variable procedural standardization. All studies demonstrated a low risk of bias due to missing data, with high participant retention rates. For outcome measurement, risk was typically moderate because blinding of ultrasonography assessors was rarely implemented or insufficiently reported; nevertheless, the reliance on objective ultrasound parameters partially mitigated this concern. Selective reporting was also judged as moderate, given the absence of preregistered protocols and the heterogeneous depth of reporting across multiple outcomes.
Three additional observational studies [
12,
15,
17] were evaluated using the Newcastle–Ottawa Scale. Soares 2015 showed a moderate risk of bias (5/10), with adequate standardization of imaging procedures but limited control of confounding and a small sample size. Mlosek 2024 also demonstrated moderate risk (6/10), characterized by clear eligibility criteria but the absence of multivariable adjustment, leading to residual confounding. Intagliata 2025 exhibited the lowest risk among the observational studies (7/9), supported by well-defined inclusion criteria, blinded clinical classification, and consistent ultrasound acquisition; however, some residual confounding could not be ruled out.
3.4. Results of Individual Studies
3.4.1. Diagnostic Applications of Ultrasound
Five studies investigated the diagnostic applications of ultrasound, encompassing a total of 412 participants (
Table 2). In 2008, Bielfeldt and colleagues[
33] employed 20-MHz B-mode imaging combined with a 22-MHz 3D applicator to characterize irregularities at the dermis–subcutis interface in 70 women with varying degrees of cellulite, with the aim of standardizing non-invasive methods for evaluating anti-cellulite treatments. The primary ultrasound-derived metric, the roughness index (Ra
m), showed a moderate correlation with clinical severity (r ≈ 0.64; R² ≈ 0.41) when compared with the Smalls 0–9 photonumeric scale. Additional features, such as borderline length and the depth of adipose protrusions, increased progressively with higher cellulite grades, indicating that high-frequency ultrasound can reliably capture structural abnormalities corresponding to clinically visible changes.
In 2014, Yoo et al.[
34] conducted a two-phase validation study assessing dermo–subcutaneous interface length, subcutaneous thickness, and dermal thickness with the objective of identifying quantitative parameters that closely reflect visual evaluation in cosmetic anti-cellulite research. In the cross-sectional phase, subcutaneous thickness demonstrated a moderate correlation with clinical scores (r = 0.502), whereas interface length showed a weaker association (r = 0.355). These relationships persisted after a 6-week intervention, with r ≈ 0.311 for subcutaneous thickness and r ≈ 0.275 for interface length. All assessments were performed under tightly standardized environmental conditions (22 ± 2 °C; 45–55% humidity), strengthening the reliability of the measurements.
In 2015, Soares et al.[
12] applied 20-MHz high-frequency ultrasound in 26 women with grade I–III cellulite to compare ultrasound-derived measures with standardized photographic grading. Interface length at the dermo–hypodermal border increased with clinical severity, while dermal echogenicity decreased, suggesting progressive tissue disorganization. Both fat-herniation depth and BMI correlated positively with clinical grade. Although correlations between ultrasound parameters and cellulite severity were generally modest, high-frequency ultrasound consistently identified microstructural alterations not always detectable on surface inspection.
In 2024, Mlosek and Malinowska[
15] conducted a large cross-sectional study involving 114 women, using an 18-MHz linear probe and a skin-dedicated scanner operating at 20–100 MHz to determine whether high-frequency ultrasound can assist in cellulite assessment and reliably correlate with clinical scores. Several ultrasound parameters showed strong associations with clinical severity: subcutaneous thickness (r = 0.63), the surface area of fat protrusions (r = 0.64), the elastographic strain ratio (r = 0.51), and thigh circumference with subcutaneous thickness (r = 0.48). These represent some of the strongest imaging–clinical correlations reported to date and support ultrasound as a robust tool for objective grading.
Most recently, Intagliata et al. (2025)[
17] used 20-MHz ultrasound to evaluate stage III cellulite in 150 Italian women with the aim of developing and validating an ultrasound-based subclassification system. Parameters assessed included superficial and deep fat thickness, fibrous septa thickness and density, edema, and vascularity on color Doppler. Ultrasound-based classification achieved 79.2% agreement with clinical staging (Gwet’s AC1 = 0.444; Cohen’s κ = 0.286; Krippendorff’s α = 0.203), indicating moderate concordance. The authors identified a superficial-fat threshold of approximately 7 mm as a potential discriminator between ultrasound phenotypes 3A and 3B and described a mixed phenotype not detectable by clinical examination alone.
3.4.2. Ultrasound for Treatment Monitoring
Four studies, including a total of 373 participants, evaluated the usefulness of ultrasound for monitoring treatment response (
Table 3). In 2011, Mlosek et al.[
30] examined 61 women using both 35-MHz high-frequency ultrasound and 18-MHz conventional ultrasound to assess the effects of an anti-cellulite cream compared with placebo. Ultrasound imaging revealed clear structural improvements in the active-treatment group, including reductions in subcutaneous tissue thickness, shortening and decreased surface area of hypodermal fascicles, and a measurable reduction in edema. No meaningful ultrasonographic changes were observed in placebo recipients. Although numerical correlations with clinical scales were not reported, the ultrasound-detected modifications closely paralleled improvements observed during palpatory assessment.
In a subsequent study in 2013, Mlosek et al.[
18] investigated 84 women undergoing topical, oral, or combined treatments for cellulite over a 30-day period, using a 35-MHz mechanical high-frequency probe for all assessments. Treatment produced consistent improvements across several architectural parameters: epidermal thickness decreased from 0.16 to 0.14 mm, dermal thickness from 1.68 to 1.41 mm, and the mean length of subcutaneous septa projecting into the dermis from 0.83 to 0.48 mm. The area of fat protrusions declined from 0.82 to 0.45 mm², the edema index from 0.77 to 0.55, and thigh circumference from 56.89 to 54.81 cm. Clinical severity, assessed using the Nürnberger–Müller scale, improved from 2.89 to 1.36. Taken together, these findings confirmed that high-frequency ultrasound is sensitive to treatment-induced microstructural remodeling and that ultrasound changes closely mirror improvements in clinical severity.
Tomaszewicz et al. (2021)[
31] evaluated 144 women using a combined approach that included 12-MHz ultrasound, high-frequency ultrasound, and strain elastography. Measurements included epidermal and dermal thickness, the length of subcutaneous bands projecting into the dermis, and subcutaneous tissue elasticity. The main quantitative findings consisted of a decrease in elasticity of 0.05 units (p = 0.032) and a reduction in thigh circumference of −0.76 cm (p < 0.001) following one month of topical anti-cellulite product use. Although the absence of a control group limits causal inference, high-frequency ultrasound clearly detected structural changes consistent with clinical improvement.
Finally, in 2025, Mlosek and Malinowska[
32] conducted a comparative study of 84 women undergoing ALIDYA® mesotherapy, body wraps, or Endermologie®. High-frequency ultrasound performed before treatment and 14–18 days after completion demonstrated significant reductions in subcutaneous thickness, echogenicity, and the surface area of fat protrusions across all treatment groups. Dermal thickness also decreased in the body-wrap and Endermologie® groups. Although correlations with clinical scales were not reported, high-frequency ultrasound again showed high responsiveness to treatment-related tissue remodeling.
3.5. Results of Syntheses
Across the nine included studies, substantial methodological and clinical heterogeneity was evident. The investigations differed in ultrasound devices and probe frequencies (ranging from approximately 12 to 35 MHz, with some scanners operating across wider bandwidths), acquisition protocols (including probe pressure, imaging planes, patient positioning, and environmental conditions), and post-processing procedures. Clinical comparators and outcome measures were likewise heterogeneous, encompassing multiple cellulite scoring systems, anthropometric measures, and a broad array of quantitative ultrasound parameters. Given these pronounced differences in interventions, measurement scales, and reporting formats, statistical harmonization and quantitative meta-analysis were deemed inappropriate; therefore, a qualitative synthesis was undertaken. Despite this variability, a coherent pattern emerged indicating that ultrasound consistently identified structural features associated with both cellulite severity and treatment-induced tissue remodeling.
All studies were concordant in demonstrating that ultrasound reliably quantified dermal and subcutaneous morphological characteristics relevant to cellulite. The most frequently assessed parameters included dermal and epidermal thickness, subcutaneous tissue thickness, the length and area of adipose protrusions into the dermis, the morphology and density of fibrous septa, and the presence of edema. Treatment-monitoring studies consistently reported pre–post reductions in dermal and subcutaneous thickness, decreased fat-lobule protrusion, increased dermal echogenicity, and improved tissue elasticity, effects that were detectable even when clinical improvement was modest.
Diagnostic investigations similarly showed consistent associations between ultrasound-derived parameters and clinical cellulite severity. Correlation coefficients ranged from moderate to strong across most cohort studies. One diagnostic study, Intagliata et al. (2025), specifically examined the ability of ultrasound to subclassify stage III cellulite, reporting a satisfactory degree of agreement between ultrasound-based and clinically determined grades, along with acceptable intermethod reliability. The identification of a superficial-fat threshold of approximately 7 mm provided meaningful discrimination between phenotypes 3A and 3B, and the authors additionally described a mixed phenotype that was not detectable through clinical inspection alone.
Across all studies, clinical comparators, including the Nürnberger–Müller scale, photonumeric grading systems, thigh circumference, and standardized photographic assessments, showed moderate alignment with ultrasound findings. Notably, ultrasound often revealed structural abnormalities not captured by clinical grading alone, underscoring its incremental diagnostic value. Elastography further demonstrated the potential to enhance sensitivity by detecting subtle variations in tissue stiffness.
4. Discussion
This systematic review synthesizes evidence from nine studies investigating the role of ultrasound in the assessment and monitoring of cellulite. Despite notable methodological heterogeneity, the overall body of evidence consistently supports ultrasound as a reliable and sensitive imaging modality capable of characterizing dermal and subcutaneous structural alterations that mirror clinically relevant features of cellulite. Across observational and interventional designs, ultrasound distinguished between different degrees of severity and quantified treatment-induced tissue remodeling, underscoring its potential as an objective outcome measure in routine clinical practice and research settings [
18,
35].
From a diagnostic perspective, the available evidence demonstrates that ultrasound can visualize key structural parameters implicated in the pathophysiology of cellulite. Early work by Bielfeldt et al. (2008) showed that three-dimensional ultrasound combined with 20–22 MHz ultrasound could detect dermal–subcutaneous interface irregularities moderately correlated with clinical severity. Subsequent investigations confirmed significant associations between ultrasound-derived metrics and validated grading scales, indicating that quantitative ultrasound measurements reflect clinically meaningful differences in tissue architecture. While some recent work has explored the potential for ultrasound to refine classification of advanced cellulite stages, the broader diagnostic literature—spanning dermatologic imaging and aesthetic medicine—consistently highlights the capacity of ultrasound to resolve fine-scale architectural features of the epidermis, dermis, and upper hypodermis with high reproducibility and quantitative precision [
36,
37].
The diagnostic value of ultrasound is further reflected in the structural patterns consistently observed across interventional studies. A reduction in dermal thickness among women with cellulite, quantifiable with high reliability, is one of the most reproducible findings [
15,
30]. Subcutaneous depth also varies with severity, reinforcing the ability of ultrasound to capture clinically relevant morphological differences. Of particular importance is the capacity of ultrasound to delineate adipose protrusions extending into the dermis, key contributors to the characteristic dimpled appearance of cellulite. Ultrasound also provides detailed visualization of fibrous septa morphology, a central component of cellulite architecture. Existing evidence shows that septal orientation, thickness, and spatial distribution meaningfully influence severity [
38,
39], with increased septal thickness and altered configurations frequently documented in affected areas. In addition, ultrasound reliably detects dermal and subcutaneous edema, offering insight into interstitial and inflammatory processes relevant to cellulite pathophysiology [
40,
41].
A second major theme concerns the responsiveness of ultrasound to therapeutic interventions. Interventional studies consistently reported measurable reductions in dermal and subcutaneous thickness, decreased adipose protrusion height, and improvements in dermal echogenicity following topical, mechanical, or injectable treatments. Mlosek et al. (2013) documented substantial quantitative changes in multiple ultrasound parameters after only 30 days of therapy, while Tomaszewicz (2021) observed significant improvements in skin elasticity and circumferential measures. Similarly, Mlosek & Malinowska (2025) reported ultrasound-detected improvements across mesotherapy, body wrapping, and Endermologie®. Several investigations emphasized that ultrasound can identify early microstructural remodeling even when clinical changes are subtle, reinforcing its sensitivity to subclinical tissue dynamics, underscoring the utility of ultrasound in real-time visualization of skin structures and in evaluating parameters such as thickness and vascularity [
42], which are essential for accurate monitoring of treatment response.
Notwithstanding convergence in findings, considerable variability in ultrasound methodologies was observed. Probe frequencies ranged from 12 to 35 MHz, with some devices offering broader bandwidths (20–100 MHz). Imaging modalities varied from conventional two-dimensional B-mode acquisition to three-dimensional reconstruction, strain elastography, and Doppler evaluation. Standardization of acquisition conditions was inconsistent, and differences in operator experience, probe pressure, scanning planes, and analysis procedures contributed substantially to methodological heterogeneity. Despite these variations, concordance between ultrasound findings and clinical evaluations was generally moderate to strong. Correlation coefficients between ultrasound-derived parameters and clinical severity ranged from approximately r = 0.31 to r = 0.64, supporting the ability of ultrasound to capture structural changes aligned with clinical assessments. Agreement statistics reported in diagnostic research further suggest that ultrasound may enhance classification accuracy beyond visual inspection alone, although such applications require additional validation in larger, well-controlled cohorts. Such heterogeneity not only limits comparability across studies, but also represents a major barrier to the development of robust computational and artificial intelligence–based imaging pipelines, which critically depend on standardized acquisition and analytic procedures to ensure reproducibility and clinical reliability.
Ultrasound offers several advantages: it is non-invasive, reproducible, and capable of identifying microstructural changes not appreciable on visual examination. These characteristics position ultrasound as a promising tool for baseline evaluation and longitudinal monitoring. Its high sensitivity to remodeling processes is particularly valuable in interventional research and aesthetic practice, where early detection of treatment response is critical.
To our knowledge, this review offers one of the most comprehensive synthesis to date focused exclusively on ultrasound for cellulite assessment, covering nearly two decades of diagnostic and interventional research. However, limitations remain. Significant methodological heterogeneity precluded quantitative meta-analysis. Many studies were small, uncontrolled or with limited adjustment for confounders such as age, BMI, baseline severity, and hormonal status. Reporting gaps were common, particularly regarding probe pressure control, scanning-plane standardization, operator blinding, and quality-assurance procedures. Environmental factors such as room temperature and humidity were inconsistently controlled, and sonographers were frequently unblinded to treatment allocation or time point, raising the possibility of measurement bias. The predominance of volunteers recruited from aesthetic settings further limits generalizability. A major recurring challenge is the absence of standardized ultrasound criteria for cellulite evaluation, with inconsistent definitions and measurement approaches across studies.
The clinical implications of these findings are noteworthy. Ultrasound enables clinicians to move beyond conventional visual grading by providing objective, quantifiable metrics that reflect underlying tissue architecture. The body of evidence synthesized in this review demonstrates that ultrasound introduces a degree of objectivity and morphological resolution not attainable through traditional clinical scales alone. Although tools such as the Nürnberger–Müller scale or photo numeric grading systems remain useful for a global assessment of cutaneous appearance, they are intrinsically influenced by subjective and inter-observer variability. In contrast, ultrasound allows reproducible measurement of structural parameters including dermal thickness, the depth and area of adipose protrusions, the density and architecture of fibrous septa, and the presence of edema or subtle echo structural alterations. Across included studies, correlations between ultrasound parameters and clinical severity indicate that imaging not only mirrors visible cutaneous differences but also captures microstructural modifications that cannot be assessed through external inspection. This capability is particularly valuable in treatment monitoring, where ultrasound has shown sensitivity in detecting early tissue remodeling even in the absence of overt clinical improvement. From a clinical standpoint, such features allow practitioners to surpass the limitations of purely visual evaluation and to obtain objective metrics that can be contextualized across distinct ultrasonographic phenotypes. Importantly, these insights can be derived without recourse to invasive procedures such as histopathological analysis, which—although potentially informative in selected research scenarios—cannot be justified for routine characterization of cellulite. Ultrasound thus represents a practical, sustainable, and ethically appropriate modality for capturing relevant morphological features in both everyday clinical management and observational research. The ability to identify distinct morphological patterns, including the emerging 3A, 3B, and mixed phenotypes, further expands opportunities for individualized assessment and for deepening understanding of cellulite pathophysiology. It must be acknowledged, however, that the intrinsic phenotypic variability of cellulite and the heterogeneity of available studies do not yet allow a one-to-one correspondence between every ultrastructural parameter and each ecographic subtype. This limitation does not diminish the clinical value of ultrasound; rather, it underscores its strategic role as a tool capable of revealing structural differences that would otherwise remain undetected, providing the preliminary framework upon which more granular and standardized future classifications may be built. In this sense, ultrasound does not replace clinical examination, but enhances it, and, crucially, lays the scientific foundation for increasingly refined ultrasonographic phenotyping.
As ultrasound technology evolves, with increasing integration of elastography, Doppler imaging, and advanced computational tools, its diagnostic and prognostic capabilities are expected to further expand. In this context, the availability of standardized, high-frequency ultrasound datasets may enable the safe implementation of artificial intelligence and deep learning approaches for automated image interpretation, feature extraction, and longitudinal monitoring. Importantly, such developments should prioritize transparency, interpretability, and patient safety to support responsible clinical translation.
5. Conclusions
This systematic review shows that ultrasound is a valuable imaging modality for characterizing the structural features of cellulite and for monitoring treatment-related tissue remodeling. Across heterogeneous study designs, ultrasound consistently detected changes in dermal and subcutaneous thickness, adipose protrusions, and fibrous-septal architecture corresponding to clinically relevant variations in severity. However, the evidence base remains limited by small sample sizes, methodological heterogeneity, and variable reporting quality. Future research should prioritize standardized imaging protocols, rigorous methodological control, and adequately powered controlled trials incorporating validated clinical and patient-reported outcomes. The establishment of consensus-based ultrasound acquisition and interpretation standards will be essential not only for improving clinical adoption, but also for enabling future integration of reproducible and ethically sound AI-based image analysis in cellulite assessment.
Author Contributions
All authors contributed equally to this work. Conceptualization, D.I. and M.L.G.; methodology, D.I. and M.L.G.; investigation, D.I. and M.L.G.; data curation, D.I. and M.L.G.; formal analysis, D.I. and M.L.G.; writing—original draft preparation, D.I. and M.L.G.; writing—review and editing, D.I. and M.L.G.; supervision, M.L.G.; project administration, M.L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. All data supporting the findings of this systematic review are derived from published studies cited in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI |
Artificial Intelligence |
| BMI |
Body Mass Index |
| CENTRAL |
Cochrane Central Register of Controlled Trials |
| HFUS |
High-Frequency Ultrasound |
| MRI |
Magnetic Resonance Imaging |
| NOS |
Newcastle-Ottawa Scale |
| PRISMA |
Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO |
International Prospective Register of Systematic Reviews |
| ROBINS-I |
Risk Of Bias In Non-randomized Studies of Interventions |
| US |
Ultrasound |
References
- Ferzli, G.; Sadick, N.S. A Review of Current Modalities to Treat Cellulite Effectively. Dermatological Reviews 2020, 1, 123–127. [Google Scholar] [CrossRef]
- Emanuele, E.; Minoretti, P.; Altabas, V.; Gaeta, E. Adiponectin Expression in Subcutaneous Adipose Tissue Is Reduced in Women With Cellulite. International Journal of Dermatology 2011, 50, 412–416. [Google Scholar] [CrossRef]
- Whipple, L.A.; Fournier, C.; Heiman, A.J.; Awad, A.N.; Roth, M.Z.; Cotofana, S.; Ricci, J.A. The Anatomical Basis of Cellulite Dimple Formation: An Ultrasound-Based Examination. Plast. Reconstr. Surg. 2021, 148, 375e–381e. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.d.l.C.; Suárez-Serrano, C.; Roldán, J.R.; Jiménez-Rejano, J.J. Cellulite’s Aetiology: A Review. J Eur Acad Dermatol Venereol 2012, 27, 273–278. [Google Scholar] [CrossRef]
- Green, J.B.; Cohen, J.L.; Kaufman, J.; Metelitsa, A.I.; Kaminer, M.S. Therapeutic Approaches to Cellulite. Seminars in Cutaneous Medicine and Surgery 2015, 34, 140–143. [Google Scholar] [CrossRef]
- Sadick, N.S. Treatment for Cellulite. International Journal of Women’s Dermatology 2019, 5, 68–72. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Pathophysiology of Cellulite: Possible Involvement of Selective Endotoxemia. Obesity Reviews 2022, 24. [Google Scholar] [CrossRef]
- Davis, D.S.; Boen, M.; Fabi, S.G. Cellulite: Patient Selection and Combination Treatments for Optimal Results—A Review and Our Experience. Dermatol Surg 2019, 45, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Calopresti, A.; Marafioti, S.; Nicolai, G.; Qorri, E. Open-Label Uncontrolled, Monocentric Study to Evaluate the Efficacy and Safety of the Electromagnetic Field and Negative Pressure in the Treatment of Cellulite. Life 2025, 15, 1148. [Google Scholar] [CrossRef]
- Zerini, I.; Sisti, A.; Cuomo, R.; Ciappi, S.; Russo, F.; Brandi, C.; D’Aniello, C.; Nisi, G. Cellulite Treatment: A Comprehensive Literature Review. J Cosmet Dermatol 2015, 14, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Bass, L.S.; Kaminer, M.S. Insights Into the Pathophysiology of Cellulite: A Review. Dermatol Surg 2020, 46, S77–S85. [Google Scholar] [CrossRef]
- Soares, J.L.; Miot, H.A.; Sanudo, A.; Bagatin, E. Cellulite: poor correlation between instrumental methods and photograph evaluation for severity classification. Int J Cosmet Sci 2015, 37, 134–140. [Google Scholar] [CrossRef]
- Ponto, T.; Benson, H.A.E.; Wright, A. Reliability of a Standardized Tool for Evaluating Severity of Cellulite in the Female Posterior Thigh. J Cosmet Dermatol 2022, 22, 890–896. [Google Scholar] [CrossRef]
- LaTowsky, B.; Jacob, C.; Hibler, B.P.; Lorenc, P.; Petraki, C.; Palm, M.D. Cellulite: Current Treatments, New Technology, and Clinical Management. Dermatol Surg 2023, 49, S8–S14. [Google Scholar] [CrossRef] [PubMed]
- Mlosek, K.; Malinowska, S. High-Frequency Ultrasound in the Assessment of Cellulite—Correlation Between Ultrasound-Derived Measurements, Clinical Assessment, and Nürnberger–Müller Scale Scores. Diagnostics 2024, 14, 1878. [Google Scholar] [CrossRef]
- Rao, J.; Gold, M.H.; Goldman, M.P. A Two-center, Double-blinded, Randomized Trial Testing the Tolerability and Efficacy of a Novel Therapeutic Agent for Cellulite Reduction. J Cosmet Dermatol 2005, 4, 93–102. [Google Scholar] [CrossRef]
- Intagliata, D.; Priolo, M.; Molinari, P. High-Frequency Ultrasound Imaging for Stage III Cellulite: A Three-Subtype Structural Classification from an Observational Cohort Study. Dermatology and Therapy 2025. [Google Scholar] [CrossRef]
- Mlosek, R.K.; Malinowska, S.; Dębowska, R.; Lewandowski, M.; Nowicki, A. The High Frequency (HF) Ultrasound as a Useful Imaging Technique for the Efficacy Assessment of Different Anti-Cellulite Treatments. Journal of Cosmetics, Dermatological Sciences and Applications 2013, 3, 90–98. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Fazeli, A.; Berlin, A.L. Clinical, Laboratory, and MRI Analysis of Cellulite Treatment With a Unipolar Radiofrequency Device. Dermatol Surg 2007, 34, 204–209. [Google Scholar] [CrossRef]
- Guardo, A.D.; Solito, C.; Cantisani, V.; Rega, F.; Gargano, L.; Rossi, G.; Musolff, N.; Azzella, G.; Paolino, G.; Losco, L.; et al. Clinical and Ultrasound Efficacy of Topical Hypertonic Cream (Jovita Osmocell®) in the Treatment of Cellulite: A Prospective, Monocentric, Double-Blind, Placebo-Controlled Study. Medicina 2024, 60, 781. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hu, X.; Li-qing, H. Intestinal Obstruction Due to Congenital Bands From Vitelline Remnants. Journal of Ultrasound in Medicine 2012, 31, 2035–2038. [Google Scholar] [CrossRef]
- Rostom, E.H.; Salama, A. Vodder Manual Lymphatic Drainage Technique Versus Casley-Smith Manual Lymphatic Drainage Technique for Cellulite After Thigh Liposuction. Advances in Dermatology and Allergology 2022, 39, 362–367. [Google Scholar] [CrossRef]
- Troia, S.; Moreira, A.M.; Pisco, D.; Noites, A.; Vale, A.L.; Carvalho, P.; Vilarinho, R. Effect of Shock Wave Therapy Associated With Aerobic Exercise on Cellulite: A Randomized Controlled Trial. J Cosmet Dermatol 2020, 20, 1732–1742. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Halabchi, F.; Mazaheri, R.; Abolhasani, M.; Tabesh, M.R. Review of the Mechanisms and Effects of Noninvasive Body Contouring Devices on Cellulite and Subcutaneous Fat. International Journal of Endocrinology and Metabolism 2016, 14. [Google Scholar] [CrossRef]
- Agochukwu-Nwubah, N.; Mentz, H.A. Paradoxical Adipose Hyperplasia After Noninvasive Radiofrequency Treatment: A Novel Report and Review. J Cosmet Dermatol 2019, 19, 866–868. [Google Scholar] [CrossRef]
- Modena, D.A.O.; Nogueira da Silva, C.; Delinocente, T.C.P.; Bianca de Araujo, T.; de Carvalho, T.M.; Grecco, C.; Moreira, R.G.; Campos, G.; de Souza, J.R.; Michelini Guidi, R. Effectiveness of the Electromagnetic Shock Wave Therapy in the Treatment of Cellulite. Dermatol Res Pract 2019, 2019, 8246815. [Google Scholar] [CrossRef] [PubMed]
- Siems, W.; Grune, T.; Voß, P.; Brenke, R. Anti-fibrosclerotic Effects of Shock Wave Therapy in Lipedema and Cellulite. Biofactors 2005, 24, 275–282. [Google Scholar] [CrossRef]
- Trelles, M.A.; Martínez-Carpio, P.A. Clinical and Histological Results in the Treatment of Atrophic and Hypertrophic Scars Using a Combined Method of Radiofrequency, Ultrasound, and Transepidermal Drug Delivery. International Journal of Dermatology 2016, 55, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mlosek, R.K.; Debowska, R.M.; Lewandowski, M.; Malinowska, S.; Nowicki, A.; Eris, I. Imaging of the skin and subcutaneous tissue using classical and high-frequency ultrasonographies in anti-cellulite therapy. Skin Res Technol 2011, 17, 461–468. [Google Scholar] [CrossRef]
- Tomaszewicz, V.; Bach, A.M.; Tafil-Klawe, M.; Klawe, J.J. Non-invasive evaluation techniques to efficacy of anti-cellulite treatment: the high frequency (HF) ultrasound as a useful imaging technique of the skin and subcutaneous tissue. J Cosmet Laser Ther 2021, 23, 72–80. [Google Scholar] [CrossRef]
- Mlosek, R.K.; Malinowska, S.P. Using High Frequency Ultrasound to Assess the Efficacy of Anti-Cellulite Treatments. Clin Cosmet Investig Dermatol 2025, 18, 2869–2885. [Google Scholar] [CrossRef]
- Bielfeldt, S.; Buttgereit, P.; Brandt, M.; Springmann, G.; Wilhelm, K.P. Non-invasive evaluation techniques to quantify the efficacy of cosmetic anti-cellulite products. Skin Res Technol 2008, 14, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.A.; Seo, Y.K.; Ryu, J.H.; Back, J.H.; Koh, J.S. A validation study to find highly correlated parameters with visual assessment for clinical evaluation of cosmetic anti-cellulite products. Skin Res Technol 2014, 20, 200–207. [Google Scholar] [CrossRef]
- Chervinskaya, I.; Kuprina, N.I.; Kruglikov, I. A Retrospective Pragmatic Longitudinal Case-Series Clinical Study to Evaluate the Clinical Outcome of Triple-Frequency Ultrasound in Treatment of Cellulite. Clinical, Cosmetic and Investigational Dermatology 2024, 17, 2779–2794. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Barrett, D.L.; Harris, N.; Jeong, J.J.; Yang, X.; Chen, S.C. High-frequency ultrasound in clinical dermatology: a review. The Ultrasound Journal 2021, 13, 24. [Google Scholar] [CrossRef]
- Vergilio, M.M.; Monteiro e Silva, S.A.; Jales, R.M.; Leonardi, G.R. High-frequency ultrasound as a scientific tool for skin imaging analysis. Experimental Dermatology 2021, 30, 897–910. [Google Scholar] [CrossRef]
- Amore, R.; Amuso, D.; Leonardi, V.; Sbarbati, A.; Conti, G.; Albini, M.; Leva, F.d.; Terranova, F.; Guida, A.; Gkritzalas, K.; et al. Treatment of Dimpling From Cellulite. Plastic & Reconstructive Surgery Global Open 2018, 6, e1771. [Google Scholar] [CrossRef]
- Hexsel, D.; Abreu, M.R.d.; Rodrigues, T.d.C.; Soirefmann, M.; Prado, D.Z.d.; Gamboa, M.M.L. Side-by-Side Comparison of Areas With and Without Cellulite Depressions Using Magnetic Resonance Imaging. Dermatol Surg 2009, 35, 1471–1477. [Google Scholar] [CrossRef]
- Nobile, V.; Cestone, E.; Puoci, F.; Ponti, I.D.; Pisati, M.; Michelotti, A. In Vitro and in Vivo Study on Humans of Natural Compound Synergy as a Multifunctional Approach to Cellulite-Derived Skin Imperfections. Cosmetics 2020, 7, 48. [Google Scholar] [CrossRef]
- Perciun, R.-E.; Telcian, A. A Diabetic Woman With Soft Tissue Complex Pathology. International Journal of Case Reports and Images 2016, 7, 65. [Google Scholar] [CrossRef]
- Cisoń, H.; Jankowska-Konsur, A.; Białynicki-Birula, R. The Evaluation of Skin Infiltration in Mycosis Fungoides/Sézary Syndrome Using the High-Frequency Ultrasonography. J Clin Med 2025, 14, 7143. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).