1. Introduction
Sjogren’s Disease, a chronic autoimmune disorder characterized primarily by the infiltration of lymphocytes into exocrine glands, leading to dryness of the mucosal surfaces, but it also has impact on cardiac function [
1]. While not a primary manifestation, cardiac involvement in Sjogren’s Disease can manifest as conduction abnormalities, such as atrioventricular block, as well as an increased risk of heart failure and pulmonary hypertension [
2].
Hematologic indices, particularly the hemoglobin-to-RDW (HRR),
have emerged as potential biomarkers for assessing disease activity and cardiovascular outcomes in various populations, including those with inflammatory and autoimmune diseases [
3]. The HRR not only reflects oxygen-carrying capacity but also serves as a composite measure of inflammation, potentially correlating with cardiovascular disease (CVD) risk [
4].
Preliminary evidence indicates that vitamin D deficiency is prevalent among patients with autoimmune disorders, including SD, and has been implicated in the modulation of immune responses and cardiovascular health. The role of adequate vitamin D levels, as immunomodulatory markers, in SS patients was assessed against chronic inflammation and cardiovascular risks, suggesting that vitamin D deficiency may exacerbate the disease's manifestations and possibly influence HRR and cardiovascular outcomes [
2]
In the context of SS, the link between HRR and cardiac function becomes crucial, given that patients with severe manifestations often exhibit elevated inflammatory markers and altered hematologic profiles. Studies have shown that lower levels of HRR are significantly associated with increased risks of mortality and CVD [
5]. Moreover, the role of vitamin D status in SS has gained attention due to its immunomodulatory functions, suggesting that vitamin D deficiency may exacerbate the disease's manifestations and potentially influence HRR and cardiovascular outcomes [
5].
Despite emerging evidence linking hematological indices and vitamin D with cardiovascular outcomes in autoimmune diseases, their interplay in the context of SD remains underexplored [
6]. Studies have shown that lower levels of HRR are significantly associated with increased risks of mortality and CVD. Furthermore, the significant role of vitamin D in modulating immune responses suggests that vitamin D deficiency may exacerbate the disease's manifestations and possibly influence HRR and cardiovascular outcomes [
7]. In this context, understanding the relationship between the Hb-to-RDW ratio, cardiac function, and vitamin D status could provide insights into the pathophysiological mechanisms of cardiovascular involvement in SS and identify potential biomarkers for early risk stratification. Therefore, this study aims to elucidate the relationship between the HRR and cardiac function in the setting of Sjogren’s disease while exploring the implications of vitamin D status.
2. Methods
2.1. Study Population and Data Collection
This cross-sectional study included 61 patients diagnosed with primary Sjogren’s syndrome (pSS). All patients met the 2016 ACR/EULAR classification criteria for Sjogren’s Syndrome (pSS) [
8]
. Data collection took place from November 2022 to April 2024 at the F. Coppi et al. [
9] International Journal of Cardiology 430 (2025) 1331852Cardiology Unit of the University Hospital of Modena and included 2D/3D echocardiography, ECG, clinical and biochemical assessments, HRCT, and pulmonary function tests. Inclusion criteria comprised patients aged 18 years or older with a confirmed diagnosis of Sjogren’s syndrome. Patients had no data of hematology parameters and Echocardiographic features were excluded. Baseline demographic, biochemical, and hematological data were systematically recorded for all patients enrolled. Hemoglobin-to-RDW ratio calculated by dividing Hb by RDW. HRR is categorized based on its tertiles.
2.2. Ethics Statement
The study protocol was conducted based on the Helsinki Declaration and was approved by the local ethics committee Area Vasta Emilia Nord (protocol no. 275/16). Written informed consent was obtained from all study participants [
9,
10]
2.3. Cardiological Evaluation
Cardiovascular risk factors, such as obesity, diabetes, dyslipidemia, smoking, chronic renal failure, and systemic hypertension, were recorded. ECGs were conducted to detect arrhythmias, and 2D/3D echocardiography was assessed with a Philips EPIQ ultrasound by 2 experienced cardiologists in specialized echocardiography. Standardized echocardiographic measurements and interpretations were provided by the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Structural and functional parameters of both right and left heart chambers were assessed [
10].
2.4. Vitamin D Assessment
Serum 25-hydroxyvitamin D levels were measured at the Clinical Laboratory using an enzyme-linked immunosorbent assay (ELISA), specifically designed for vitamin D quantification. Serum levels were set to <30 ng/mL as vitamin D inadequacies according to general clinical guidelines. Levels above this value were deemed adequate [
10].
2.5. Statistical Analysis
Statistical analysis was performed using SPSS version 26 (IBM Corporation, Chicago, IL). Data were reported as mean ± SD for quantitative and number (%) qualitative data. Normal distribution was determined by the Kolmogorov-Smirnov test. A chi-square test was used to compare categorical groups, and the Kruskal-Wallis H test was utilized to compare quantitative data between groups. To evaluate the association between echocardiographic parameters as dependent variables and HRR tertiles as independent variables (with T3 as the reference group compared to T1 and T2), β-coefficients were calculated using univariate linear regression models. The data were adjusted for potential confounding factors, including age, sex, smoking status, BMI, DM, HTN, Dyslipidemia, ILD, and duration of disease. A P-value<0.05 was considered significant. GraphPad was used to create a graph.
3. Results
The study involved 61 participants with Sjogren’s disease, with a mean age of 59.8 ± 14.4 years. The majority were female (88.9%)
, and 11.1% were male. Nineteen percent were current smokers, while 38.1% had interstitial lung disease (ILD). A mean body mass index (BMI) was 25.1 ± 4.8 kg/m², and mean serum vitamin D was 32.4 ± 14.6 ng/mL. The prevalence of comorbidities was 32.8% for hypertension, 23.4% for dyslipidemia, and 6.7% for diabetes mellitus; the mean hemoglobin concentration was 12.95 ± 1.51 g/dL, red cell distribution width (RDW) was 14.53 ± 1.67%, and the mean HRR was 0.90 ± 0.16 (
Table 1).
Echocardiographic assessments revealed a mean TAPSE of 23.39 ± 4.14 mm, STDI of 13.40 ± 8.45 cm/s, PAPs of 28.08 ± 11.64 mmHg, and a TAPSE/PAPs ratio of 0.93 ± 0.35. The mean FAC_RV and EF_RV were 40.17 ± 10.2% and 48.23 ± 6.68%, respectively (
Table 1).
The echocardiographic parameters were analyzed according to HRR tertiles (T3>0.98, T2: 0.87-0.98, T1 <0.98) in the total patients with Sjogren’s disease, as well as stratified by vitamin D status (≥30 ng/mL and <30 ng/mL) (
Table 2 and
Figure 1). In the total patient group (N=61), PAPs were significantly lower in patients with HRR >0.98 compared to other groups (22.95±7.02 mmHg vs. 22.95±7.02 and 29.68±8.63 mmHg, p=0.017). Additionally, the TAPSE/PAPs were significantly higher in patients with HRR >0.98 (p: 0.009). All other parameters, including TAPSE, STDI, FAC_RV, and EF_RV, did not differ significantly between the HRR groups (
Figure 1).
Likewise, comparing patients with vitamin D <30 ng/mL ranked in order of vitamin D levels, this trend matched, PAPs was significantly lower (p=0.016), and TAPSE/PAPs ratio significantly higher (p=0.004) in the highest HRR tertile compared to lower tertiles. In comparison, PAPs and TAPSE/PAPs were not statistically different for patients with vitamin D ≥30 ng/mL between HRR tertiles (p = 0.16, p = 0.33, respectively). No differences were seen in other parameter values in HRR tertiles with respect to each group in which there was a vitamin D subgroup (
Table 2).
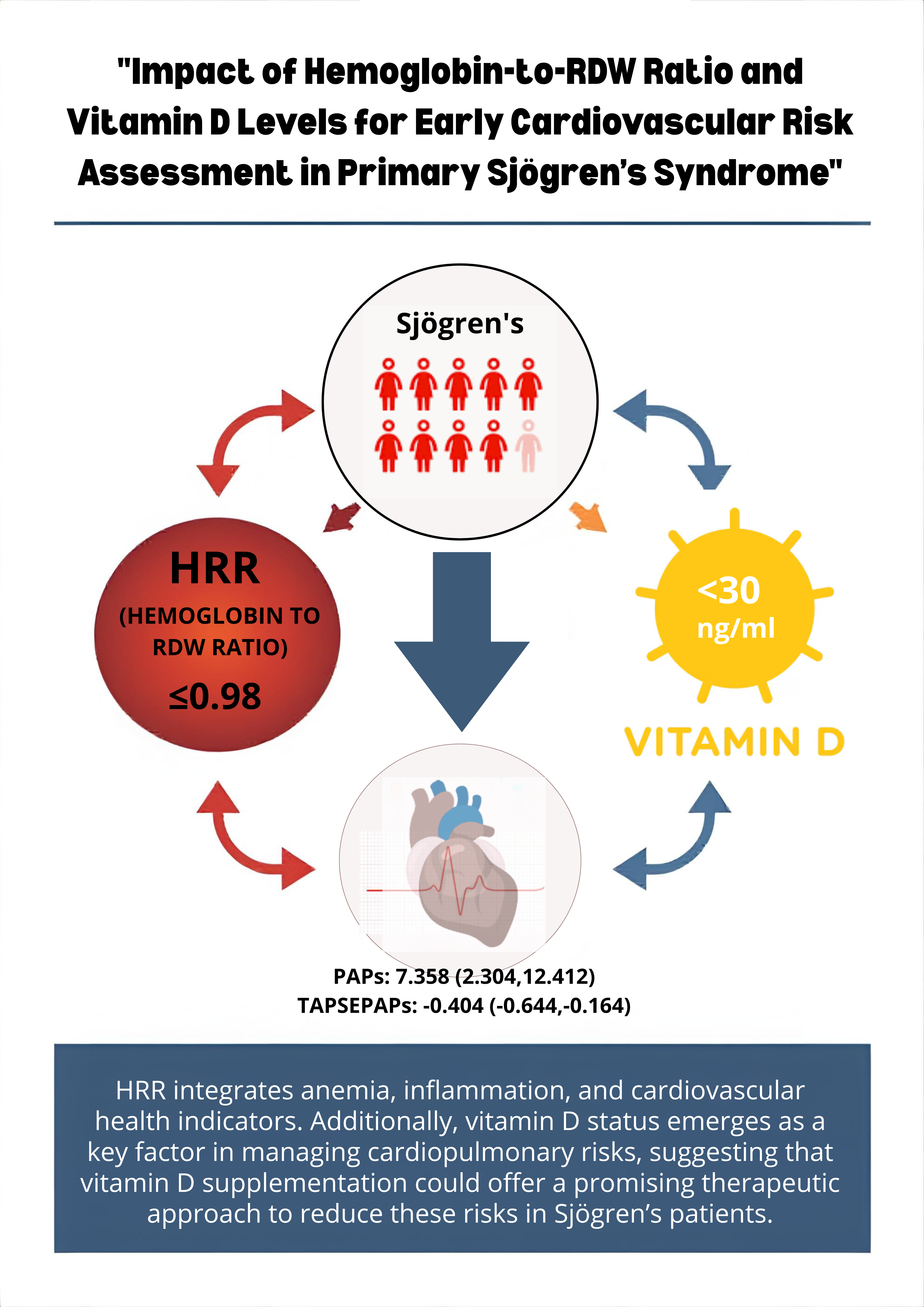
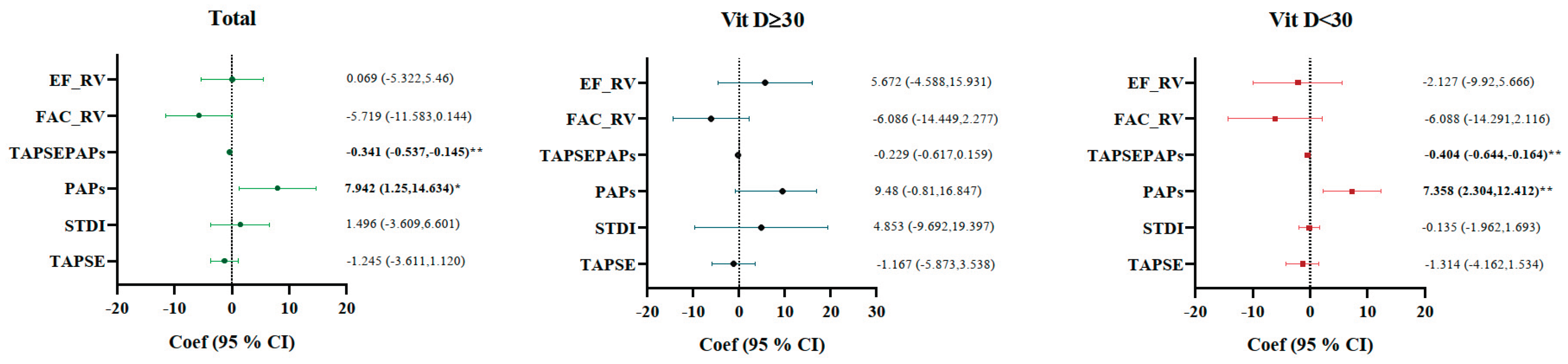
We used linear regression analysis to identify the association between echocardiographic parameters and HRR tertiles among Sjogren’s disease patients, stratified by vitamin D status. The highest tertiles (T3, HRR > 0.98) were defined as the reference group, and the model was adjusted for age, BMI, smoking, hypertension, diabetes, and dyslipidemia. Patients in the lower HRR tertiles (T1 + T2 ≤ 0.98) had significantly higher PAPs (β = 7.94, 95% CI 1.25–14.63, p = 0.021) and lower TAPSE/PAPs ratio (β = –0.341, 95% CI –0.537 to –0.145, p = 0.001) when compared with the reference group. PAPs: β = 7.36 (95% CI 2.30–12.41, p = 0.006), TAPSE/PAPs: β = −0.404 (95% CI –0.644 to –0.164, p = 0.002), these associations remained significant in the subgroup having vitamin D < 30 ng/mL, while no significant effects were observed in patients with vitamin D > 30 ng/mL. Besides TAPSE, S′ (STDI), FAC, and EF showed no statistically relevant differences in HRR groups, yet FAC exhibited a trend toward reduction in patients with low HRR (β = –5.72, p = 0.056) (
Figure 2).
4. Discussion
Our findings demonstrate that a lower HRR is associated with adverse echocardiographic markers of right ventricular-pulmonary vascular coupling, particularly increased pulmonary artery pressures and decreased TAPSE/PAPs ratio, in patients with Sjogren’s disease. This relationship is more pronounced in patients with concomitant vitamin D insufficiency, suggesting a potential modulatory role of vitamin D status in cardiopulmonary manifestations of Sjogren’s disease.
Elevated RDW and altered HRR have previously been implicated as prognostic hematologic indices in various cardiovascular conditions and inflammatory diseases [
3]. Higher HRR values are generally associated with lower risks of CVD and mortality. Findings suggest that the HRR provides a more reliable predictive value compared to hemoglobin and RDW alone. A cohort study indicated that a unit increase in HRR is linked to significant reductions in both 28-day and 90-day hospital mortality rates [
5]
SS induces a persistent pro-inflammatory state mediated by cytokines (IL-1, IL-6, TNF-α) [
11]. These conditions inhibit erythropoietin production and iron metabolism (hepcidin increase), contributing to anemia of chronic disease (lower hemoglobin). Inflammation also causes increased oxidative stress, damaging red blood cells and leading to greater heterogeneity in size, reflected as elevated RDW [
12,
13]. Studies indicate that Sjogren’s patients with higher systemic inflammation may exhibit elevated RDW [
14,
15]. Anemia and increased RDW, and consequently low HRR, are frequently observed in SS. This impaired loop may lead to worsened inflammation, oxidative stress, and heart failure [
16]
Several studies have shown that reduced hemoglobin-to-RDW ratio (HRR) has been associated with negative outcomes seen in heart failure, coronary artery disease, and acute coronary syndrome [
17,
18]. This ratio is associated with higher mortality, increased subsequent rehospitalization, and adverse cardiac events. The underlying mechanisms may involve chronic inflammation that stimulates cytokines (such as IL-6 and TNF-α) and impairs iron metabolism and erythropoiesis. This causes an increased RDW and decreased hemoglobin production [
19]. Anemia exacerbates the problem by reducing oxygen delivery to myocardial and peripheral tissues, making ischemia continue to worsen [
20]. Further, higher RDW levels induce inflammation and oxidative stress that lead to endothelial dysfunction, atherosclerosis, and myocardial remodeling. The consequences of these conditions include poorer outcomes in heart failure, coronary artery disease, and acute coronary syndrome [
20].
Cardiac tissue expressed Vitamin D receptors highly, so that vitamin D regulates cardiomyocyte hypertrophy, fibrosis, and myocardial contractility [
21]. On the other hand, Vitamin D inhibits NF-κB signaling, a central pathway in inflammatory gene activation in SS [
22]. Reducing VitD in SS leads to Chronic inflammation, endothelial dysfunction, and accelerated atherosclerosis [
23,
24]. Furthermore, it can exacerbate anemia and alter hematologic indices. This complex increases the risk of hypertension, coronary artery disease, heart failure, and adverse cardiac remodeling
Mechanistically, chronic inflammation characteristic of Sjögren’s disease promotes cytokine-mediated disruptions in erythropoiesis and iron metabolism, leading to anemia and increased red blood cell size heterogeneity. This results in decreased HRR, which correlates with intensified oxidative stress, endothelial dysfunction, and myocardial injury, thereby contributing to adverse cardiac remodeling and heart failure.
The study has several limitations. The small sample size and single center are the most important constraints. A multicenter study would be beneficial in collecting more samples from a diverse range of races and populations. Another concern is the cross-sectional approach, which could be enhanced by longitudinally tracking patients over time and evaluating their vitamin D levels, hematological parameters, and cardiac function.
5. Conclusions
This study highlights the clinical significance of the hemoglobin-to-RBC distribution width (HRR) ratio as a comprehensive biomarker in Sjogren’s disease. The HRR effectively captures the complex relationship between anemia, inflammation, and cardiovascular health. Furthermore, the research indicates that vitamin D levels may play a crucial role in managing cardiopulmonary risks within this patient population. This finding proposes designing a prospective and interventional study to confirm the role of vitamin D supplementation and the potential role of HRR in cardiovascular risk among patients with pSS.
Author Contributions
Aurora Vicenzi, Leila Bigdelu, Federica Fantuzzi (writing). Francesca Coppi, Francesco Sbarra, Cecilia Campani, Daniela Aschieri, Francesco Fedele, Gianluca Pagnoni: cardiologists, visit the patients. Martina Moretti, Dilia Giuggioli, Caterina Vacchi, Amelia Spinella, Alessandra Dei Cas: Rheumatologist and Endocrinologist, visit and follow the patients .Anna Vittoria Mattioli, Alessio Baccarani8, Marcello Pinti: scientific and grammatical editing .Susan Darroudi: data analysis. Susan Darroudi and Gianluca Pagnoni: Corresponding author.
Funding
The European Union - NextGenerationEU through the Italian Ministry of University and Research under PNRR - M4C2–I1.3 Project PE_00000019 "HEAL ITALIA" (D.G., A.V.M., M.P., and F.C.). National Institute for Cardiovascular Research (INRC), Via Irnerio 48, 40126, Bologna (Italy).
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors acknowledge the use of ChatGPT for editing the writing.
Conflicts of Interest
The authors declare no conflicts of interest related to this work.
References
- Melissaropoulos K, Bogdanos D, Dimitroulas T, Sakkas LI, Kitas GD, Daoussis D. Primary Sjögren’s Syndrome and Cardiovascular Disease. Curr Vasc Pharmacol [Internet]. 2020 Aug 10 [cited 2025 Nov 26];18(5):447–54. Available from: https://www.eurekaselect.com/178806/article.
- Pagnoni G, Giuggioli D, de Pinto M, Maini A, Battigaglia E, Macripò P, et al. Vitamin D insufficiency and cardiovascular involvement in systemic sclerosis: Association with echocardiographic parameters and risk factors. Int J Cardiol Cardiovasc Risk Prev [Internet]. 2025 [cited 2025 Nov 26];200502. Available from: https://www.sciencedirect.com/science/article/pii/S2772487525001400. [CrossRef]
- Li M, Li H, Zhong W, Wang S, Liu R, Cheng H, et al. Hemoglobin-to-red cell distribution width ratio was associated with cardiovascular diseases and death. J Clin Med [Internet]. 2025 [cited 2025 Nov 26];14(13):4464. Available from: https://www.mdpi.com/2077-0383/14/13/4464. [CrossRef]
- Feng X, Zhang Y, Li Q, Wang B, Shen J. Hemoglobin to red cell distribution width ratio as a prognostic marker for ischemic stroke after mechanical thrombectomy. Front Aging Neurosci [Internet]. 2023 [cited 2025 Nov 26];15:1259668. Available from: https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2023.1259668/full.
- Lai T, Liang Y, Guan F, Hu K. Trends in hemoglobin-to- red cell distribution width ratio and its prognostic value for all-cause, cancer, and cardiovascular mortality: a nationwide cohort study. Sci Rep [Internet]. 2025 Mar 5 [cited 2025 Dec 3];15(1):7685. Available from: https://www.nature.com/articles/s41598-025-92228-w. [CrossRef]
- Li M, Li H, Zhong W, Wang S, Liu R, Cheng H, et al. Hemoglobin-to-red cell distribution width ratio was associated with cardiovascular diseases and death. J Clin Med [Internet]. 2025 [cited 2025 Nov 26];14(13):4464. Available from: https://www.mdpi.com/2077-0383/14/13/4464. [CrossRef]
- Athanassiou P, Mavragani C, Athanassiou L, Kostoglou-Athanassiou I, Koutsilieris M. Vitamin D deficiency in primary Sjögren’s syndrome: association with clinical manifestations and immune activation markers. Mediterr J Rheumatol [Internet]. 2022 [cited 2025 Nov 26];33(1):106–8. Available from: https://cyberleninka.ru/article/n/vitamin-d-deficiency-in-primary-sj-gren-s-syndrome-association-with-clinical-manifestations-and-immune-activation-markers. [CrossRef]
- Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018 Feb;6(2):138–53. [CrossRef]
- Coppi F, Pagnoni G, Campani C, Grossule F, Vacchi C, Giuggioli D, et al. Sjögren’s syndrome and pulmonary hypertension: Exploring the intricate link with interstitial lung disease. Int J Cardiol [Internet]. 2025 [cited 2025 Dec 3];430:133185. Available from: https://www.sciencedirect.com/science/article/pii/S0167527325002281.
- Coppi F, Cavalletti A, Pagnoni G, Campani C, Grossule F, Maini A, et al. Pulmonary hypertension in patients with Sjögren’s syndrome: Gender differences in cardiovascular risk factors and instrumental data. Int J Cardiol [Internet]. 2025 June 1 [cited 2025 Dec 3];428:133131. Available from: https://www.sciencedirect.com/science/article/pii/S0167527325001743.
- Li Y, Zhang J, Liu X, Ganesan K, Shi G. Identification of inflammatory markers as indicators for disease progression in primary Sjögren syndrome. Eur Cytokine Netw [Internet]. 2024 [cited 2025 Dec 3];35(1):1–12. Available from: https://stm.cairn.info/revue-european-cytokine-network-2024-1-page-1?tab=texte-integral.
- Clinical and laboratory features of primary Sjögren’s syndrome complicated with mild to severe thrombocytopenia - PMC [Internet]. [cited 2025 Dec 3]. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC9011250/.
- Hematological Manifestations among Patients with Rheumatic Diseases | Acta Haematologica | Karger Publishers [Internet]. [cited 2025 Dec 3]. Available from: https://karger.com/aha/article/144/4/403/16187.
- Moghaddam AA, Saremi Z, Atabati E, Sharifzadeh G. Hematologic parameters and disease activity in patients with primary Sjögren’s syndrome. Egypt Rheumatol [Internet]. 2022 [cited 2025 Dec 3];44(4):329–32. Available from: https://www.sciencedirect.com/science/article/pii/S1110116422000813.
- Hu ZD, Sun YI, Guo J, Huang YL, Qin BD, Gao Q, et al. Red blood cell distribution width and neutrophil/lymphocyte ratio are positively correlated with disease activity in primary Sjögren’s syndrome. Clin Biochem [Internet]. 2014 [cited 2025 Dec 3];47(18):287–90. Available from: https://www.sciencedirect.com/science/article/pii/S0009912014006572.
- Long-term risk of heart failure and other adverse cardiovascular outcomes in primary Sjögren’s syndrome - Sun - 2023 - Journal of Internal Medicine - Wiley Online Library [Internet]. [cited 2025 Dec 3]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/joim.13595. [CrossRef]
- Feng X, Zhang Y, Li Q, Wang B, Shen J. Hemoglobin to red cell distribution width ratio as a prognostic marker for ischemic stroke after mechanical thrombectomy. Front Aging Neurosci [Internet]. 2023 [cited 2025 Dec 3];15:1259668. Available from: https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2023.1259668/full. [CrossRef]
- Li M, Li H, Zhong W, Wang S, Liu R, Cheng H, et al. Hemoglobin-to-red cell distribution width ratio was associated with cardiovascular diseases and death. J Clin Med [Internet]. 2025 [cited 2025 Dec 3];14(13):4464. Available from: https://www.mdpi.com/2077-0383/14/13/4464. [CrossRef]
- He Y, Liu C, Zeng Z, Ye W, Lin J, Ou Q. Red blood cell distribution width: a potential laboratory parameter for monitoring inflammation in rheumatoid arthritis. Clin Rheumatol [Internet]. 2018 Jan [cited 2025 Dec 3];37(1):161–7. Available from: http://link.springer.com/10.1007/s10067-017-3871-7. [CrossRef]
- Namazi G, Heidar Beygi S, Vahidi MH, Asa P, Bahmani F, Mafi A, et al. Relationship Between Red Cell Distribution Width and Oxidative Stress Indexes in Patients with Coronary Artery Disease. Rep Biochem Mol Biol [Internet]. 2023 July [cited 2025 Dec 3];12(2):241–50. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC10838587/. [CrossRef]
- Vitamin D deficiency and cardiovascular pathology - Podzolkov - Terapevticheskii arkhiv [Internet]. [cited 2025 Dec 3]. Available from: https://edgccjournal.org/0040-3660/article/view/33003.
- Role of the Innate Immunity Signaling Pathway in the Pathogenesis of Sjögren’s Syndrome [Internet]. [cited 2025 Dec 3]. Available from: https://www.mdpi.com/1422-0067/22/6/3090.
- Vitamin D in Primary Sjogren’s Syndrome (pSS) and the Identification of Novel Single-Nucleotide Polymorphisms Involved in the Development of pSS-Associated Diseases [Internet]. [cited 2025 Dec 3]. Available from: https://www.mdpi.com/2075-4418/14/18/2035.
- Sjögren’s syndrome and pulmonary hypertension: Exploring the intricate link with interstitial lung disease - ScienceDirect [Internet]. [cited 2025 Dec 3]. Available from: https://www.sciencedirect.com/science/article/pii/S0167527325002281.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |