1. Introduction
In device development, a well-defined characterization platform is a prerequisite for achieving an efficient and optimized final product. MN development is no exception. Given their intended applications, it is essential to conduct systematic evaluations of their performance in order to achieve the most effective MN design [
1]. Since MNs primarily interact with biological tissue, an appropriate tissue model for initial evaluation is critical. Humans represent the primary target group for these devices, making human skin the ideal model. However, ethical constraints and the limited availability of fresh human tissue make comprehensive testing across different tissue types nearly impossible. Moreover, recent studies have highlighted the potential of MNs not only for transdermal use but also for applications involving internal tissues [
2,
3], further underscoring the impracticality of obtaining all relevant biological samples. This creates a pressing need for models that closely replicate the structural and mechanical properties of real tissues. Thus, alongside optimizing MN design and materials, it is equally essential to ensure that the tissue models employed are as physiologically relevant as possible.
In recent years, artificial tissues, particularly polymer-based models such as agarose gel, polydimethylsiloxane (PDMS), and Parafilm, have provided new opportunities to characterize MN systems and analyze diverse aspects of their performance [
4,
5]. Their significance has become increasingly evident. Without such models, the ability to systematically evaluate MN performance would have been severely limited, hindering progress in biomedical applications. Artificial tissues have been particularly valuable for assessing mechanical performance, such as insertion and extraction behavior. For example, agarose gel has been widely used to investigate MN penetration capabilities, and it has also been employed in drug delivery studies, where release and diffusion processes can be assessed in vitro [
6,
7,
8]. Similarly, artificial tissues have been used in biosensing applications, further demonstrating their versatility and importance. Three-dimensional microfluidic culture systems have also been widely employed as engineered tissue analogues, as they enable controlled investigation of oxygen and nutrient transport, shear stress, and cellular organisation under well-defined geometric and perfusion conditions [
9].
This review explores the diverse applications of artificial tissues in MN research, with a particular emphasis on their role as platforms for characterization and performance evaluation rather than as biological substitutes alone. While numerous studies have reported individual tissue-mimicking models for specific MN applications, a consolidated perspective linking model selection to the particular performance metrics being evaluated remains limited. Here, artificial tissue models are examined across mechanical testing, therapeutic delivery, and biosensing applications, highlighting how different material choices and platform designs influence experimental outcomes. By organising the literature around the functional role of artificial tissues in MN development, this review aims to support more informed selection of tissue models and to clarify their advantages and limitations at different stages of MN research. The review concludes with a discussion of future perspectives and directions for research in this area.
2. Why Artificial Tissues?
The rationale for employing artificial tissue models is to establish reliable, accessible platforms for MN testing and evaluation. The use of real tissue samples often entails significant challenges. Ethical approval and regulatory requirements for animal models create delays, which, in turn, affect research timelines and resource allocation [
10]. In addition, animal testing requires strict storage and maintenance conditions, with high associated costs that pose a significant obstacle, particularly during the early stages of MN development.
Artificial synthetic tissue models, by contrast, are readily available and offer a high degree of flexibility. Their properties can be customized to mimic the mechanical and structural characteristics of target tissues, a level of control that is not achievable with in vivo or ex vivo samples. For instance, animal skin, e.g., porcine skin, commonly used as one of the closest analogues to human skin, still exhibits variability in properties depending on factors such as storage conditions, animal age, and anatomical origin. These variations may not align with the specific tissue type for which MNs are designed, limiting the reliability of results.
Artificial models are scalable and suitable for industrial and commercial applications. Their ease of preparation and tunability make them highly practical for multiple testing purposes. Furthermore, they enable the modeling of extreme physiological conditions that would be unsafe or impractical to replicate in vivo. In drug delivery applications, these models provide a valuable platform for early-stage formulation optimization, including dosage determination, prior to extensive in vivo studies.
3. Applications of Artificial Tissue Models
As tools for MN testing and characterization, artificial tissue-mimetic models have been employed for various evaluation purposes. These models provide a means to study microneedle–tissue interactions across a wide range of applications. Their ability to replicate the mechanical and diffusional characteristics of real tissue samples makes them particularly valuable for assessing MN performance in therapeutic and biosensing fields. In the following subsections, selected studies in this area are presented.
3.1. MN-Tissue Mechanical Interaction Analysis
In MN applications, tissue serves as the primary pathway, and all factors influencing MN–tissue interactions directly affect device performance. These include insertion force, penetration depth, and extraction force in the case of non-dissolvable microneedles. To facilitate systematic experimentation and iterative optimization, a variety of artificial skin models have been developed.
Parafilm
® M, composed of hydrocarbon wax and polyolefin, is commonly employed as a membrane model to simulate layered tissue structures in MN penetration studies. For instance, eight layers of Parafilm were used in vitro to mimic the thickness of human skin, with each layer averaging 126 µm in thickness. The insertion capability of microneedle patches with different formulations was quantified by measuring the number of layers penetrated [
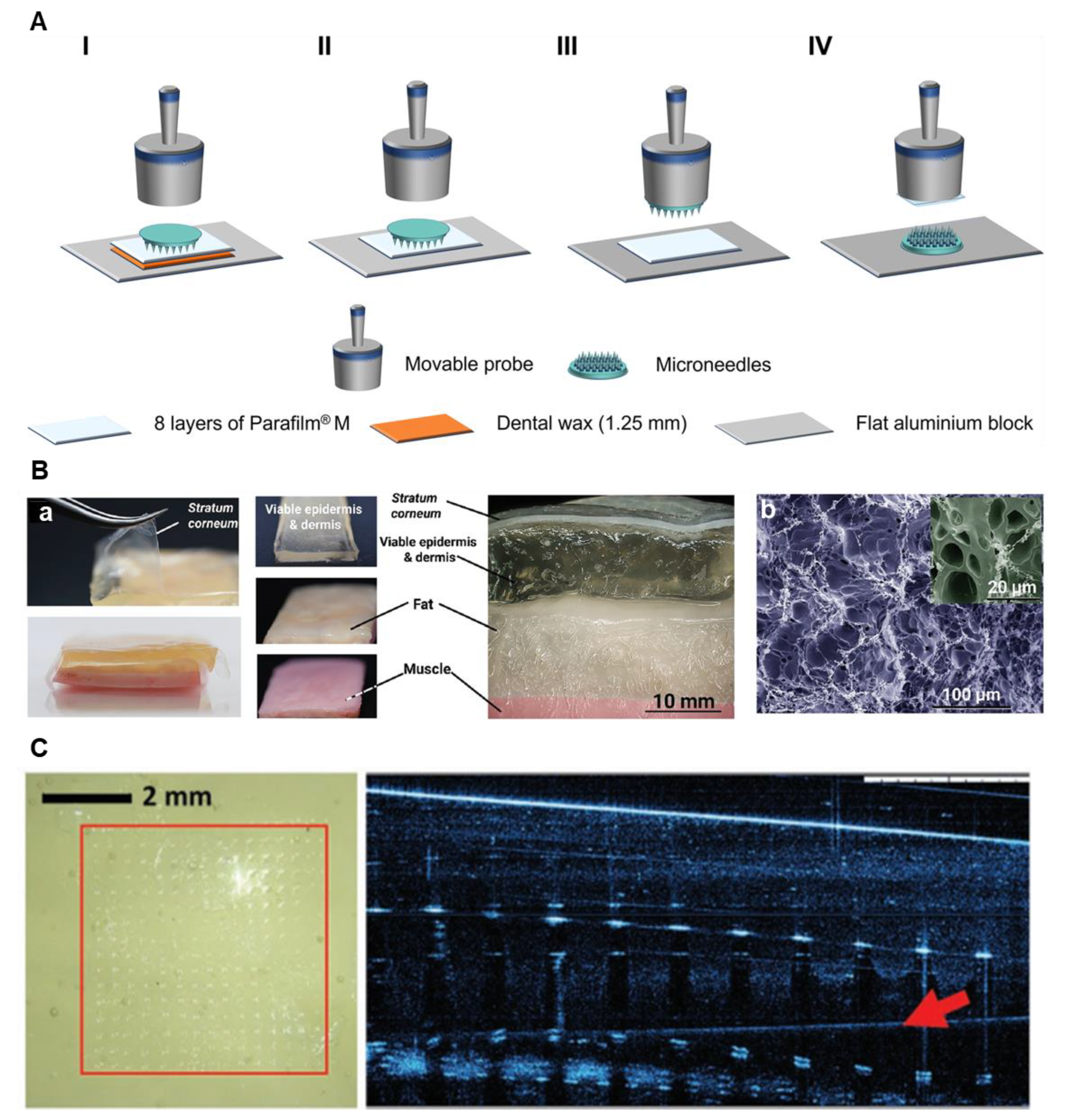
11]. In another study, an MN insertion setup was used to analyze MN penetration depth, as shown in
Figure 1A. As in the previous approach, eight layers of Parafilm
® M were stacked together as the tissue model. Penetration depth was evaluated by calculating the percentage of holes created by the MNs at each layer of Parafilm [
12].
Agarose gel, a hydrogel widely used in biomedical applications [
13], has also demonstrated utility as a tissue-mimetic model due to its viscoelastic properties, which resemble those of hydrated biological tissues. In MN studies, agarose gels have been employed to measure MN insertion and extraction forces, as well as to assess MN tissue adhesiveness through quantification of extraction behavior [
6,
14]. These characteristics make agarose gel a valuable model for studying MN performance in hydrated tissue environments [
14].
In another study, Makvandi et al. developed a composite tissue model composed of a silicone layer and a gel layer, designed to mimic the stratum corneum and the hydrated viable epidermis/dermis, respectively [
15]. The tissue structure is shown in
Figure 1B. This artificial model was proposed as an alternative to in vivo or ex vivo tissue samples for testing MN penetration. The silicone layer, incorporated to replicate the stratum corneum, was used to evaluate the insertion performance of hydrogel microneedle arrays across subcutaneous tissue layers. Penetration tests were conducted using three substrates: porcine skin, agarose gel, and the engineered tissue model. The findings showed that, with respect to MN penetrability, the tissue model yielded results more comparable to those from porcine skin than agarose gel. The channel widths formed by MN insertion were ≈300 µm in ex vivo porcine skin and ≈280 µm in the tissue model, both significantly wider than those generated in agarose gel (>10 µm).
Figure 1C shows the MNs penetration into the tissue model.
3.2. Drug Delivery and Therapeutic Evaluation
The use of artificial tissue models has created new opportunities for MN testing in therapeutic applications such as drug delivery. These tissue-mimicking platforms enable comprehensive analysis of various aspects of the drug release process. For instance, the transparency of agarose gel makes it a suitable candidate for visualizing drug diffusion pathways. Ramalheiro et al. demonstrated the release profile of fluorescently labeled theranomycin-loaded cubosome-like particles using agarose gel as a tissue model [
16]. The particles were incorporated into dissolving microneedles, which fully dissolved in 3% agarose gel within one minute, releasing their cargo into the gel matrix.
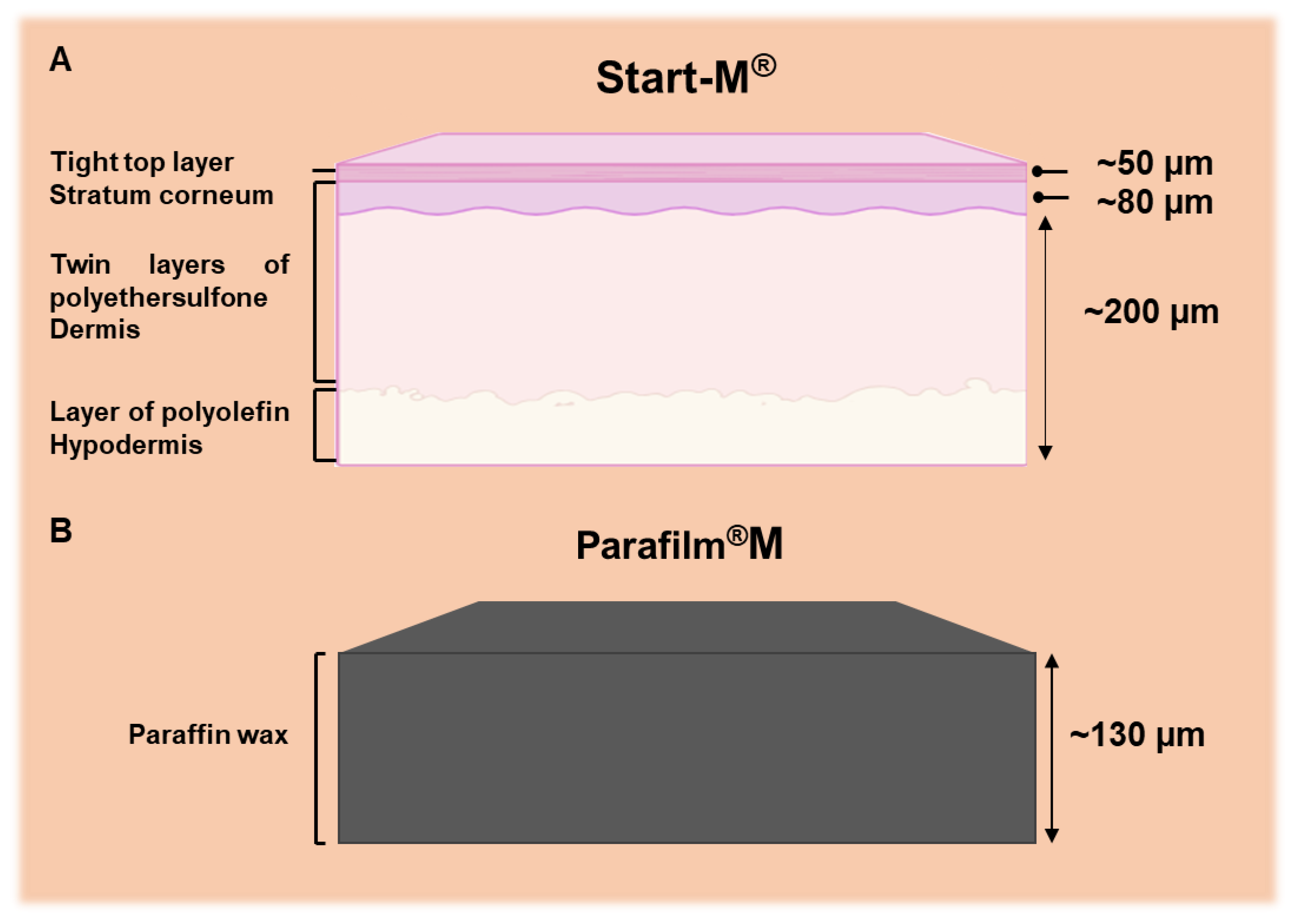
Biological variability in real tissues often masks the effects of experimental parameters, thereby directly affecting the reliability and reproducibility of results. Since these artificial tissues are free of biological interference, the analysis can focus solely on the intended aspects of the platform without concern for undesired or uncontrolled biological factors. This is particularly important in cases such as protein or peptide transdermal delivery, where the use of real biological tissues can complicate analysis due to protein interference. Artificial models such as Parafilm
® M and Strat-M
® were proposed as reliable alternatives to dermatomed porcine skin [
17]. Strat-M
®, engineered to mimic human skin, consists of multiple layers: a lipid-based outer layer representing the stratum corneum; a porous polyether sulfone middle layer simulating the dermis; and a polyolefin non-fabric support layer mimicking the subcutaneous tissue.
Figure 2 schematically illustrates the structure of these tissue models. These artificial models were employed to evaluate dissolving microneedle patches for transdermal delivery of proteins such as ovalbumin, bovine serum albumin, and metabolites derived from amniotic mesenchymal stem cells. By circumventing the challenges of protein interference in biological tissues, they enhance the reliability of experimental data.
Sometimes, the importance and accuracy of a particular aspect of MN performance necessitate the use of artificial tissue models alongside animal tissues. For example, in a recent study by Leite et al., the dissolution performance of single-component polysaccharide-based, lidocaine-loaded dissolving MNs (PL- Lid MNs) was evaluated using two different tissue models: agarose hydrogel and porcine ear skin [
18]. The dissolution assay conducted on agarose gel showed results comparable to those on porcine skin tissue, with a similar dissolution rate and loss of integrity over the same time points. In both models, the microneedle tips were fully dissolved within 10 minutes of insertion. 26% and 32% of the lidocaine remained in the patch after insertion into agarose hydrogel and porcine skin, respectively.
3.2.1. Cell–Laden Artificial Tissue Models
To evaluate cellular responses to therapeutic agents and assess treatment effectiveness, it is common to extend MN testing to cell-based platforms. To date, cytotoxicity analyses have primarily been conducted using 2D culture media. However, recent studies have begun to advance this approach by culturing cells within 3D tissue models, making the testing environment more complex and closely aligned with real physiological conditions.
3.2.1.1. 3D Bioprinted Models
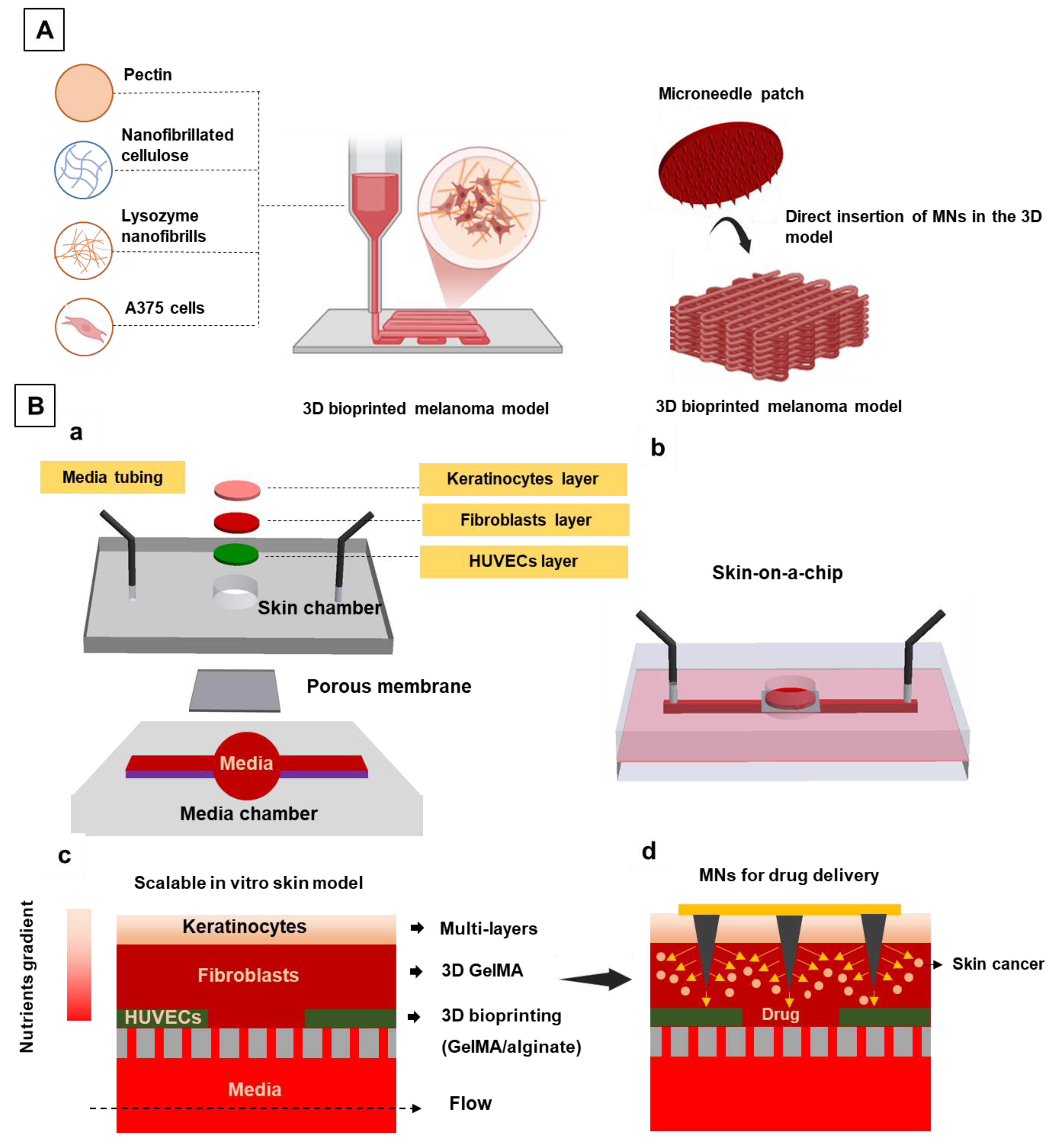
The antitumor activity of the dissolvable carboxymethylcellulose–fucoidan (CMC_Fuc) MNs against melanoma cells (A375 cell line) was evaluated using a 3D bioprinted cell-laden culture model (
Figure 3A) [
19]. The 3D in vitro model was fabricated using a bioink composed of a pectin nanocomposite hydrogel containing 4.7% (w/v) pectin, 2.0% (w/v) nanofibrillated cellulose (NFC), and 0.75% (w/v) lysozyme nanofibrils (LNFs), loaded with A375 cells at a density of 3 × 10
6 cells mL⁻¹. Using this structure offers several advantages, as it better mimics the natural physiological conditions of the tumor microenvironment, including cell–cell interactions, spatial complexity, and cellular communication, which are not adequately represented in conventional 2D cultures. The antitumor activity of the MNs was assessed in both 2D cell culture media and the 3D bioprinted cell-laden tissue model. The MN array exhibited a clear cytotoxic effect on A375 cells after 24 and 48 hours of application in both culture systems, as reflected by the observed reduction in cell viability. In the 2D culture, cell viability decreased to 59 ± 4% after 24 h and 17 ± 7% after 48 h. In the 3D tissue model, viability was reduced to 68 ± 9% after 24 h and 44 ± 4% after 48 h. The comparatively stronger cytotoxic response in the 2D model is attributed to its lower physiological complexity relative to the 3D environment, which reduces cellular resistance to treatment and thereby increases susceptibility to the MN-induced antitumor effect.
3.2.1.2. Wound Models
Another key concern with artificial skin models is their ability to replicate pathological or damaged tissue conditions. Addressing this issue, Silve et al. developed a reproducible wound model using a 3D skin construct [
20]. The system consisted of a fully differentiated, stratified squamous epithelium derived from human keratinocytes grown at an air–liquid interface on a type I collagen scaffold seeded with human dermal fibroblasts. Wound conditions were simulated using a microneedle stamping method, allowing the creation of length-tailored wound areas for therapeutic evaluation. The morphology, cell viability, and immunological responses at the simulated wound site were evaluated using histological staining, metabolic assays, and cytokine expression analysis.
3.2.1.3. Skin-On-a-Chip Platforms
Skin-on-a-chip platforms have been introduced as robust model systems that provide physiologically relevant conditions for disease modeling and drug efficacy analysis [
21,
22]. To investigate the progression of skin-related diseases and evaluate the effectiveness of drug treatments, a skin-on-a-chip model was developed by Barros et al. [
21]. The system was designed on a PDMS-based microfluidic platform consisting of a microchannel for media perfusion, a porous polyester membrane (0.4 μm pore size), and a PDMS skin chamber, as shown in
Figure 3B. The engineered skin model incorporated vascular layer, dermis and epidermis presenting enhanced functionality and maturation. Dermal layer development was confirmed by Collagen I and Fibronectin expression (after 7 days), while epidermal layer maturation was validated by the expression of Filaggrin and Keratin 10, 14, and 19 at the air–liquid interface (ALI) over 21 days. To simulate melanoma invasion into the dermis, melanoma cells were placed 400 µm below the epidermal layer, adjacent to the dermis. The skin-on-a-chip platform was subsequently utilized to assess the therapeutic efficacy of doxorubicin (DOX)-loaded gelatin methacryloyl (GelMA) MNs for targeted treatment of melanoma cancer cells.
3.3. Biosensing
As for another application regarding the MN system, MNs present potential as biosensory system. The detection platform used in this system can be based on two main principles of on-site and off-site mechanisms. Similar to the drug delivery performance analysis, the biosensing function of the MN array system needs to be monitored for well-established results. Use of artificial tissue models for the preliminary steps of in vitro test series for microneedle performance assessment could be as a reference for sensing function analysis. Employing artificial tissue samples allows the option of sensitivity analysis of the MN testing system under different conditions for a variety of biomarkers. Analyzing extreme conditions and manipulating the sensing environment are among the options offered through using these tissue-like platforms.
A patch of porous microneedles containing glucose oxidase (GOx) and horseradish peroxidase (HRP), and a colorimetric sensing layer containing 3, 30, 5, 50-tetramethylbenzidine (TMB) on the back of the MNs was prepared for rapid glucose sampling and sensing [
23]. For testing and validating the MN’s performance in rapid fluid sampling and collection, an agarose gel was used as a tissue model containing simulated fluid. The volume of the fluid extracted from agarose gel was measured in different time intervals. 34.57 ± 4.76 mg of the loaded fluid in agarose gel was extracted in 1 minute of patch wearing. Effects of different glucose concentrations on the performance of microneedles was also analyzed. A linear relationship was observed between the glucose content into the tissue structure and the recovered liquid volume by porous microneedles.
Manssouri Majd reported the preparation of a hydrogel-coated stainless-steel MN biosensor capable of selective and low-potential electrochemical glucose detection directly in skin-mimicking phantom [
24]. For in vitro testing of electrochemical functionality, a phantom-gel tissue was modeled to simulate the mechanical and diffusional properties of human tissue. Two different phantom gels were formulated using either Phosphate Buffered Saline (PBS) or interstitial fluid (ISF) to replicate the transdermal condition. Defined glucose concentrations, ranging from low (normal) to high (pathological) levels, were injected into the tissue models, and the electrochemical biosensing performance of the microneedles was evaluated under both conditions. The results demonstrated that both tissue models provided a promising skin-mimicking platform. However, the microneedle biosensor exhibited higher sensitivity in PBS than in ISF, attributed to the presence of multivalent ions and organic constituents in ISF that may interfere with detection. The biosensor’s selectivity, an essential feature given that microneedles operate in a complex microenvironment, was further evaluated by introducing interfering analytes, such as ascorbic acid, uric acid, and acetaminophen, at physiological or supraphysiological levels.
A summary of the reported studies is presented in
Table 1.
4. Limitations and Challenges
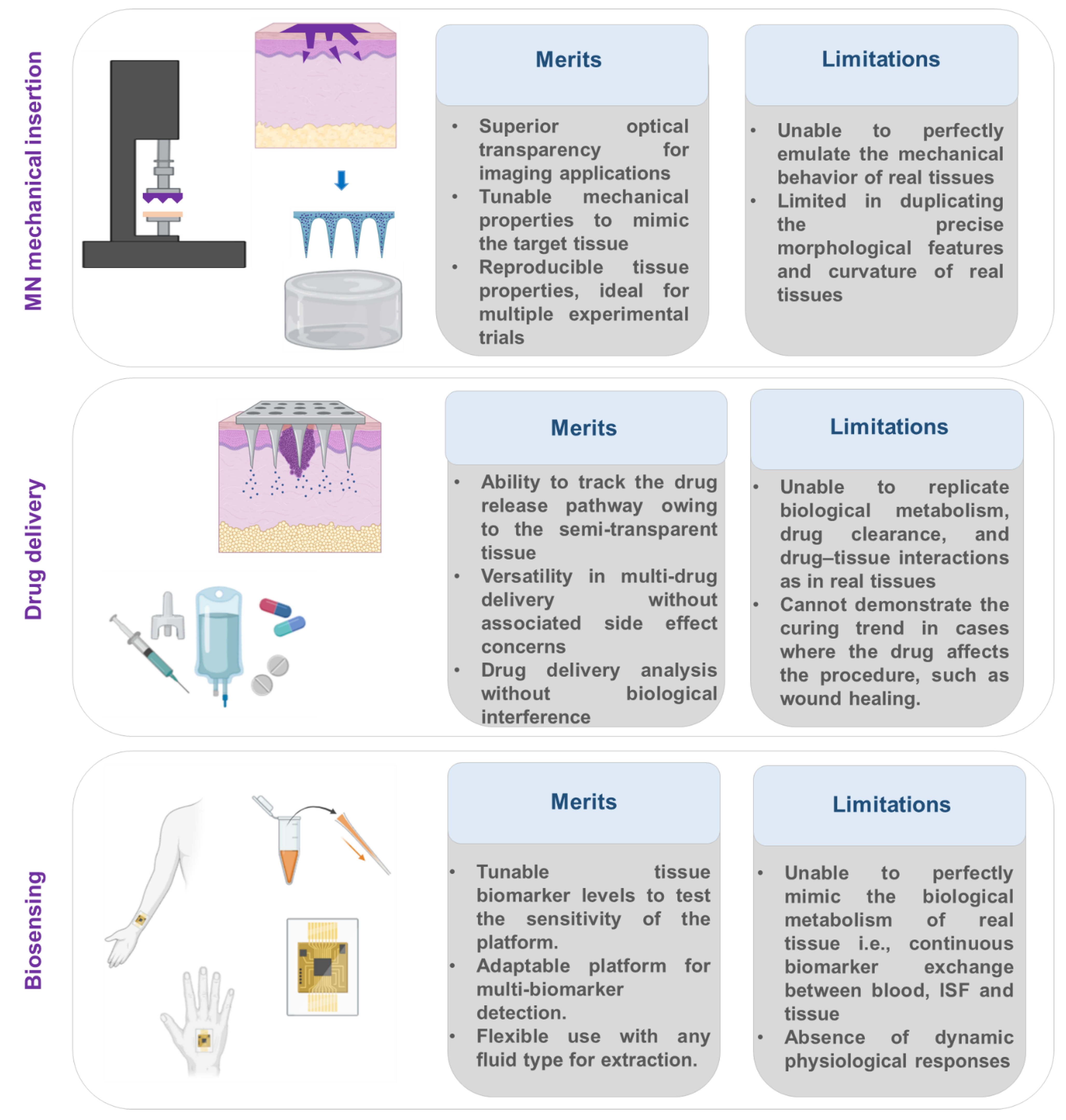
Despite the significant advantages of artificial tissue models for MN testing and post-analysis, several challenges persist. These models cannot fully replicate the structural, mechanical, and functional characteristics of real biological tissues. The unique morphology of each tissue type plays a crucial role in microneedle penetration and overall performance, which may not be accurately simulated in artificial platforms.
For instance, the curvature and delicate structure of ocular tissue pose specific challenges for artificial reproduction of ocular MN applications. In addition, physiological responses, such as tearing induced by sustained absence of blinking, cannot be mimicked in vitro. Similarly, if the target tissue is the breast, natural movements associated with respiration (e.g., chest expansion and relaxation) introduce dynamic mechanical conditions that are not easily simulated in artificial environments.
Another major limitation is the absence of systemic physiological components. Artificial tissue models lack integration with blood circulation, immune responses, and drug clearance pathways, all of which play critical roles in drug delivery and therapeutic outcomes. Likewise, for fluid extraction applications, replicating the dynamics of ISF flow remains a significant challenge.
It can be confidently stated that artificial tissue platforms are essential for in vitro microneedle testing and analysis during the early stages of MN development. Given the high costs of microneedle design and fabrication, these models offer a practical, cost-effective approach to overcoming challenges associated with repeated testing and design iteration. However, despite their advantages, artificial tissue platforms may still have limitations when used in the final stages of microneedle evaluation test series.
Figure 4.
Key information on the merits and limitations of artificial tissue models in microneedle studies. Elements were created with BioRender.
https://BioRender.com/.
Figure 4.
Key information on the merits and limitations of artificial tissue models in microneedle studies. Elements were created with BioRender.
https://BioRender.com/.
5. Commercial Readiness of Artificial Tissue Platforms
Looking ahead, and with heightened awareness following recent global health crises, microneedle-based medical technologies are poised to play an increasingly important role in healthcare delivery. As standardized testing workflows and safe production pathways become increasingly critical, robust, reliable characterization platforms have become a high priority. Consequently, there is a clear and growing need to advance the commercialization of artificial tissue models as realistic tissue surrogates, in parallel with the rapid technological progress of MN systems.
A useful framework for describing and benchmarking the maturity of such emerging characterization platforms is the Technology Readiness Level (TRL) scale, originally developed by the National Aeronautics and Space Administration (NASA) and now widely adopted across engineering, biomedical, and translational research domains. TRL defines technology maturation across 9 levels, ranging from fundamental concept formulation (TRL 1) to fully deployed, commercially operational systems (TRL 9) [
25]. Within translational research, this framework has proven particularly valuable for identifying gaps between laboratory proof-of-concept and practical deployment—commonly referred to as the “valley of death” [
26].
In the context of artificial tissue models for MN evaluation, most reported platforms currently reside in the mid-TRL range (approximately TRL 3–5), where feasibility has been demonstrated through laboratory validation and controlled testing. Progression toward higher TRLs requires not only improved physiological relevance but also clear evidence of reproducibility, robustness, scalability in manufacturing, and alignment with regulatory and industrial testing requirements. Importantly, TRL-based assessment does not imply replacement of in vivo studies. Instead, it provides a structured means of qualifying artificial tissue platforms as reliable preclinical screening and benchmarking tools within standardized development pipelines.
It is also essential to recognise that TRL, while widely used, is not without limitations. Studies examining TRL implementation across multiple industries have highlighted challenges related to assessment validity, system complexity, and contextual interpretation, particularly as technologies approach higher readiness levels [
25]. These findings emphasise that TRL alone cannot fully capture integration requirements, workflow compatibility, or the robustness of validation protocols, all of which are critical for commercial deployment. Consequently, the practical application of TRL frameworks in translational contexts often requires adaptation to sector-specific constraints and the provision of supporting evidence beyond nominal readiness classification.
For artificial tissue surrogates, this suggests that advancement toward commercial readiness will depend on demonstrating consistent performance across multiple microneedle designs, compatibility with industry-standard testing workflows, and documentation practices aligned with recognised quality and safety frameworks, such as those established by the International Organization for Standardization and the Food and Drug Administration. Ultimately, the successful commercialization of artificial tissue platforms has the potential to accelerate microneedle development, reduce development risk, and enable faster, safer market entry for microneedle-based technologies.
6. Conclusions and Future Perspective
With the rapid advancement in microneedle research, the need for a reliable, scalable characterization platform has become increasingly evident. From penetration to application (e.g., therapy or sensing), microneedle functionality must be rigorously evaluated with respect to insertion mechanics, drug delivery efficiency, and biosensing capabilities. Given the need for multiple characterization tests, artificial tissue platforms that closely simulate the properties of real tissues offer an excellent alternative for microneedle performance assessment, as they circumvent several challenges associated with in vivo and ex vivo tissue models.
This paper aimed to provide a distinct perspective on these artificial surrogates, which have consistently complemented in vivo tests during the microneedle evaluation process. It should be noted that, despite their flexibility and demonstrated potential as tissue mimics, the use of artificial models has thus far been largely restricted to the early stages of microneedle development and has not yet found widespread adoption in final evaluation stages. This limitation is likely due to their inability to fully replicate the complexity of real tissues.
Such dissimilarity, however, can be considered a double-edged sword. On one hand, simplified models are advantageous for preliminary testing, as they allow researchers to isolate and investigate specific aspects of microneedle functionality without interference. On the other hand, the absence of these biological interferences, which are an inseparable part of real tissues, can sometimes lead to findings that diverge from in vivo performance.
Looking forward, bridging this gap between early-stage screening and advanced evaluation will be critical not only for improving physiological relevance but also for enabling broader adoption of artificial tissue platforms in translational and industrial settings. In this context, continued development of standardized, scalable, and application-relevant artificial tissue models will be essential to support the reliable advancement of microneedle technologies toward clinical use and commercial deployment.
Therefore, it is worthwhile to direct greater efforts in research and development toward creating more realistic artificial samples for advanced microneedle evaluation. For instance, three-dimensional bioprinting technologies capable of generating functional tissue-like architectures represent a promising approach in this direction [
27].
Author Contributions
Conceptualization, N.K. and E.L.Z.; Literature review and analysis, E.L.Z.; Writing—original draft preparation, E.L.Z.; Writing—review and editing, N.K. Visualization, E.L.Z.; Supervision, N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian Research Council (ARC) Discovery Early Career Research Award (DECRA) (DE220100205).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the School of Engineering and Built Environment (EBE) at Griffith University for providing a supportive research environment and institutional support during the preparation of this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lori Zoudani, E.; Nguyen, N.T.; Kashaninejad, N. Microneedle optimization: Toward enhancing microneedle’s functionality and breaking the traditions. Small Structures 2024, 5, 2400121. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Pei, X.; Chen, J.; Wei, X.; Liu, Y.; Xia, P.; Wan, Q.; Gu, Z.; He, Y. Blue-ringed octopus-inspired microneedle patch for robust tissue surface adhesion and active injection drug delivery. Science advances 2023, 9, eadh2213. [Google Scholar] [CrossRef]
- Long, L.; Ji, D.; Hu, C.; Yang, L.; Tang, S.; Wang, Y. Microneedles for in situ tissue regeneration. Materials today bio 2023, 19, 100579. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, J.; Xiang, Q.; Dong, H. Hollow microneedle microfluidic paper-based chip for biomolecules rapid sampling and detection in interstitial fluid. Analytica Chimica Acta 2023, 1255, 341101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, X.; Kim, H.J.; Qu, M.; Jiang, X.; Lee, K.; Ren, L.; Wu, Q.; Wang, C.; Zhu, X. Gelatin methacryloyl microneedle patches for minimally invasive extraction of skin interstitial fluid. Small 2020, 16, 1905910. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Morde, R.S.; Mariani, S.; La Mattina, A.A.; Vignali, E.; Yang, C.; Barillaro, G.; Lee, H. 4D printing of a bioinspired microneedle array with backward-facing barbs for enhanced tissue adhesion. Advanced Functional Materials 2020, 30, 1909197. [Google Scholar] [CrossRef]
- Muresan, P.; McCrorie, P.; Smith, F.; Vasey, C.; Taresco, V.; Scurr, D.J.; Kern, S.; Smith, S.; Gershkovich, P.; Rahman, R. Development of nanoparticle loaded microneedles for drug delivery to a brain tumour resection site. European Journal of Pharmaceutics and Biopharmaceutics 2023, 182, 53–61. [Google Scholar] [CrossRef]
- Detamornrat, U.; Parrilla, M.; Domínguez-Robles, J.; Anjani, Q.K.; Larrañeta, E.; De Wael, K.; Donnelly, R.F. Transdermal on-demand drug delivery based on an iontophoretic hollow microneedle array system. Lab on a Chip 2023, 23, 2304–2315. [Google Scholar] [CrossRef]
- Barisam, M.; Saidi, M.S.; Kashaninejad, N.; Vadivelu, R.; Nguyen, N.-T. Numerical Simulation of the Behavior of Toroidal and Spheroidal Multicellular Aggregates in Microfluidic Devices with Microwell and U-Shaped Barrier. Micromachines 2017, 8, 358. [Google Scholar] [CrossRef]
- Makvandi, P.; Kirkby, M.; Hutton, A.R.; Shabani, M.; Yiu, C.K.; Baghbantaraghdari, Z.; Jamaledin, R.; Carlotti, M.; Mazzolai, B.; Mattoli, V. Engineering microneedle patches for improved penetration: analysis, skin models and factors affecting needle insertion. Nano-Micro Letters 2021, 13, 93. [Google Scholar] [CrossRef]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. International journal of pharmaceutics: X 2020, 2, 100047. [Google Scholar] [CrossRef]
- Alrimawi, B.H.; Lee, J.Y.; Ng, K.W.; Goh, C.F. In vitro evaluation of microneedle strength: a comparison of test configurations and experimental insights. RSC Pharmaceutics 2024, 1, 227–233. [Google Scholar] [CrossRef]
- Karunanithi, S.; Rajappan, K. Biofunctionalized agarose-based biopolymer composites for advanced biomedical applications: a review. Polymer Bulletin 2025, 1–43. [Google Scholar] [CrossRef]
- Zoudani, E.L.; De Saram, P.; Engel, K.; Nguyen, N.-T.; Kashaninejad, N. Microneedle–Tissue Interaction Across Varying Biological and Mechanical Conditions. Biosensors 2025, 15, 521. [Google Scholar] [CrossRef]
- Makvandi, P.; Shabani, M.; Rabiee, N.; Anjani, Q.K.; Maleki, A.; Zare, E.N.; Sabri, A.H.B.; De Pasquale, D.; Koskinopoulou, M.; Sharifi, E. Engineering and development of a tissue model for the evaluation of microneedle penetration ability, drug diffusion, photothermal activity, and ultrasound imaging: a promising surrogate to ex vivo and in vivo tissues. Advanced Materials 2023, 35, 2210034. [Google Scholar] [CrossRef] [PubMed]
- Ramalheiro, A.; Paris, J.L.; Silva, B.F.; Pires, L.R. Rapidly dissolving microneedles for the delivery of cubosome-like liquid crystalline nanoparticles with sustained release of rapamycin. International Journal of Pharmaceutics 2020, 591, 119942. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Nainggolan, A.D.C.; Li, H.; Miatmoko, A.; Larrañeta, E.; Donnelly, R.F. Parafilm® M and Strat-M® as skin simulants in in vitro permeation of dissolving microarray patches loaded with proteins. International Journal of Pharmaceutics 2024, 655, 124071. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.M.; Silva, A.C.; Jesus, A.; da Cruz, B.C.; Vieira, S.I.; Dias-Pereira, P.; Costa, P.C.; Almeida, I.F.; Correia-Sá, I.; Silvestre, A.J. Single Polysaccharide Dissolvable Microneedles for Painless Local Anesthesia: Fabrication, Characterization, and In Vitro Neuronal Imaging. ACS Biomaterials Science & Engineering 2025, 11, 5426–5439. [Google Scholar] [CrossRef]
- Silva, A.C.; Teixeira, M.C.; Jesus, A.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Correia-Sá, I.; Oliveira, H.; Silvestre, A.J.; Vilela, C. Carboxymethylcellulose-fucoidan dissolvable microneedle patches for minimally invasive melanoma treatment: Demonstration on a 3D bioprinted A375 cell line model. International Journal of Biological Macromolecules 2025, 319, 145320. [Google Scholar] [CrossRef] [PubMed]
- Salazar Silva, C.S.; Petzold, W.; Hirsch, U.; Schmelzer, C.E.; Friedmann, A. A standardized in vitro bioengineered skin for penetrating wound modeling. In vitro models 2025, 4, 15–30. [Google Scholar] [CrossRef]
- Barros, N.R.; Kang, R.; Kim, J.; Ermis, M.; Kim, H.-J.; Dokmeci, M.R.; Lee, J. A human skin-on-a-chip platform for microneedling-driven skin cancer treatment. Materials Today Bio 2025, 30, 101399. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Gore, M.; Yadav, S.; Majumder, A.; Jain, R.; Dandekar, P. A 3D microfluidic model for preclinical drug permeation studies: Advancing validation of skin-on-chip technology. Journal of Pharmaceutical and Biomedical Analysis 2025, 117187. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, M.; Hu, W.; Cao, W.; Zhan, Y.; Zhang, Y.; Wang, Q.; Ma, T. Porous colorimetric microneedles for minimally invasive rapid glucose sampling and sensing in skin interstitial fluid. Biosensors 2023, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Majd, S.M. Hydrogel-coated microneedle biosensor for selective and low-potential glucose detection in skin-mimicking interstitial fluid phantoms. Talanta 2026, 297, 128555. [Google Scholar] [CrossRef] [PubMed]
- Olechowski, A.L.; Eppinger, S.D.; Joglekar, N.; Tomaschek, K. Technology readiness levels: Shortcomings and improvement opportunities. Systems Engineering 2020, 23, 395–408. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Kumar, V.; Nishad, S.N. Technology Readiness Level: An Assessment of the Usefulness of this Scale for Translational Research. Productivity 2022, 62, 112–124. [Google Scholar] [CrossRef]
- Yadav, S.; Nguyen, N.-T. Three-dimensional models of human skin for biomedical applications. Biophysics Reviews 2025, 6. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |