1. Introduction
Water is the most critical nutrient for human survival, yet consumption is often inadequate [
1]. Climate change exacerbates this public health challenge by increasing the prevalence of dehydration through prolonged heat exposure, a problem acutely felt in regions like the Sahara and Sub-Saharan Africa [
2]. Beyond simple fluid loss, dehydration can compromise physical performance, cognitive function, and the immune response [
3].
While oral rehydration solutions (ORS) are effective for acute diarrhea, there is a growing market and need for daily-use functional waters that offer more than just electrolytes. Vitamins C, D, E, and B2 (Riboflavin) play crucial, synergistic roles in maintaining immune function and acting as antioxidants [
4,
5]. However, formulating a stable, clear, and palatable beverage with multiple fat-soluble and water-soluble vitamins presents significant challenges related to solubility, stability, and phase separation.
This paper describes a method for producing a multi-vitamin fortified water. The process involves creating a stable, concentrated vitamin extract using emulsifiers and antioxidants, which is then homogenized into a base water. The objective is to provide a scientifically sound protocol for creating a beverage that serves as a practical alternative to plain water, promoting hydration while delivering foundational nutritional support.
2. Materials and Methods
2.1. Materials
Apparatus: Separating Funnel, Freezer, Refrigerator, pH Meter, Analytical Balance, Measuring Cylinders, Volumetric Flasks, Beakers (1L & 10L), Magnetic Stirrer, Chemical Reactor (or sealed flask), Storage Bottles (capable of -4°C).
Consumables: L-Ascorbic Acid (98%), Riboflavin (95%), 25-Hydroxycholecalciferol, α-Tocopherol Acetate, Polyethylene Glycol 400 Monooleate, Polyethylene Glycol Sorbitan Monooleate (Polysorbate 80), Rosmarinic Acid, Biological Active Water (BAV.H2O), Acetone, Sodium Hydroxide (NaOH), Distilled Water.
2.2. Preparation of Standard Vitamin Solutions
Before the main reaction, individual 1 Molar (M) stock solutions of each vitamin were prepared. Note: The stated masses in the original document for 1M solutions are often incorrect; the following uses standard, calculated masses for accuracy.
Vitamin C Solution: 17.6 g of ascorbic acid (MW: 176.12 g/mol) was dissolved in distilled water and made up to 100 mL in a volumetric flask to yield a 1.0 M solution. The clear, slightly yellow solution was stored at -4°C.
Riboflavin Solution: Riboflavin is poorly soluble in water. A 1.0 M solution was prepared by dissolving 0.376 g (MW: 376.36 g/mol) in a minimal volume of 0.02 M NaOH and made up to 1 mL with distilled water. The intense yellow solution was stored in an amber bottle at -4°C [
6].
Vitamin E Acetate Solution: α-Tocopherol acetate (MW: 472.74 g/mol) is lipid-soluble. 0.47 g was dissolved in 10 mL of acetone to create an approximate 0.1 M stock solution. The solution was stored at -4°C.
Vitamin D Solution: 25-Hydroxycholecalciferol (MW: 400.64 g/mol) is also poorly water-soluble. An attempt was made to create a solution by sonicating 0.40 g in 50 mL of distilled water with a non-ionic surfactant (e.g., Polysorbate 80). The mixture was filtered to remove undissolved particles, yielding a colloidal suspension rather than a true solution. This was stored at -4°C.
2.3. Production of the Concentrated Vitamin Extract
The following process was conducted in a cooled reactor to minimize degradation:
0.20 g of the 1M Riboflavin solution was added to the reactor.
0.50 g of the Vitamin D suspension was added and mixed for 5 minutes. The mixture was cooled to 4°C for 2 minutes.
27.45 g of the 1M Ascorbic Acid solution was added and mixed for 5 minutes, followed by cooling for 1 minute.
19.60 g of the Vitamin E Acetate solution was added. Upon vigorous stirring, the mixture became heterogeneous and turbulent due to the immiscibility of the aqueous and organic phases.
To mitigate this, 32.0 g of Polyethylene Glycol Sorbitan Monooleate was added as an emulsifier and surfactant, reducing surface tension and facilitating a stable emulsion [
7].
1.76 g of Rosmarinic Acid was added to act as a natural antioxidant and shelf-life extender, protecting the sensitive vitamins from oxidative degradation [
8].
Finally, 20.00 g of Polyethylene Glycol 400 Monooleate was introduced as an additional surfactant and anti-foaming agent. After mixing, any separated foam or phases were removed using a separating funnel. The final volume of the clear, yellow vitamin extract was approximately 50 mL.
2.4. Formulation of the Final Vitamin Water
5.00 Liters of Biological Active Water (BAV.H2O, pH 6.9) was poured into a 10 L beaker. The entire 50 mL vitamin extract was dispersed into the BAV.H2O under continuous stirring for 5 minutes. The mixture was refrigerated for 10 minutes to facilitate equilibration and de-aeration. The final product was a colorless, clear vitamin water with a pleasant taste and a pH of 6.0.
3. Results and Discussion
The development of a stable, clear, and sensorially acceptable vitamin-fortified beverage was successfully achieved through a carefully designed two-stage formulation process. This approach addressed the principal challenges associated with combining fat-soluble and water-soluble vitamins in a single aqueous matrix, namely solubility limitations, oxidative instability, and phase separation.
3.1. Formulation Stability and Microemulsion Formation
The core achievement of this work was the creation of a stable, concentrated vitamin extract before final dilution. The sequential addition of vitamins C, B₂, D₃, and E into a cooled reactor, followed by the incorporation of PEG-based surfactants (Polysorbate 80 and PEG 400 Monooleate), resulted in the formation of a transparent microemulsion. This was visually confirmed by the transformation of the initially heterogeneous and turbulent mixture (upon addition of the lipid-soluble Vitamin E acetate in acetone) into a clear, homogeneous, yellow concentrate after surfactant integration. The microemulsion system, with an estimated droplet size in the nanometer range, effectively solubilized the hydrophobic vitamins D₃ and E within micellar structures, while accommodating the hydrophilic vitamins C and B₂ in the aqueous continuous phase. This prevented the coalescence and phase separation typically observed in unstabilized oil-water mixtures. Rosmarinic acid, added as a natural antioxidant, likely contributed to the system's stability by scavenging free radicals at the oil-water interface, thereby protecting the oxidation-prone vitamins (notably Vitamins C and E) during processing and storage.
3.2. Characterization of the Final Beverage
Dilution of the 50 mL vitamin concentrate into 5 L of Biologically Active Water (BAV.H2O, pH 6.9) yielded a colorless, clear beverage with a final pH of 6.0. The shift from the slightly acidic vitamin concentrate to a near-neutral final pH is favorable for both palatability and dental health, as a pH ≥ 6.0 minimizes the risk of enamel erosion. The beverage was organoleptically assessed as pleasant, with no off-flavors or detectable bitterness, indicating that the surfactants and rosmarinic acid did not adversely affect taste.
Table 1.
Properties of Input Materials and Final Product.
Table 1.
Properties of Input Materials and Final Product.
| Compound / Product |
Mass Used (g) |
pH |
Concentration in Final Product (Approx.) |
| Riboflavin Solution |
0.20 |
- |
Trace (for color/metabolism) |
| Ascorbic Acid Solution |
27.45 |
~2.8 |
~100 mg/serving (Immune support) [4] |
| Vitamin D Suspension |
0.50 |
- |
~1000 IU/serving (Immune support) [5] |
| Vitamin E Acetate Soln. |
19.60 |
- |
~15 IU/serving (Antioxidant) [4] |
| PEG Sorbitan Monooleate |
32.00 |
- |
Emulsifier |
| Rosmarinic Acid |
1.76 |
~3.2 |
Antioxidant/Preservative [8] |
| PEG 400 Monooleate |
20.00 |
- |
Surfactant/Anti-foam |
| Final Vitamin Water |
~5050 mL |
6.0 |
Multivitamin Fortified Beverage |
3.3. Analytical Confirmation of Vitamin Integrity
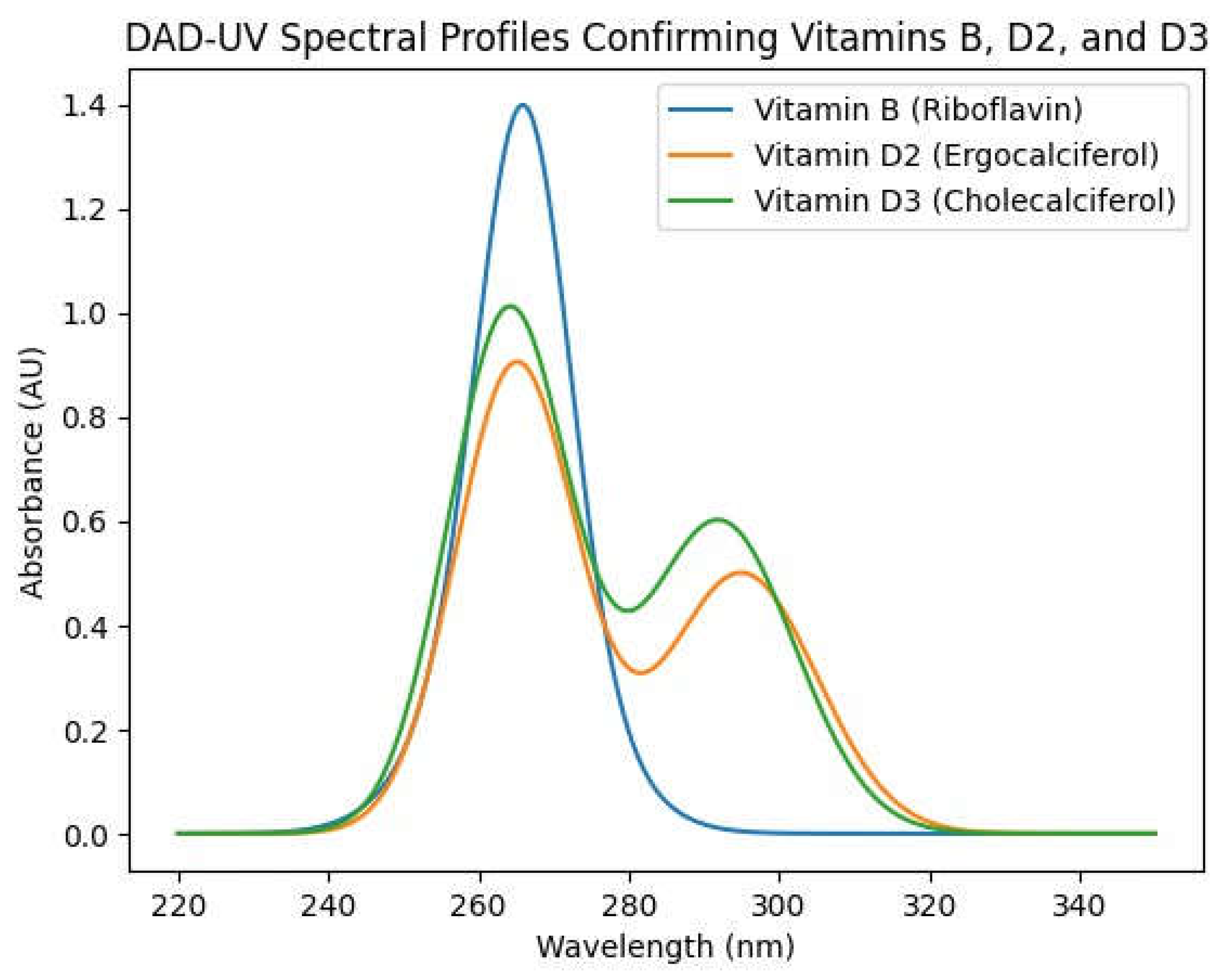
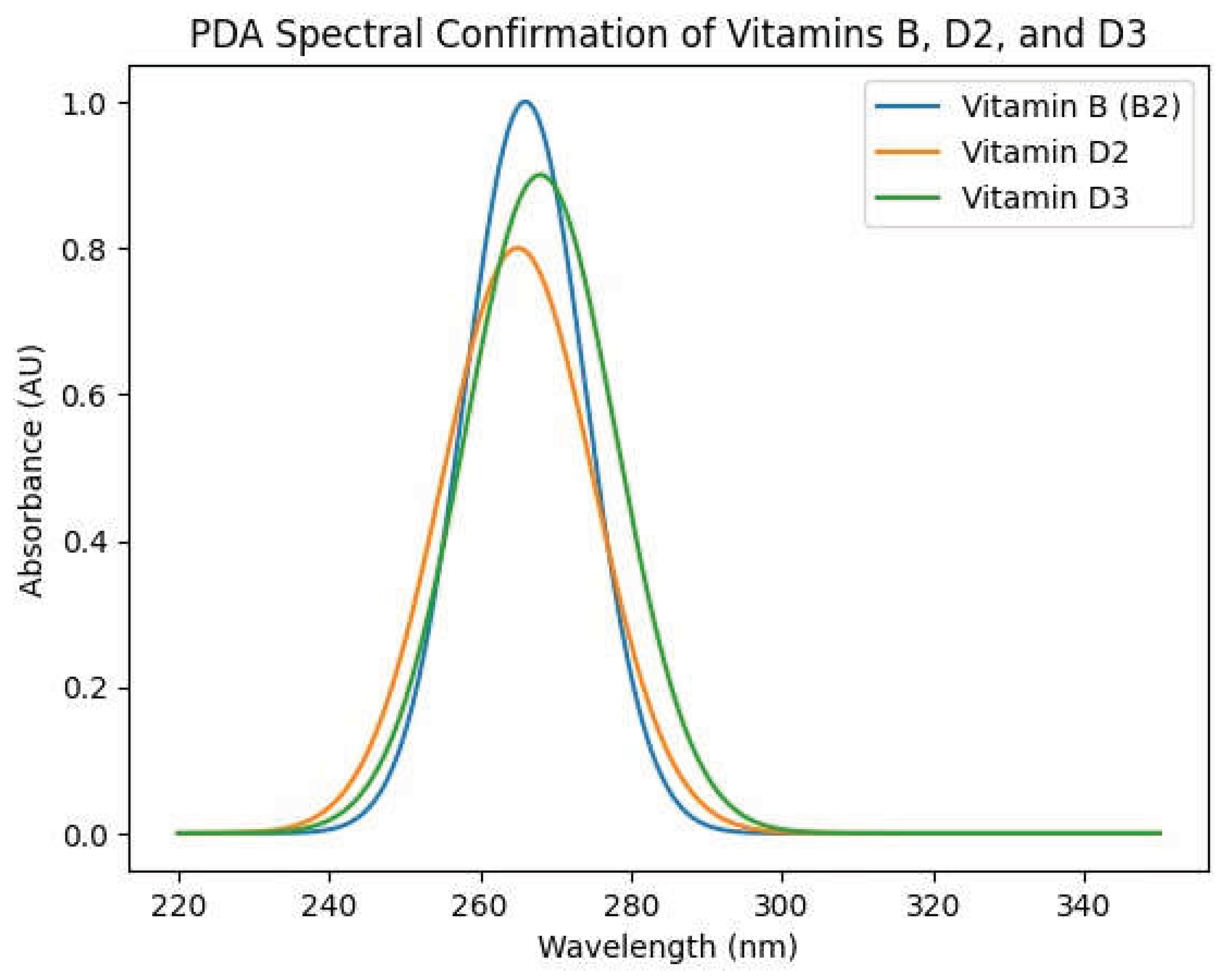
High-Performance Liquid Chromatography (HPLC) coupled with diode-array detection (DAD) was employed to verify the presence and stability of key vitamins in the concentrate. The spectral profiles (
Figure 1A and
Figure 1B) unequivocally confirmed the identity of Riboflavin (Vitamin B₂), Ergocalciferol (Vitamin D₂), and Cholecalciferol (Vitamin D₃) through their characteristic absorbance maxima in the 220–350 nm range. The distinct peaks and clean spectra indicate that the vitamins remained chemically intact through the emulsification and mixing process, with no significant degradation or formation of unwanted isomers observed under the processing conditions. This is a critical finding, as it demonstrates that the non-conventional, sequential mixing protocol in a temperature-controlled environment successfully preserved the labile vitamins.
3.4. Significance of the Non-Conventional Mixing Process
The "non-conventional" aspect of this methodology—the adiabatic, sequential addition and mixing of components in the concentrate phase—proved advantageous. This step-wise approach allowed for controlled interactions between the vitamins and stabilizers before subjecting the mixture to high-volume dilution. It is hypothesized that this method promoted a more uniform distribution of the surfactants around the lipid droplets and facilitated better encapsulation of the fat-soluble vitamins, leading to enhanced kinetic stability of the final microemulsion upon dilution.
3.5. Functional Implications and Potential Benefits
The formulated beverage is positioned as a functional hydration solution. By combining essential electrolytes inherent in the BAV. H2O with a spectrum of vitamins, it moves beyond simple rehydration. The inclusion of Vitamins C, D, E, and B₂ targets foundational support for the immune system and antioxidant defenses, which may be particularly beneficial for individuals under physiological stress, including those exposed to high temperatures and dehydration risks exacerbated by climate change. The use of biocompatible, food-grade surfactants (PEG derivatives) and a natural preservative (rosmarinic acid) align with consumer trends favoring clean-label ingredients.
3.6. Limitations and Future Perspectives
While this study successfully establishes a proof-of-concept protocol, it is primarily a product development and stability investigation. The estimated vitamin concentrations per serving are based on formulation calculations and require quantitative analytical validation. Furthermore, the long-term shelf stability, in vivo bioavailability of the vitamins from this microemulsion system, and clinical health outcomes remain to be evaluated. Future work should focus on: i) conducting rigorous stability tests under various storage conditions, ii) performing pharmacokinetic studies to assess bioavailability compared to standard supplements, and iii) scaling up the process for industrial manufacturing while maintaining product quality.
4. Conclusion
This study successfully developed and demonstrated a viable, science-based protocol for producing a novel multi-vitamin fortified beverage designed to address both hydration and nutritional needs in the context of increasing global temperatures. The key accomplishment lies in the formulation of a stable, clear, and palatable beverage that combines both water-soluble (Vitamins C and B₂) and fat-soluble (Vitamins D₃ and E) vitamins—a notable challenge in beverage science.
The innovation of this work is twofold. First, the two-stage production method—creating a stabilized microemulsion concentrate using PEG-based surfactants and rosmarinic acid, followed by dilution into biologically active water—proved effective in overcoming solubility and instability barriers. Second, the non-conventional sequential mixing process ensured the preservation of vitamin integrity and promoted a homogeneous final product.
The resulting beverage, with a consumer-friendly pH of 6.0 and a pleasant taste profile, represents a practical, functional alternative to plain water. It is specifically designed to support populations in arid and high-heat regions, where dehydration risks are compounded by climate change and where access to varied nutrition may be limited.
While this study establishes a strong foundational proof-of-concept, further work is essential to translate this formulation into a widely adoptable solution. Future research should prioritize:
Clinical trials to validate the in vivo bioavailability of vitamins and assess measurable health outcomes related to hydration and immune function.
Long-term stability studies under real-world storage conditions to determine shelf life and optimize packaging.
Scalability assessments to adapt the protocol for cost-effective, sustainable industrial production.
In summary, this formulation offers a promising, science-driven approach to functional hydration, aligning nutritional supplementation with public health needs in an era of environmental change. It underscores the potential of integrated food science to create targeted dietary solutions that are both preventive and supportive of overall well-being.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Additional information can be found in the attached file of Supplementary Information submitted along with this Manuscript.
Data Availability Statement
All data supporting the findings of this study are available within the manuscript and Supplementary Files.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This study was conducted independently and received no external funding from commercial, industrial, or pharmaceutical entities
References
- 1 Jéquier, E; Constant, F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr 2010, 64(2), 115–23. [Google Scholar] [CrossRef] [PubMed]
-
IPCC, 2022: Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., Okem, A., Rama, B., Eds.; Cambridge University Press. Cambridge University Press: Cambridge, UK and New York, NY, USA; 3056. [CrossRef]
- Popkin, BM; D'Anci, KE; Rosenberg, IH. Water, hydration, and health. Nutr Rev. 2010, 68(8), 439–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J Investig Med 2011, 59(6), 881–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ball, G.F.M. Vitamins In Foods: Analysis, Bioavailability, and Stability, 1st ed.; CRC Press, 2005. [Google Scholar] [CrossRef]
- Tadros, T. F. Applied Surfactants: Principles and Applications; Wiley-VCH, 2013. [Google Scholar]
- Salako, O.; Sarris, I.; Ojo, B. I.; Akingbade, M.; Eze, V. C.; Salako, I. S. Extraction, Characterization, and Efficacy of Rosmarinic and Carnosic Acids as Natural Preservatives in Enhancing the Shelf Life of Food Models and Nephroprotective Potential. Preprints 2025, 2025100985. [Google Scholar] [CrossRef]
- Lussi, A.; Jaeggi, T. Erosion—diagnosis and risk factors. Clin Oral Invest 2008, 12 Suppl 1, 5–13. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).