Submitted:
31 December 2025
Posted:
01 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Prosections and illustrative graphic proposal.
2.2. Introduction of the antigen test swab.
2.3. Review of the instructions per patients of COVID antigen test
3. Results
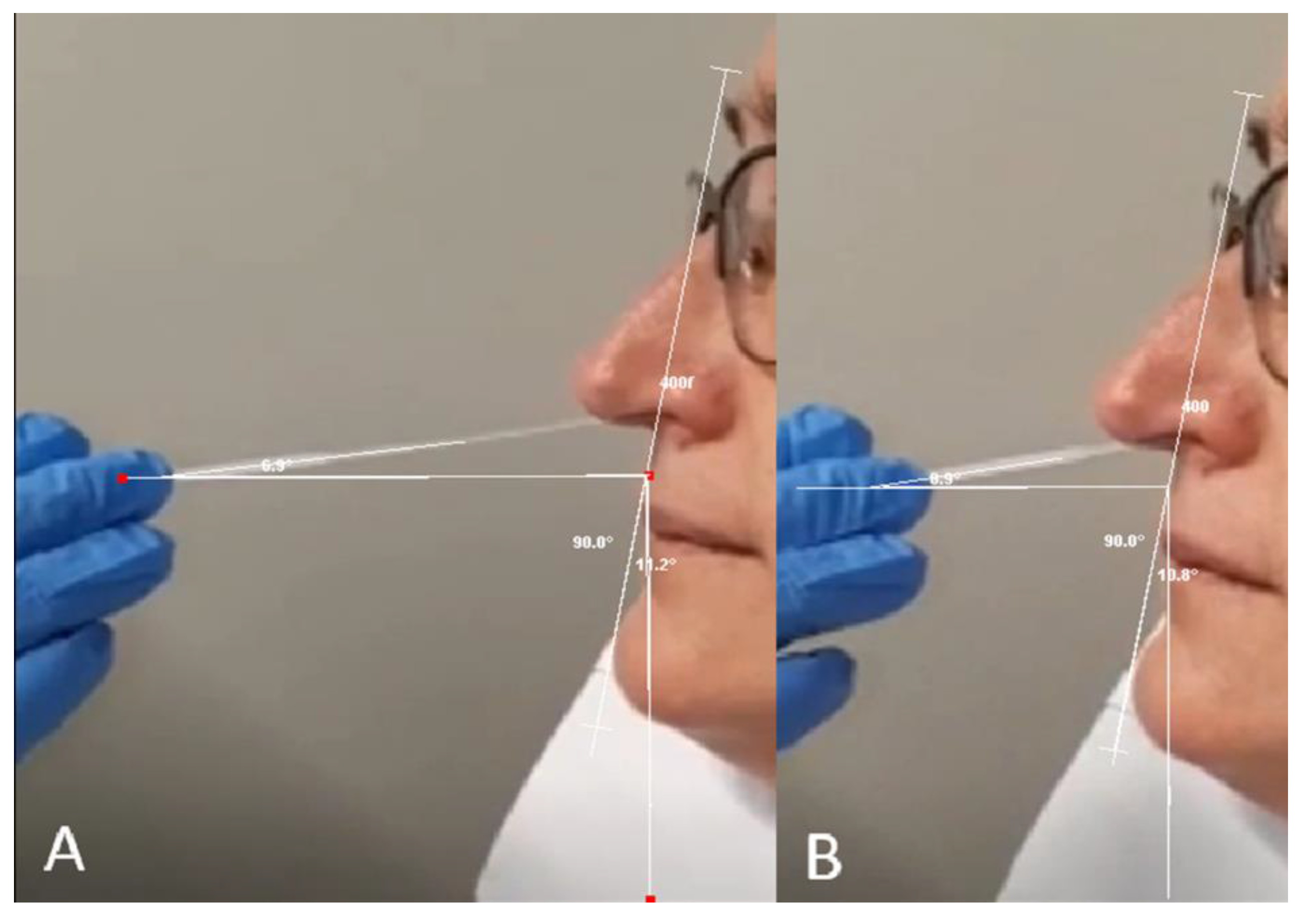
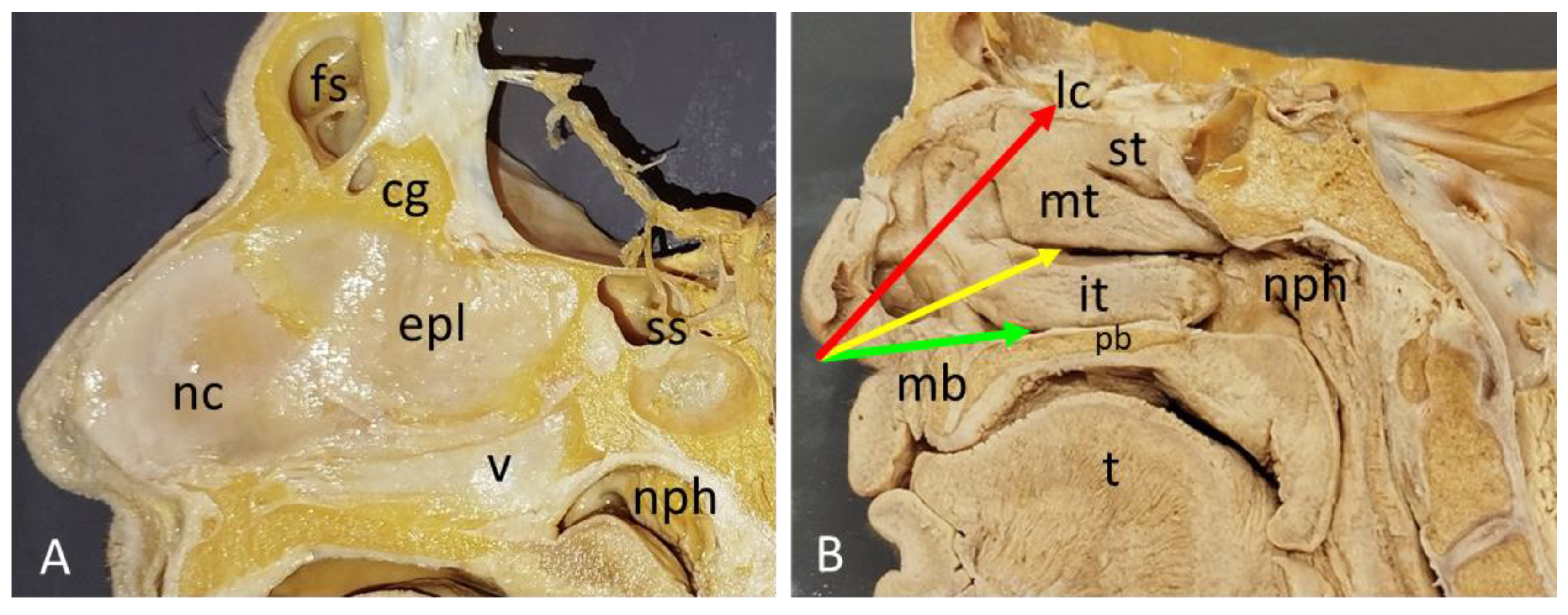
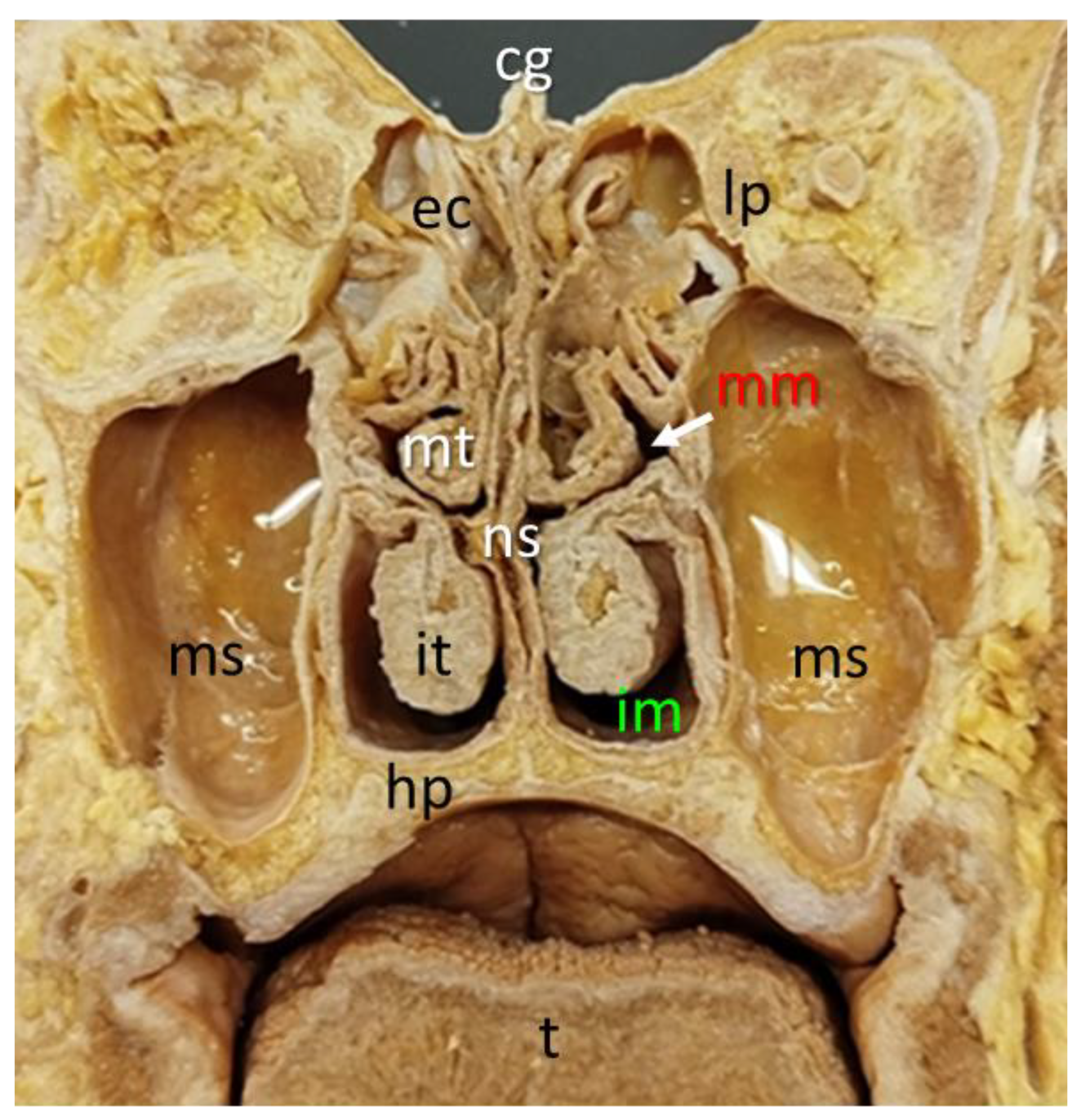
3.1. Nasal cavity anatomical dissections.
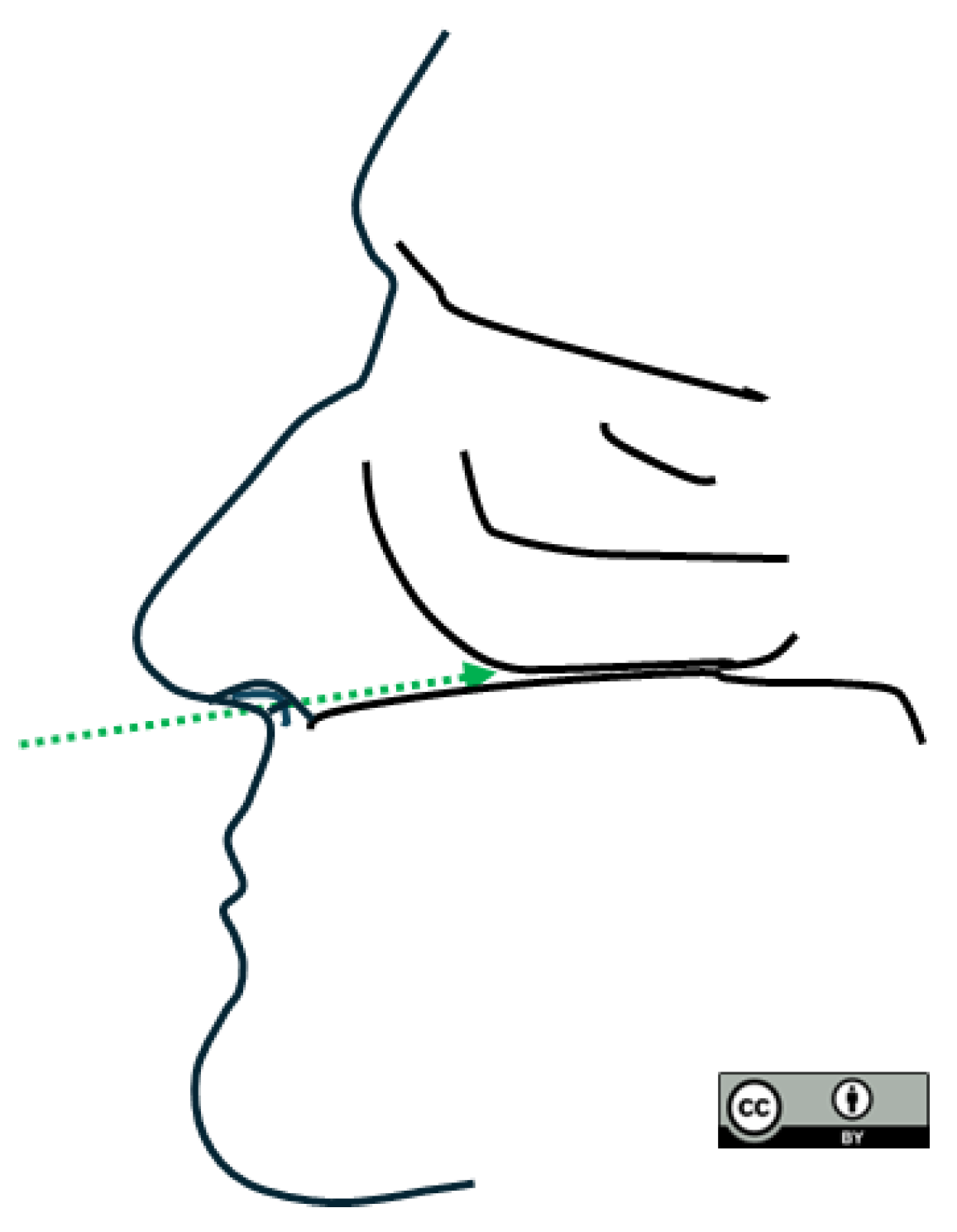
3.2. Swab Insertion Technique
3.3. Review of the instructions for available antigen tests
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CG | Crista galli |
| EPL | Ethmoidal perpendicular lamina |
| EC | Ethmoidal cells |
| FS | Frontal sinus |
| IM | Inferior meatus |
| IT | Inferior turbinate |
| MM | Middle meatus |
| MT | Mid-turbinate |
| NC | Nasal cartilage |
| NPH | Nasopharynx |
| PB | Horizontal plate of palatine bone |
| ST | Superior turbinate |
| SS | Sphenoidal sinus |
| V | Vomer bone |
References
- Marty FM, Chen K, Verrill KA. How to Obtain a Nasopharyngeal Swab Specimen. N Engl J Med. 2020 May 28;382(22):e76.
- NEJM Group. https://youtu.be/DVJNWefmHjE?feature=shared [accessed September 21, 2025].
- Itamura K, Wu A, Illing E, Ting J, Higgins T. YouTube Videos Demonstrating the Nasopharyngeal Swab Technique for SARS-CoV-2 Specimen Collection: Content Analysis. JMIR Public Health Surveill. 2021 Jan 14;7(1):e24220. PMID: 33406478; PMCID: PMC7813578. [CrossRef]
- Ciència i Tecnologia. Demostració: com s'ha de col·locar el palet en un test d'antígens? https://www.regio7.cat/videos/ciencia-i-tecnologia/2021/12/23/demostracio-com-sha-collocar-palet-60978647.html [accessed December 21, 2025.
- https://www.aemps.gob.es/la-aemps/ultima-informacion-de-la-aemps-acerca-del-covid-19/informacion-general-sobre-tests-de-diagnostico-de-covid-19/?lang=en [accessed on December 21, 2025].
- https://www.assuretech-product.com/infectious-disease-tests/covid-19-tests/sars-cov-2-antigen-rapid-tests-for-self.html, minute 2:00, [accessed on December 21, 2025].
- https://farmaferoles.com/wp-content/uploads/2022/12/fluorecare_test_nasal_rapido_antigenos_covid19__gripe_a_b__vrs_instrucciones.pdf [accessed on December 21, 2025].
- https://www.medomics-dx.net/product/SARS-CoV-2--26-Influenza-AB--26-RSV-Antigen-Combo-Rapid-Test-Kit--LFIA--518.html [accessed on December 21, 2025].
- https://aidentiapreventix.com/wp-content/uploads/ES-IFU-Rapid-COVID-19-Antigen-Test-Colloidal-Gold-Nasal-Swab-V2.16.pdf [accessed on December 21, 2025].
- https://www.grupotristan.es/wp-content/uploads/2022/09/IFU-P213106-P213107-P213109-EZER-Flu-COVID-19-Antigen-Duo-ES.pdf [accessed on December 21, 2025].
- https://unahealth.co.uk/wp-content/uploads/146555702-ISIR-535-Beright-CE-EN-PI.pdf [accessed on December 21, 2025].
- https://cordx.com/wp-content/uploads/2022/07/CorDx-FluABCOVID-19RSV-4%E5%90%881-%E8%81%94%E5%8D%A1-210x285mm.pdf [accessed on December 21, 2025].
- https://www.certest.es/wp-content/uploads/2021/12/IU-SC80001P-Self-Test-ES-23.12.21.pdf [accessed on December 21, 2025].
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020 Mar 19;382(12):1177-1179. Epub 2020 Feb 19. PMID: 32074444; PMCID: PMC7121626. [CrossRef]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Cochrane COVID-19 Diagnostic Test Accuracy Group 2. Rapid point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705.
- Dinnes J, Sharma P, Berhane S, van Wyk SS, Nyaaba N, Domen J, Taylor M, Cunningham J, Davenport C, Dittrich S, Emperador D, Hooft L, Leeflang MM, McInnes MD, Spijker R, Verbakel JY, Takwoingi Y, Taylor-Phillips S, Van den Bruel A, Deeks JJ; Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. Update in: Cochrane Database Syst Rev. 2025 Nov 28;11:CD013705. doi: 10.1002/14651858.CD013705.pub4. [CrossRef]
- Kohmer N, Eckermann L, Böddinghaus B, Götsch U, Berger A, Herrmann E, Kortenbusch M, Tinnemann P, Gottschalk R, Hoehl S, Ciesek S. Self-Collected Samples to Detect SARS-CoV-2: Direct Comparison of Saliva, Tongue Swab, Nasal Swab, Chewed Cotton Pads and Gargle Lavage. J Clin Med. 2021 Dec 8;10(24):5751. PMID: 34945047; PMCID: PMC8709431. [CrossRef]
- Paquin R, Ryan L, Vale FL, Rutkowski M, Byrd JK. CSF Leak After COVID-19 Nasopharyngeal Swab: A Case Report. Laryngoscope. 2021 Sep;131(9):1927-1929. Epub 2021 Feb 20. [CrossRef]
- Ku J, Chen CY, Ku J, Chang HK, Wu JC, Yen YS. Iatrogenic cerebrospinal fluid leak after repeated nasal swab tests for COVID-19: illustrative case. J Neurosurg Case Lessons. 2021 Oct 25;2(17):CASE21421. [CrossRef]
- Sullivan CB, Schwalje AT, Jensen M, Li L, Dlouhy BJ, Greenlee JD, Walsh JE. Cerebrospinal Fluid Leak After Nasal Swab Testing for Coronavirus Disease 2019. JAMA Otolaryngol Head Neck Surg. 2020 Dec 1;146(12):1179-1181. doi: 10.1001/jamaoto.2020.3579. Erratum in: JAMA Otolaryngol Head Neck Surg. 2020 Dec 1;146(12):1181. [CrossRef]
- Samadian M, Maroufi SF, Taheri MS, Jafari A. CSF rhinorrhea after nasopharyngeal swab testing for COVID-19: A case report and review of literature. Otolaryngol Case Rep. 2021 Nov;21:100370. [CrossRef]
- Alberola-Amores FJ, Valdeolivas-Urbelz E, Torregrosa-Ortiz M, Álvarez-Sauco M, Alom-Poveda J. Meningitis due to cerebrospinal fluid leak after nasal swab testing for COVID-19. Eur J Neurol. 2021 Nov;28(11):e91-e92. Epub 2021 Feb 12. [CrossRef]
- Samargandy SA, Fritz CG, Ahmadian D, Bhalla V, Lee JM, Le CH. Traumatic CSF rhinorrhea associated with COVID-19 testing: a case series and systematic review. Eur Arch Otorhinolaryngol. 2025 Jan;282(1):193-205. [CrossRef]
- Vos MB, Gonzalez MD, Stone C, Cleeton R, Figueroa J, Jerris R, Park SI, Heilman S, Nayee R, Chahroudi A, Schoof N, Mavigner M, Morris CR, Leong T, Grindle A, Westbrook A, Lam W, Rogers BB. Comparison of Mid-turbinate Nasal Swabs, Saliva, and Nasopharyngeal Swabs for SARS-CoV-2 Reverse Transcription-Polymerase Chain Reaction Testing in Pediatric Outpatients. Arch Pathol Lab Med. 2022 Sep 1;146(9):1056-1061. [CrossRef]
- Frezza D, Fabbris C, Franz L, Vian E, Rigoli R, De Siati R, Emanuelli E, Bertinato L, Boscolo-Rizzo P, Spinato G. A Severe Acute Respiratory Syndrome Coronavirus 2 detection method based on nasal and nasopharyngeal lavage fluid: A pilot feasibility study. Laryngoscope Investig Otolaryngol. 2021 Jul 27;6(4):646-649.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




