Submitted:
25 December 2025
Posted:
29 December 2025
You are already at the latest version
Abstract
Keywords:
Section 1: Introduction; Definition and Description of HPV
1.1. Basic Properties of HPV
1.2. Proposed Models of O2 Sensing in HPV
- According to the Redox theory, introduced by Kenneth Weir and Stephen Archer in 1995 [36], hypoxia decreases the production of ROS by the mitochondrial electron transport chain (ETC), leading to a fall in [ROS] and/or the reduction of redox couples in the cytoplasm. This evokes the closure of voltage gated K+ (KV) channels which are activated by basal ROS production under normoxic conditions, thus causing membrane depolarization, the opening of voltage-gated Ca2+ channels (VGCC), and therefore contraction. Although the Redox Theory stresses the importance of KV channels, there are also other effectors which could potentially respond to a fall in ROS or a reduction of cytoplasmic redox couples in such a way as to cause HPV. For example, there is evidence that the reduction of Cys42 on PKG1a (protein kinase G a1), which may exert a tonic vasorelaxing influence on PASMC, diminishes its activity [37].
- A proposal which we will refer to as the Mitochondrial ROS hypothesis, developed by Paul Schumacker, Naveen Chandal and colleagues and first described in 2001 [38], proposes that hypoxia causes an increase in mitochondrial ROS production, leading to a higher [ROS], and/or oxidation of redox couples, in the cytoplasm. This hypoxia-induced rise in cytoplasmic ROS may be supplemented by a PKCϵ-mediated stimulation of Nox which is triggered by the mitochondrial ROS [39]. The rise in cytoplasmic [ROS] might evoke contraction through multiple effector pathways, potentially including Ca2+ release from the sarcoplasmic reticulum, an increase in store operated Ca2+ influx, and RhoA/ Rho kinase-mediated Ca2+ sensitization [13,14,15].
- The processes responsible for O2 sensing and HPV have also been the subject of an extensive series of papers by the laboratories of Michael Wolin and Sachin Gupte (see [40] for a review ). In agreement with the Redox theory, these authors propose that hypoxia causes contraction by removing a normoxic vasodilating influence, although this is seen as being maintained largely by oxidation-induced activation of soluble guanylate cyclase and protein kinase G (PKGa1) rather than by the opening of KV channels. In addition, they have presented evidence that hypoxia activates the pentose phosphate pathway, thereby increasing the production of NADPH, and that this contributes to the inhibition of PKGa1 and also activates other Ca2+-dependent and -independent contractile mechanisms.
1.3. Normoxia, Physoxia and Hypoxia
Section 2: Reactive Oxygen Species as Signaling Molecules
2.1. ROS Definition and Function
2.2. H2O2 Signaling
Section 3: Cellular Mechanisms of ROS Production
3.1. Mitochondrial Regulation of Cytoplasmic H2O2
3.1.1. Mitochondrial ROS Production
3.1.1.1. Oxidative Phosphorylation
3.1.1.2. Electron Flow Through the ETC
3.1.1.3. Factors Governing Mitochondrial ROS Production

3.1.1.4. Mechanisms by Which Hypoxia May Increase Mitochondrial ROS Production
3.1.1.5. Possible Mechanisms by Which Hypoxia Could Decrease Mitochondrial ROS Production
3.1.2. Mitochondrial ROS Consumption
3.2. ROS Production by NADPH Oxidases
3.2.1. Nox1
3.2.2. Nox4
4. Intra- and Extracellular H2O2 Concentrations
Section 5: Models of O2 Sensing in HPV
5.1. The Redox Theory
5.1.1. Evidence for a Fall in Mitochondrial ROS Production and PASMC Reduction as the O2 Sensor in HPV
5.1.2. The Involvement of ROS in PASMC K+ Channel Inhibition During Hypoxia


5.2. The Mitochondrial ROS Theory of HPV
5.2.1. Role of Mitochondrial Ca2+ and the Rieske Iron-Sulfur Protein in Hypoxia-Induced ROS Production
5.2.2. Mitochondrial Hyperpolarization, Reduction of the Quinone Pool, and Cox4i2
5.2.2. HPV and Mitochondrial NCLX
5.2.3. Hypoxia and Increased ROS Production by Complex II
5.2.4. NAD(P)H Oxidase as a Source of Increased ROS During HPV
The Role of NADPH Oxidase in HPV: Summary and Conclusions
5.2.5. HPV Effector Mechanisms Coupled to an Increase in PASMC [ROS]
6.3. H2S, ROS and HPV
6.4. Effects of Hypoxia on Redox Couples
7. Critique of the Redox and Mitochondrial ROS Theories
- 4.
- Blockers of the ETC which induce a fall in mitochondrial ROS production should mimic hypoxia by causing inhibition of PASMC K+ currents and contraction and should also prevent HPV.
- 5.
- Similarly, anti-oxidants should cause a sustained contraction. Their effect on HPV is more difficult to predict, since a small anti-oxidant effect might add to that of hypoxia to enhance HPV, while a large anti-oxidant effect could abolish any further response to hypoxia.
- 6.
- Pro-oxidants should prevent and reverse HPV.
- 7.
- Blockers of the ETC which diminish mitochondrial ROS production should not cause contraction under normoxic conditions but should inhibit HPV.
- 8.
- ETC blockers like myxothiazol or rotenone which act at or upstream of the Qo site of complex III should inhibit HPV. Antimycin, shown by investigators to increase complex III-mediated ROS production in many types of cells, would be expected to cause PASMC to contract during normoxia, but its effects on HPV are difficult to predict.
- 9.
- Anti-oxidants should have no effect on PASMC tension in normoxia, but should block HPV.
7.1. Pulmonary Effects of ETC Blockers



7.2. Pulmonary Vascular Effects of Antioxidants





7.3. Effects of Oxidants on Basal Tone and HPV


7.4. Effects of Hypoxia on PASMC ROS Levels



7.5. Concluding Remarks: The Redox and Mitochondrial Models
8. The Role of the Pentose Phosphate Pathway and the Withdrawal of Normoxic Vasodilation Maintained by Nox4, H2O2 and Protein Kinase G in HPV
8.1. Early Studies: HPV as the Loss of Tonic H2O2 and sGC-Mediated Vasorelaxation
8.2. PPP Activity as a Determinant of the Effects of Hypoxia on Vascular Tone
8.2. Loss of Basal H2O2-Induced Stimulation of sGC and PKG as a Mechanism of HPV
8.2.1. Basal H2O2 Production and HPV
8.2.2. HPV and Regulation of sGC and PKG by H2O2
8.3. Does the Presence of Extracellular H2O2 affect HPV?
8.4. Summary and Critique: Does Activation of the PPP and the Withdrawal of H2O2/PKG – Mediated Normoxic Vasodilation Cause HPV?
- Under normoxic conditions, the production of superoxide/H2O2 by Nox4 is greater in PA than in systemic arteries because the PPP, being more active, generates more NADPH. The resulting higher level of H2O2 creates an ongoing vasodilating influence by stimulating PKG1a, both directly by oxidizing Cys42 on PKG1a, and indirectly by activating sGC. Hypoxia decreases the production of superoxide/H2O2 by Nox4, thereby raising vascular tone by inhibiting this baseline vasorelaxation.
- Hypoxia also suppresses the activity of PKG1a by stimulating G-6-PD and the PPP, causing a consequent increase in the NADPH/NADP+ ratio. This acts, at least in part, through Trx-1 and TrxR-1, to reduce PKG1a, causing it to become less active, which promotes contraction. The activation of G-6-PD is proposed to result from an increase in cytoplasmic [ROS], which promotes also HPV by stimulating rho kinase and Ca2+-dependent contractile mechanisms.
8.4.1. Decreased Production of H2O2 by Nox as a Cause of HPV
8.4.2. Does a Fall in H2O2 During Hypoxia Cause HPV by Inhibiting sGC?
8.4.3. Does a Fall in H2O2 During Hypoxia Cause HPV by Directly Inhibiting Protein Kinase G?
8.4.4. Is Activation of the PPP Important for HPV?
9. Conclusion
Acknowledgements
References
- Gierhardt, M.; Pak, O.; Walmrath, D.; Seeger, W.; Grimminger, F.; Ghofrani, H.A.; Weissmann, N.; Hecker, M.; Sommer, N. Impairment of hypoxic pulmonary vasoconstriction in acute respiratory distress syndrome. Eur Respir Rev 2021, 30. [CrossRef]
- Sommer, N.; Dietrich, A.; Schermuly, R.T.; Ghofrani, H.A.; Gudermann, T.; Schulz, R.; Seeger, W.; Grimminger, F.; Weissmann, N. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J 2008, 32, 1639-1651. [CrossRef]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic pulmonary vasoconstriction. Physiol Rev 2012, 92, 367-520. [CrossRef]
- Nagendran, J.; Stewart, K.; Hoskinson, M.; Archer, S.L. An anesthesiologist's guide to hypoxic pulmonary vasoconstriction: implications for managing single-lung anesthesia and atelectasis. Curr Opin Anaesthesiol 2006, 19, 34-43. [CrossRef]
- Dinenno, F.A. Skeletal muscle vasodilation during systemic hypoxia in humans. J Appl Physiol (1985) 2016, 120, 216-225. [CrossRef]
- Dorrington, K.L.; Clar, C.; Young, J.D.; Jonas, M.; Tansley, J.G.; Robbins, P.A. Time course of the human pulmonary vascular response to 8 hours of isocapnic hypoxia. Am J Physiol 1997, 273, H1126-1134. [CrossRef]
- Talbot, N.P.; Balanos, G.M.; Dorrington, K.L.; Robbins, P.A. Two temporal components within the human pulmonary vascular response to approximately 2 h of isocapnic hypoxia. J Appl Physiol (1985) 2005, 98, 1125-1139. [CrossRef]
- Marshall, B.E.; Clarke, W.R.; Costarino, A.T.; Chen, L.; Miller, F.; Marshall, C. The dose-response relationship for hypoxic pulmonary vasoconstriction. Respir Physiol 1994, 96, 231-247. [CrossRef]
- Fishman, A.P. Acute hypoxia and pulmonary vasoconstriction in humans: uncovering the mechanism of the pressor response. Am J Physiol Lung Cell Mol Physiol 2004, 287, L893-894. [CrossRef]
- Robertson, T.P.; Hague, D.; Aaronson, P.I.; Ward, J.P. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol 2000, 525 Pt 3, 669-680. [CrossRef]
- Weissmann, N.; Dietrich, A.; Fuchs, B.; Kalwa, H.; Ay, M.; Dumitrascu, R.; Olschewski, A.; Storch, U.; Mederos y Schnitzler, M.; Ghofrani, H.A.; et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A 2006, 103, 19093-19098. [CrossRef]
- Jain, P.P.; Hosokawa, S.; Xiong, M.; Babicheva, A.; Zhao, T.; Rodriguez, M.; Rahimi, S.; Pourhashemi, K.; Balistrieri, F.; Lai, N.; et al. Revisiting the mechanism of hypoxic pulmonary vasoconstriction using isolated perfused/ventilated mouse lung. Pulm Circ 2020, 10, 2045894020956592. [CrossRef]
- Jabr, R.I.; Toland, H.; Gelband, C.H.; Wang, X.X.; Hume, J.R. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol 1997, 122, 21-30. [CrossRef]
- Weigand, L.; Foxson, J.; Wang, J.; Shimoda, L.A.; Sylvester, J.T. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 2005, 289, L5-L13. [CrossRef]
- Robertson, T.P.; Dipp, M.; Ward, J.P.; Aaronson, P.I.; Evans, A.M. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol 2000, 131, 5-9. [CrossRef]
- Aaronson, P.I.; Robertson, T.P.; Ward, J.P. Endothelium-derived mediators and hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 2002, 132, 107-120. [CrossRef]
- Archer, S.L.; Wu, X.C.; Thebaud, B.; Nsair, A.; Bonnet, S.; Tyrrell, B.; McMurtry, M.S.; Hashimoto, K.; Harry, G.; Michelakis, E.D. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res 2004, 95, 308-318. [CrossRef]
- Connolly, M.J.; Prieto-Lloret, J.; Becker, S.; Ward, J.P.; Aaronson, P.I. Hypoxic pulmonary vasoconstriction in the absence of pretone: essential role for intracellular Ca2+ release. J Physiol 2013, 591, 4473-4498. [CrossRef]
- Moudgil, R.; Michelakis, E.D.; Archer, S.L. Hypoxic pulmonary vasoconstriction. J Appl Physiol (1985) 2005, 98, 390-403. [CrossRef]
- Wang, L.; Yin, J.; Nickles, H.T.; Ranke, H.; Tabuchi, A.; Hoffmann, J.; Tabeling, C.; Barbosa-Sicard, E.; Chanson, M.; Kwak, B.R.; et al. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest 2012, 122, 4218-4230. [CrossRef]
- Sommer, N.; Strielkov, I.; Pak, O.; Weissmann, N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. Eur Respir J 2016, 47, 288-303. [CrossRef]
- Dao, V.T.; Elbatreek, M.H.; Altenhofer, S.; Casas, A.I.; Pachado, M.P.; Neullens, C.T.; Knaus, U.G.; Schmidt, H. Isoform-selective NADPH oxidase inhibitor panel for pharmacological target validation. Free Radic Biol Med 2020, 148, 60-69. [CrossRef]
- Archer, S.L.; Gomberg-Maitland, M.; Maitland, M.L.; Rich, S.; Garcia, J.G.; Weir, E.K. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 2008, 294, H570-578. [CrossRef]
- Nozik-Grayck, E.; Stenmark, K.R. Role of reactive oxygen species in chronic hypoxia-induced pulmonary hypertension and vascular remodeling. Adv Exp Med Biol 2007, 618, 101-112. [CrossRef]
- Wang, Y.X.; Zheng, Y.M. ROS-dependent signaling mechanisms for hypoxic Ca(2+) responses in pulmonary artery myocytes. Antioxid Redox Signal 2010, 12, 611-623. [CrossRef]
- Resta, T.C.; Broughton, B.R.; Jernigan, N.L. Reactive oxygen species and RhoA signaling in vascular smooth muscle: role in chronic hypoxia-induced pulmonary hypertension. Adv Exp Med Biol 2010, 661, 355-373. [CrossRef]
- Veit, F.; Pak, O.; Brandes, R.P.; Weissmann, N. Hypoxia-dependent reactive oxygen species signaling in the pulmonary circulation: focus on ion channels. Antioxid Redox Signal 2015, 22, 537-552. [CrossRef]
- Jaitovich, A.; Jourd'heuil, D. A Brief Overview of Nitric Oxide and Reactive Oxygen Species Signaling in Hypoxia-Induced Pulmonary Hypertension. Adv Exp Med Biol 2017, 967, 71-81. [CrossRef]
- Huetsch, J.C.; Suresh, K.; Shimoda, L.A. Regulation of Smooth Muscle Cell Proliferation by NADPH Oxidases in Pulmonary Hypertension. Antioxidants (Basel) 2019, 8. [CrossRef]
- Yan, S.; Resta, T.C.; Jernigan, N.L. Vasoconstrictor Mechanisms in Chronic Hypoxia-Induced Pulmonary Hypertension: Role of Oxidant Signaling. Antioxidants (Basel) 2020, 9. [CrossRef]
- Reyes-Garcia, J.; Carbajal-Garcia, A.; Di Mise, A.; Zheng, Y.M.; Wang, X.; Wang, Y.X. Important Functions and Molecular Mechanisms of Mitochondrial Redox Signaling in Pulmonary Hypertension. Antioxidants (Basel) 2022, 11. [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol 2022, 23, 499-515. [CrossRef]
- Dunham-Snary, K.J.; Hong, Z.G.; Xiong, P.Y.; Del Paggio, J.C.; Herr, J.E.; Johri, A.M.; Archer, S.L. A mitochondrial redox oxygen sensor in the pulmonary vasculature and ductus arteriosus. Pflugers Arch 2016, 468, 43-58. [CrossRef]
- Smith, K.A.; Schumacker, P.T. Sensors and signals: the role of reactive oxygen species in hypoxic pulmonary vasoconstriction. J Physiol 2019, 597, 1033-1043. [CrossRef]
- Pak, O.; Nolte, A.; Knoepp, F.; Giordano, L.; Pecina, P.; Huttemann, M.; Grossman, L.I.; Weissmann, N.; Sommer, N. Mitochondrial oxygen sensing of acute hypoxia in specialized cells - Is there a unifying mechanism? Biochim Biophys Acta Bioenerg 2022, 1863, 148911. [CrossRef]
- Weir, E.K.; Archer, S.L. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J 1995, 9, 183-189. [CrossRef]
- Burgoyne, J.R.; Madhani, M.; Cuello, F.; Charles, R.L.; Brennan, J.P.; Schroder, E.; Browning, D.D.; Eaton, P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 2007, 317, 1393-1397. [CrossRef]
- Waypa, G.B.; Chandel, N.S.; Schumacker, P.T. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 2001, 88, 1259-1266. [CrossRef]
- Rathore, R.; Zheng, Y.M.; Niu, C.F.; Liu, Q.H.; Korde, A.; Ho, Y.S.; Wang, Y.X. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008, 45, 1223-1231. [CrossRef]
- Alruwaili, N.; Kandhi, S.; Sun, D.; Wolin, M.S. Metabolism and Redox in Pulmonary Vascular Physiology and Pathophysiology. Antioxid Redox Signal 2019, 31, 752-769. [CrossRef]
- Evans, A.M.; Mustard, K.J.; Wyatt, C.N.; Peers, C.; Dipp, M.; Kumar, P.; Kinnear, N.P.; Hardie, D.G. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem 2005, 280, 41504-41511. [CrossRef]
- Evans, A.M.; Lewis, S.A.; Ogunbayo, O.A.; Moral-Sanz, J. Modulation of the LKB1-AMPK Signalling Pathway Underpins Hypoxic Pulmonary Vasoconstriction and Pulmonary Hypertension. Adv Exp Med Biol 2015, 860, 89-99. [CrossRef]
- Moral-Sanz, J.; Lewis, S.A.; MacMillan, S.; Ross, F.A.; Thomson, A.; Viollet, B.; Foretz, M.; Moran, C.; Hardie, D.G.; Evans, A.M. The LKB1-AMPK-alpha1 signaling pathway triggers hypoxic pulmonary vasoconstriction downstream of mitochondria. Sci Signal 2018, 11. [CrossRef]
- Moral-Sanz, J.; Mahmoud, A.D.; Ross, F.A.; Eldstrom, J.; Fedida, D.; Hardie, D.G.; Evans, A.M. AMP-activated protein kinase inhibits Kv 1.5 channel currents of pulmonary arterial myocytes in response to hypoxia and inhibition of mitochondrial oxidative phosphorylation. J Physiol 2016, 594, 4901-4915. [CrossRef]
- Olson, K.R.; Dombkowski, R.A.; Russell, M.J.; Doellman, M.M.; Head, S.K.; Whitfield, N.L.; Madden, J.A. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol 2006, 209, 4011-4023. [CrossRef]
- Olson, K.R.; Whitfield, N.L.; Bearden, S.E.; St Leger, J.; Nilson, E.; Gao, Y.; Madden, J.A. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol 2010, 298, R51-60. [CrossRef]
- Olson, K.R. Are Reactive Sulfur Species the New Reactive Oxygen Species? Antioxid Redox Signal 2020, 33, 1125-1142. [CrossRef]
- Olson, K.R. A Case for Hydrogen Sulfide Metabolism as an Oxygen Sensing Mechanism. Antioxidants (Basel) 2021, 10. [CrossRef]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol Rev 2019, 99, 161-234. [CrossRef]
- Warpsinski, G.; Smith, M.J.; Srivastava, S.; Keeley, T.P.; Siow, R.C.M.; Fraser, P.A.; Mann, G.E. Nrf2-regulated redox signaling in brain endothelial cells adapted to physiological oxygen levels: Consequences for sulforaphane mediated protection against hypoxia-reoxygenation. Redox Biol 2020, 37, 101708. [CrossRef]
- Smith, M.J.; Yang, F.; Griffiths, A.; Morrell, A.; Chapple, S.J.; Siow, R.C.M.; Stewart, T.; Maret, W.; Mann, G.E. Redox and metal profiles in human coronary endothelial and smooth muscle cells under hyperoxia, physiological normoxia and hypoxia: Effects of NRF2 signaling on intracellular zinc. Redox Biol 2023, 62, 102712. [CrossRef]
- Altun, H.Y.; Secilmis, M.; Yang, F.; Akgul Caglar, T.; Vatandaslar, E.; Toy, M.F.; Vilain, S.; Mann, G.E.; Ozturk, G.; Eroglu, E. Visualizing hydrogen peroxide and nitric oxide dynamics in endothelial cells using multispectral imaging under controlled oxygen conditions. Free Radic Biol Med 2024, 221, 89-97. [CrossRef]
- Alva, R.; Wiebe, J.E.; Stuart, J.A. Revisiting reactive oxygen species production in hypoxia. Pflugers Arch 2024, 476, 1423-1444. [CrossRef]
- Kamler, M.; Nowak, K.; Bock, M.; Herold, U.; Motsch, J.; Hagl, S.; Gebhard, M.M.; Jakob, H. Bronchial artery revascularization restores peribronchial tissue oxygenation after lung transplantation. J Heart Lung Transplant 2004, 23, 763-766. [CrossRef]
- Herold, U.; Jakob, H.; Kamler, M.; Thiele, R.; Tochtermann, U.; Weinmann, J.; Motsch, J.; Gebhard, M.M.; Hagl, S. Interruption of bronchial circulation leads to a severe decrease in peribronchial oxygen tension in standard lung transplantation technique. Eur J Cardiothorac Surg 1998, 13, 176-183. [CrossRef]
- Rivera, B.K.; Naidu, S.K.; Subramanian, K.; Joseph, M.; Hou, H.; Khan, N.; Swartz, H.M.; Kuppusamy, P. Real-time, in vivo determination of dynamic changes in lung and heart tissue oxygenation using EPR oximetry. Adv Exp Med Biol 2014, 812, 81-86. [CrossRef]
- Marshall, C.; Marshall, B. Site and sensitivity for stimulation of hypoxic pulmonary vasoconstriction. J Appl Physiol Respir Environ Exerc Physiol 1983, 55, 711-716. [CrossRef]
- Grayson, C.; Mailloux, R.J. Coenzyme Q(10) and nicotinamide nucleotide transhydrogenase: Sentinels for mitochondrial hydrogen peroxide signaling. Free Radic Biol Med 2023, 208, 260-271. [CrossRef]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry 2014, 53, 5111-5120. [CrossRef]
- Knock, G.A. NADPH oxidase in the vasculature: Expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic Biol Med 2019, 145, 385-427. [CrossRef]
- Rogers, Z.J.; Flood, D.; Bencherif, S.A.; Taylor, C.T. Oxygen control in cell culture - Your cells may not be experiencing what you think! Free Radic Biol Med 2025, 226, 279-287. [CrossRef]
- Peniche Silva, C.J.; Liebsch, G.; Meier, R.J.; Gutbrod, M.S.; Balmayor, E.R.; van Griensven, M. A New Non-invasive Technique for Measuring 3D-Oxygen Gradients in Wells During Mammalian Cell Culture. Front Bioeng Biotechnol 2020, 8, 595. [CrossRef]
- Rogers, Z.J.; Colombani, T.; Khan, S.; Bhatt, K.; Nukovic, A.; Zhou, G.; Woolston, B.M.; Taylor, C.T.; Gilkes, D.M.; Slavov, N.; et al. Controlling Pericellular Oxygen Tension in Cell Culture Reveals Distinct Breast Cancer Responses to Low Oxygen Tensions. Adv Sci (Weinh) 2024, 11, e2402557. [CrossRef]
- Malconian, M.K.; Rock, P.B.; Reeves, J.T.; Cymerman, A.; Houston, C.S. Operation Everest II: gas tensions in expired air and arterial blood at extreme altitude. Aviat Space Environ Med 1993, 64, 37-42.
- Sutton, J.R.; Reeves, J.T.; Wagner, P.D.; Groves, B.M.; Cymerman, A.; Malconian, M.K.; Rock, P.B.; Young, P.M.; Walter, S.D.; Houston, C.S. Operation Everest II: oxygen transport during exercise at extreme simulated altitude. J Appl Physiol (1985) 1988, 64, 1309-1321. [CrossRef]
- West, J.B.; Hackett, P.H.; Maret, K.H.; Milledge, J.S.; Peters, R.M., Jr.; Pizzo, C.J.; Winslow, R.M. Pulmonary gas exchange on the summit of Mount Everest. J Appl Physiol Respir Environ Exerc Physiol 1983, 55, 678-687. [CrossRef]
- Grocott, M.P.; Martin, D.S.; Levett, D.Z.; McMorrow, R.; Windsor, J.; Montgomery, H.E.; Caudwell Xtreme Everest Research, G. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med 2009, 360, 140-149. [CrossRef]
- Kemp, P.J.; Telezhkin, V. Oxygen sensing by the carotid body: is it all just rotten eggs? Antioxid Redox Signal 2014, 20, 794-804. [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J Biol Chem 2019, 294, 19683-19708. [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu Rev Biochem 2017, 86, 715-748. [CrossRef]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev 2011, 2011, 809696. [CrossRef]
- Bienert, G.P.; Moller, A.L.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 2007, 282, 1183-1192. [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007, 87, 315-424. [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol 2017, 11, 613-619. [CrossRef]
- Rios, E.J.; Fallon, M.; Wang, J.; Shimoda, L.A. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2005, 289, L867-874. [CrossRef]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; Yu, Q.; Heppner, D.E.; Li, J.; Long, J.; Mills, E.L.; Szpyt, J.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 968-983 e924. [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic Biol Med 2019, 140, 14-27. [CrossRef]
- Salsbury, F.R., Jr.; Knutson, S.T.; Poole, L.B.; Fetrow, J.S. Functional site profiling and electrostatic analysis of cysteines modifiable to cysteine sulfenic acid. Protein Sci 2008, 17, 299-312. [CrossRef]
- Mailloux, R.J.; Grayson, C.; Koufos, O. Regulation of Mitochondrial Hydrogen Peroxide Availability by Protein S-glutathionylation. Cells 2022, 12. [CrossRef]
- Burgoyne, J.R.; Mongue-Din, H.; Eaton, P.; Shah, A.M. Redox signaling in cardiac physiology and pathology. Circ Res 2012, 111, 1091-1106. [CrossRef]
- Brennan, J.P.; Bardswell, S.C.; Burgoyne, J.R.; Fuller, W.; Schroder, E.; Wait, R.; Begum, S.; Kentish, J.C.; Eaton, P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem 2006, 281, 21827-21836. [CrossRef]
- Cuello, F.; Eaton, P. Cysteine-Based Redox Sensing and Its Role in Signaling by Cyclic Nucleotide-Dependent Kinases in the Cardiovascular System. Annu Rev Physiol 2019, 81, 63-87. [CrossRef]
- Kettenhofen, N.J.; Wood, M.J. Formation, reactivity, and detection of protein sulfenic acids. Chem Res Toxicol 2010, 23, 1633-1646. [CrossRef]
- Truong, T.H.; Carroll, K.S. Redox regulation of protein kinases. Crit Rev Biochem Mol Biol 2013, 48, 332-356. [CrossRef]
- Ahsan, M.K.; Lekli, I.; Ray, D.; Yodoi, J.; Das, D.K. Redox regulation of cell survival by the thioredoxin superfamily: an implication of redox gene therapy in the heart. Antioxid Redox Signal 2009, 11, 2741-2758. [CrossRef]
- Pillay, C.S.; Rohwer, J.M. Computational models as catalysts for investigating redoxin systems. Essays Biochem 2024, 68, 27-39. [CrossRef]
- Xiao, W.; Wang, R.S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid Redox Signal 2018, 28, 251-272. [CrossRef]
- Winterbourn, C.C. Biological Production, Detection, and Fate of Hydrogen Peroxide. Antioxid Redox Signal 2018, 29, 541-551. [CrossRef]
- Scherschel, M.; Niemeier, J.O.; Jacobs, L.; Hoffmann, M.D.A.; Diederich, A.; Bell, C.; Hohne, P.; Raetz, S.; Kroll, J.B.; Steinbeck, J.; et al. A family of NADPH/NADP(+) biosensors reveals in vivo dynamics of central redox metabolism across eukaryotes. Nat Commun 2024, 15, 10704. [CrossRef]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J Inorg Biochem 2019, 197, 110699. [CrossRef]
- Netto, L.E.; Antunes, F. The Roles of Peroxiredoxin and Thioredoxin in Hydrogen Peroxide Sensing and in Signal Transduction. Mol Cells 2016, 39, 65-71. [CrossRef]
- Booth, D.M.; Enyedi, B.; Geiszt, M.; Varnai, P.; Hajnoczky, G. Redox Nanodomains Are Induced by and Control Calcium Signaling at the ER-Mitochondrial Interface. Mol Cell 2016, 63, 240-248. [CrossRef]
- Stocker, S.; Van Laer, K.; Mijuskovic, A.; Dick, T.P. The Conundrum of Hydrogen Peroxide Signaling and the Emerging Role of Peroxiredoxins as Redox Relay Hubs. Antioxid Redox Signal 2018, 28, 558-573. [CrossRef]
- Sobotta, M.C.; Liou, W.; Stocker, S.; Talwar, D.; Oehler, M.; Ruppert, T.; Scharf, A.N.; Dick, T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol 2015, 11, 64-70. [CrossRef]
- Portillo-Ledesma, S.; Randall, L.M.; Parsonage, D.; Dalla Rizza, J.; Karplus, P.A.; Poole, L.B.; Denicola, A.; Ferrer-Sueta, G. Differential Kinetics of Two-Cysteine Peroxiredoxin Disulfide Formation Reveal a Novel Model for Peroxide Sensing. Biochemistry 2018, 57, 3416-3424. [CrossRef]
- Trujillo, M.; Ferrer-Sueta, G.; Radi, R. Kinetic studies on peroxynitrite reduction by peroxiredoxins. Methods Enzymol 2008, 441, 173-196. [CrossRef]
- Rhee, S.G.; Woo, H.A. Multiple functions of 2-Cys peroxiredoxins, I and II, and their regulations via post-translational modifications. Free Radic Biol Med 2020, 152, 107-115. [CrossRef]
- van Dam, L.; Pages-Gallego, M.; Polderman, P.E.; van Es, R.M.; Burgering, B.M.T.; Vos, H.R.; Dansen, T.B. The Human 2-Cys Peroxiredoxins form Widespread, Cysteine-Dependent- and Isoform-Specific Protein-Protein Interactions. Antioxidants (Basel) 2021, 10. [CrossRef]
- Mailloux, R.J.; Treberg, J.R. Protein S-glutathionlyation links energy metabolism to redox signaling in mitochondria. Redox Biol 2016, 8, 110-118. [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431-435. [CrossRef]
- Pelletier, M.; Lepow, T.S.; Billingham, L.K.; Murphy, M.P.; Siegel, R.M. New tricks from an old dog: mitochondrial redox signaling in cellular inflammation. Semin Immunol 2012, 24, 384-392. [CrossRef]
- Starkov, A.A.; Andreyev, A.Y.; Zhang, S.F.; Starkova, N.N.; Korneeva, M.; Syromyatnikov, M.; Popov, V.N. Scavenging of H2O2 by mouse brain mitochondria. J Bioenerg Biomembr 2014, 46, 471-477. [CrossRef]
- Treberg, J.R.; Munro, D.; Banh, S.; Zacharias, P.; Sotiri, E. Differentiating between apparent and actual rates of H2O2 metabolism by isolated rat muscle mitochondria to test a simple model of mitochondria as regulators of H2O2 concentration. Redox Biol 2015, 5, 216-224. [CrossRef]
- Dey, S.; Sidor, A.; O'Rourke, B. Compartment-specific Control of Reactive Oxygen Species Scavenging by Antioxidant Pathway Enzymes. J Biol Chem 2016, 291, 11185-11197. [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005, 70, 200-214. [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem J 2009, 417, 1-13. [CrossRef]
- Drose, S.; Brandt, U.; Wittig, I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim Biophys Acta 2014, 1844, 1344-1354. [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Murphy, A.N.; Starkov, A.A. Mitochondrial ROS Metabolism: 10 Years Later. Biochemistry (Mosc) 2015, 80, 517-531. [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 2016, 100, 14-31. [CrossRef]
- Hernansanz-Agustin, P.; Morales-Vidal, C.; Calvo, E.; Natale, P.; Marti-Mateos, Y.; Jaroszewicz, S.N.; Cabrera-Alarcon, J.L.; Acin-Perez, R.; Lopez-Montero, I.; Vazquez, J.; et al. A transmitochondrial sodium gradient controls membrane potential in mammalian mitochondria. Cell 2024, 187, 6599-6613 e6521. [CrossRef]
- Nicholls, D.G.; Ferguson, S.J. Bioenergetics 3, 3rd ed.; Academic Press: San Diego, Calif., 2002; pp. xviii, 297 p.
- Gauthier, L.D.; Greenstein, J.L.; Cortassa, S.; O'Rourke, B.; Winslow, R.L. A computational model of reactive oxygen species and redox balance in cardiac mitochondria. Biophys J 2013, 105, 1045-1056. [CrossRef]
- Castro, L.; Tortora, V.; Mansilla, S.; Radi, R. Aconitases: Non-redox Iron-Sulfur Proteins Sensitive to Reactive Species. Acc Chem Res 2019, 52, 2609-2619. [CrossRef]
- Tahara, E.B.; Navarete, F.D.; Kowaltowski, A.J. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 2009, 46, 1283-1297. [CrossRef]
- Hoffman, D.L.; Brookes, P.S. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J Biol Chem 2009, 284, 16236-16245. [CrossRef]
- Balmaceda, V.; Komlodi, T.; Szibor, M.; Gnaiger, E.; Moore, A.L.; Fernandez-Vizarra, E.; Viscomi, C. The striking differences in the bioenergetics of brain and liver mitochondria are enhanced in mitochondrial disease. Biochim Biophys Acta Mol Basis Dis 2024, 1870, 167033. [CrossRef]
- Slade, L.; Chalker, J.; Kuksal, N.; Young, A.; Gardiner, D.; Mailloux, R.J. Examination of the superoxide/hydrogen peroxide forming and quenching potential of mouse liver mitochondria. Biochim Biophys Acta Gen Subj 2017, 1861, 1960-1969. [CrossRef]
- Wong, H.S.; Benoit, B.; Brand, M.D. Mitochondrial and cytosolic sources of hydrogen peroxide in resting C2C12 myoblasts. Free Radic Biol Med 2019, 130, 140-150. [CrossRef]
- Chandel, N.S. Mitochondria. Cold Spring Harb Perspect Biol 2021, 13. [CrossRef]
- Han, D.; Canali, R.; Rettori, D.; Kaplowitz, N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol 2003, 64, 1136-1144. [CrossRef]
- Hoehne, M.N.; Jacobs, L.; Lapacz, K.J.; Calabrese, G.; Murschall, L.M.; Marker, T.; Kaul, H.; Trifunovic, A.; Morgan, B.; Fricker, M.; et al. Spatial and temporal control of mitochondrial H(2) O(2) release in intact human cells. EMBO J 2022, 41, e109169. [CrossRef]
- Goncalves, R.L.S.; Watson, M.A.; Wong, H.S.; Orr, A.L.; Brand, M.D. The use of site-specific suppressors to measure the relative contributions of different mitochondrial sites to skeletal muscle superoxide and hydrogen peroxide production. Redox Biol 2020, 28, 101341. [CrossRef]
- Bleier, L.; Wittig, I.; Heide, H.; Steger, M.; Brandt, U.; Drose, S. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic Biol Med 2015, 78, 1-10. [CrossRef]
- Gao, L.; Gonzalez-Rodriguez, P.; Ortega-Saenz, P.; Lopez-Barneo, J. Redox signaling in acute oxygen sensing. Redox Biol 2017, 12, 908-915. [CrossRef]
- Dunham-Snary, K.J.; Wu, D.; Potus, F.; Sykes, E.A.; Mewburn, J.D.; Charles, R.L.; Eaton, P.; Sultanian, R.A.; Archer, S.L. Ndufs2, a Core Subunit of Mitochondrial Complex I, Is Essential for Acute Oxygen-Sensing and Hypoxic Pulmonary Vasoconstriction. Circ Res 2019, 124, 1727-1746. [CrossRef]
- Paddenberg, R.; Ishaq, B.; Goldenberg, A.; Faulhammer, P.; Rose, F.; Weissmann, N.; Braun-Dullaeus, R.C.; Kummer, W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 2003, 284, L710-719. [CrossRef]
- Korde, A.S.; Yadav, V.R.; Zheng, Y.M.; Wang, Y.X. Primary role of mitochondrial Rieske iron-sulfur protein in hypoxic ROS production in pulmonary artery myocytes. Free Radic Biol Med 2011, 50, 945-952. [CrossRef]
- Waypa, G.B.; Marks, J.D.; Guzy, R.D.; Mungai, P.T.; Schriewer, J.M.; Dokic, D.; Ball, M.K.; Schumacker, P.T. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 2013, 187, 424-432. [CrossRef]
- Sommer, N.; Alebrahimdehkordi, N.; Pak, O.; Knoepp, F.; Strielkov, I.; Scheibe, S.; Dufour, E.; Andjelkovic, A.; Sydykov, A.; Saraji, A.; et al. Bypassing mitochondrial complex III using alternative oxidase inhibits acute pulmonary oxygen sensing. Sci Adv 2020, 6, eaba0694. [CrossRef]
- Sommer, N.; Huttemann, M.; Pak, O.; Scheibe, S.; Knoepp, F.; Sinkler, C.; Malczyk, M.; Gierhardt, M.; Esfandiary, A.; Kraut, S.; et al. Mitochondrial Complex IV Subunit 4 Isoform 2 Is Essential for Acute Pulmonary Oxygen Sensing. Circ Res 2017, 121, 424-438. [CrossRef]
- Gibbs, E.T.; Lerner, C.A.; Watson, M.A.; Wong, H.S.; Gerencser, A.A.; Brand, M.D. Site IQ in mitochondrial complex I generates S1QEL-sensitive superoxide/hydrogen peroxide in both the reverse and forward reactions. Biochem J 2023, 480, 363-384. [CrossRef]
- Bleier, L.; Drose, S. Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim Biophys Acta 2013, 1827, 1320-1331. [CrossRef]
- Guillaud, F.; Drose, S.; Kowald, A.; Brandt, U.; Klipp, E. Superoxide production by cytochrome bc1 complex: a mathematical model. Biochim Biophys Acta 2014, 1837, 1643-1652. [CrossRef]
- Sarewicz, M.; Osyczka, A. Electronic connection between the quinone and cytochrome C redox pools and its role in regulation of mitochondrial electron transport and redox signaling. Physiol Rev 2015, 95, 219-243. [CrossRef]
- Rottenberg, H.; Covian, R.; Trumpower, B.L. Membrane potential greatly enhances superoxide generation by the cytochrome bc1 complex reconstituted into phospholipid vesicles. J Biol Chem 2009, 284, 19203-19210. [CrossRef]
- Markevich, N.I.; Hoek, J.B. Computational modeling analysis of mitochondrial superoxide production under varying substrate conditions and upon inhibition of different segments of the electron transport chain. Biochim Biophys Acta 2015, 1847, 656-679. [CrossRef]
- Quinlan, C.L.; Gerencser, A.A.; Treberg, J.R.; Brand, M.D. The mechanism of superoxide production by the antimycin-inhibited mitochondrial Q-cycle. J Biol Chem 2011, 286, 31361-31372. [CrossRef]
- Tsubaki, M. Fourier-transform infrared study of cyanide binding to the Fea3-CuB binuclear site of bovine heart cytochrome c oxidase: implication of the redox-linked conformational change at the binuclear site. Biochemistry 1993, 32, 164-173. [CrossRef]
- Biscoe, T.J.; Duchen, M.R. Cellular basis of transduction in carotid chemoreceptors. Am J Physiol 1990, 258, L271-278. [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: regulation and role in cellular and tissue metabolism. J Physiol 2017, 595, 7023-7038. [CrossRef]
- Ward, J.P. Oxygen sensors in context. Biochim Biophys Acta 2008, 1777, 1-14. [CrossRef]
- Buckler, K.J.; Turner, P.J. Functional Properties of Mitochondria in the Type-1 Cell and Their Role in Oxygen Sensing. Adv Exp Med Biol 2015, 860, 69-80. [CrossRef]
- Wilson, D.F.; Rumsey, W.L.; Green, T.J.; Vanderkooi, J.M. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem 1988, 263, 2712-2718.
- Sommer, N.; Pak, O.; Schorner, S.; Derfuss, T.; Krug, A.; Gnaiger, E.; Ghofrani, H.A.; Schermuly, R.T.; Huckstorf, C.; Seeger, W.; et al. Mitochondrial cytochrome redox states and respiration in acute pulmonary oxygen sensing. Eur Respir J 2010, 36, 1056-1066. [CrossRef]
- Michelakis, E.D.; Hampl, V.; Nsair, A.; Wu, X.; Harry, G.; Haromy, A.; Gurtu, R.; Archer, S.L. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 2002, 90, 1307-1315. [CrossRef]
- Roy, A.; Li, J.; Al-Mehdi, A.B.; Mokashi, A.; Lahiri, S. Effect of acute hypoxia on glomus cell Em and psi m as measured by fluorescence imaging. J Appl Physiol (1985) 2002, 93, 1987-1998. [CrossRef]
- Kurokawa, H.; Ito, H.; Inoue, M.; Tabata, K.; Sato, Y.; Yamagata, K.; Kizaka-Kondoh, S.; Kadonosono, T.; Yano, S.; Inoue, M.; et al. High resolution imaging of intracellular oxygen concentration by phosphorescence lifetime. Sci Rep 2015, 5, 10657. [CrossRef]
- Gnaiger, E.; Steinlechner-Maran, R.; Mendez, G.; Eberl, T.; Margreiter, R. Control of mitochondrial and cellular respiration by oxygen. J Bioenerg Biomembr 1995, 27, 583-596. [CrossRef]
- Gnaiger, E.; Lassnig, B.; Kuznetsov, A.; Rieger, G.; Margreiter, R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol 1998, 201, 1129-1139. [CrossRef]
- Brown, G.C.; Cooper, C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 1994, 356, 295-298. [CrossRef]
- Palacios-Callender, M.; Quintero, M.; Hollis, V.S.; Springett, R.J.; Moncada, S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc Natl Acad Sci U S A 2004, 101, 7630-7635. [CrossRef]
- Edmunds, N.J.; Moncada, S.; Marshall, J.M. Does nitric oxide allow endothelial cells to sense hypoxia and mediate hypoxic vasodilatation? In vivo and in vitro studies. J Physiol 2003, 546, 521-527. [CrossRef]
- Quintero, M.; Colombo, S.L.; Godfrey, A.; Moncada, S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A 2006, 103, 5379-5384. [CrossRef]
- Galkin, A.; Higgs, A.; Moncada, S. Nitric oxide and hypoxia. Essays Biochem 2007, 43, 29-42. [CrossRef]
- Fagan, K.A.; Tyler, R.C.; Sato, K.; Fouty, B.W.; Morris, K.G., Jr.; Huang, P.L.; McMurtry, I.F.; Rodman, D.M. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am J Physiol 1999, 277, L472-478. [CrossRef]
- Leeman, M.; de Beyl, V.Z.; Delcroix, M.; Naeije, R. Effects of endogenous nitric oxide on pulmonary vascular tone in intact dogs. Am J Physiol 1994, 266, H2343-2347. [CrossRef]
- Persson, M.G.; Gustafsson, L.E.; Wiklund, N.P.; Moncada, S.; Hedqvist, P. Endogenous nitric oxide as a probable modulator of pulmonary circulation and hypoxic pressor response in vivo. Acta Physiol Scand 1990, 140, 449-457. [CrossRef]
- Vaughan, D.J.; Brogan, T.V.; Kerr, M.E.; Deem, S.; Luchtel, D.L.; Swenson, E.R. Contributions of nitric oxide synthase isozymes to exhaled nitric oxide and hypoxic pulmonary vasoconstriction in rabbit lungs. Am J Physiol Lung Cell Mol Physiol 2003, 284, L834-843. [CrossRef]
- Blitzer, M.L.; Loh, E.; Roddy, M.A.; Stamler, J.S.; Creager, M.A. Endothelium-derived nitric oxide regulates systemic and pulmonary vascular resistance during acute hypoxia in humans. J Am Coll Cardiol 1996, 28, 591-596. [CrossRef]
- Leach, R.M.; Robertson, T.P.; Twort, C.H.; Ward, J.P. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. Am J Physiol 1994, 266, L223-231. [CrossRef]
- Castello, P.R.; David, P.S.; McClure, T.; Crook, Z.; Poyton, R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab 2006, 3, 277-287. [CrossRef]
- Palacios-Callender, M.; Hollis, V.; Mitchison, M.; Frakich, N.; Unitt, D.; Moncada, S. Cytochrome c oxidase regulates endogenous nitric oxide availability in respiring cells: a possible explanation for hypoxic vasodilation. Proc Natl Acad Sci U S A 2007, 104, 18508-18513. [CrossRef]
- Amdahl, M.B.; DeMartino, A.W.; Gladwin, M.T. Inorganic nitrite bioactivation and role in physiological signaling and therapeutics. Biol Chem 2019, 401, 201-211. [CrossRef]
- Olson, K.R.; Deleon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am J Physiol Regul Integr Comp Physiol 2013, 305, R592-603. [CrossRef]
- Petersen, L.C. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim Biophys Acta 1977, 460, 299-307. [CrossRef]
- Prieto-Lloret, J.; Snetkov, V.A.; Shaifta, Y.; Docio, I.; Connolly, M.J.; MacKay, C.E.; Knock, G.A.; Ward, J.P.T.; Aaronson, P.I. Role of reactive oxygen species and sulfide-quinone oxoreductase in hydrogen sulfide-induced contraction of rat pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 2018, 314, L670-L685. [CrossRef]
- Szabo, C.; Ransy, C.; Modis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol 2014, 171, 2099-2122. [CrossRef]
- Nicholson, R.A.; Roth, S.H.; Zhang, A.; Zheng, J.; Brookes, J.; Skrajny, B.; Bennington, R. Inhibition of respiratory and bioenergetic mechanisms by hydrogen sulfide in mammalian brain. J Toxicol Environ Health A 1998, 54, 491-507. [CrossRef]
- Levitt, M.D.; Abdel-Rehim, M.S.; Furne, J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal 2011, 15, 373-378. [CrossRef]
- Wilson, D.F.; Harrison, D.K.; Vinogradov, A. Mitochondrial cytochrome c oxidase and control of energy metabolism: measurements in suspensions of isolated mitochondria. J Appl Physiol (1985) 2014, 117, 1424-1430. [CrossRef]
- Harrison, D.K.; Fasching, M.; Fontana-Ayoub, M.; Gnaiger, E. Cytochrome redox states and respiratory control in mouse and beef heart mitochondria at steady-state levels of hypoxia. J Appl Physiol (1985) 2015, 119, 1210-1218. [CrossRef]
- Wilson, D.F.; Erecinska, M.; Drown, C.; Silver, I.A. Effect of oxygen tension on cellular energetics. Am J Physiol 1977, 233, C135-140. [CrossRef]
- Wilson, D.F.; Erecinska, M.; Drown, C.; Silver, I.A. The oxygen dependence of cellular energy metabolism. Arch Biochem Biophys 1979, 195, 485-493. [CrossRef]
- Pajuelo Reguera, D.; Cunatova, K.; Vrbacky, M.; Pecinova, A.; Houstek, J.; Mracek, T.; Pecina, P. Cytochrome c Oxidase Subunit 4 Isoform Exchange Results in Modulation of Oxygen Affinity. Cells 2020, 9. [CrossRef]
- Huttemann, M.; Kadenbach, B.; Grossman, L.I. Mammalian subunit IV isoforms of cytochrome c oxidase. Gene 2001, 267, 111-123. [CrossRef]
- Buckler, K.J.; Turner, P.J. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol 2013, 591, 3549-3563. [CrossRef]
- Scialo, F.; Fernandez-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front Physiol 2017, 8, 428. [CrossRef]
- Treberg, J.R.; Quinlan, C.L.; Brand, M.D. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I). J Biol Chem 2011, 286, 27103-27110. [CrossRef]
- Fernandez-Aguera, M.C.; Gao, L.; Gonzalez-Rodriguez, P.; Pintado, C.O.; Arias-Mayenco, I.; Garcia-Flores, P.; Garcia-Perganeda, A.; Pascual, A.; Ortega-Saenz, P.; Lopez-Barneo, J. Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab 2015, 22, 825-837. [CrossRef]
- Arias-Mayenco, I.; Gonzalez-Rodriguez, P.; Torres-Torrelo, H.; Gao, L.; Fernandez-Aguera, M.C.; Bonilla-Henao, V.; Ortega-Saenz, P.; Lopez-Barneo, J. Acute O2 Sensing: Role of Coenzyme QH2/Q Ratio and Mitochondrial ROS Compartmentalization. Cell Metab 2018, 28, 145-158 e144. [CrossRef]
- Swiderska, A.; Coney, A.M.; Alzahrani, A.A.; Aldossary, H.S.; Batis, N.; Ray, C.J.; Kumar, P.; Holmes, A.P. Mitochondrial Succinate Metabolism and Reactive Oxygen Species Are Important but Not Essential for Eliciting Carotid Body and Ventilatory Responses to Hypoxia in the Rat. Antioxidants (Basel) 2021, 10. [CrossRef]
- Torres-Lopez, M.; Spiller, P.F.; Gao, L.; Garcia-Flores, P.; Murphy, M.P.; Ortega-Saenz, P.; Lopez-Barneo, J. Acute oxygen sensing by arterial chemoreceptors with a mutant mitochondrial complex I ND6 subunit lacking reverse electron transport. FEBS Lett 2025, 599, 1122-1134. [CrossRef]
- Hernansanz-Agustin, P.; Choya-Foces, C.; Carregal-Romero, S.; Ramos, E.; Oliva, T.; Villa-Pina, T.; Moreno, L.; Izquierdo-Alvarez, A.; Cabrera-Garcia, J.D.; Cortes, A.; et al. Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature 2020, 586, 287-291. [CrossRef]
- Hernansanz-Agustin, P.; Ramos, E.; Navarro, E.; Parada, E.; Sanchez-Lopez, N.; Pelaez-Aguado, L.; Cabrera-Garcia, J.D.; Tello, D.; Buendia, I.; Marina, A.; et al. Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biol 2017, 12, 1040-1051. [CrossRef]
- Vinogradov, A.D. Catalytic properties of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) and the pseudo-reversible active/inactive enzyme transition. Biochim Biophys Acta 1998, 1364, 169-185. [CrossRef]
- Roberts, P.G.; Hirst, J. The deactive form of respiratory complex I from mammalian mitochondria is a Na+/H+ antiporter. J Biol Chem 2012, 287, 34743-34751. [CrossRef]
- Babot, M.; Birch, A.; Labarbuta, P.; Galkin, A. Characterisation of the active/de-active transition of mitochondrial complex I. Biochim Biophys Acta 2014, 1837, 1083-1092. [CrossRef]
- Babot, M.; Galkin, A. Molecular mechanism and physiological role of active-deactive transition of mitochondrial complex I. Biochem Soc Trans 2013, 41, 1325-1330. [CrossRef]
- Maklashina, E.; Kotlyar, A.B.; Karliner, J.S.; Cecchini, G. Effect of oxygen on activation state of complex I and lack of oxaloacetate inhibition of complex II in Langendorff perfused rat heart. FEBS Lett 2004, 556, 64-68. [CrossRef]
- Chalmers, S.; Nicholls, D.G. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem 2003, 278, 19062-19070. [CrossRef]
- Gherardi, G.; Monticelli, H.; Rizzuto, R.; Mammucari, C. The Mitochondrial Ca(2+) Uptake and the Fine-Tuning of Aerobic Metabolism. Front Physiol 2020, 11, 554904. [CrossRef]
- Wescott, A.P.; Kao, J.P.Y.; Lederer, W.J.; Boyman, L. Voltage-energized Calcium-sensitive ATP Production by Mitochondria. Nat Metab 2019, 1, 975-984. [CrossRef]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225-236. [CrossRef]
- Lee, I.; Bender, E.; Arnold, S.; Kadenbach, B. New control of mitochondrial membrane potential and ROS formation--a hypothesis. Biol Chem 2001, 382, 1629-1636. [CrossRef]
- Starkov, A.A.; Fiskum, G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 2003, 86, 1101-1107. [CrossRef]
- Ramzan, R.; Vogt, S.; Kadenbach, B. Stress-mediated generation of deleterious ROS in healthy individuals - role of cytochrome c oxidase. J Mol Med (Berl) 2020, 98, 651-657. [CrossRef]
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ Res 2018, 122, 1460-1478. [CrossRef]
- Duong, Q.V.; Hoffman, A.; Zhong, K.; Dessinger, M.J.; Zhang, Y.; Bazil, J.N. Calcium overload decreases net free radical emission in cardiac mitochondria. Mitochondrion 2020, 51, 126-139. [CrossRef]
- Grivennikova, V.G.; Kareyeva, A.V.; Vinogradov, A.D. Oxygen-dependence of mitochondrial ROS production as detected by Amplex Red assay. Redox Biol 2018, 17, 192-199. [CrossRef]
- Stepanova, A.; Konrad, C.; Manfredi, G.; Springett, R.; Ten, V.; Galkin, A. The dependence of brain mitochondria reactive oxygen species production on oxygen level is linear, except when inhibited by antimycin A. J Neurochem 2019, 148, 731-745. [CrossRef]
- Hoffman, D.L.; Salter, J.D.; Brookes, P.S. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol 2007, 292, H101-108. [CrossRef]
- Archer, S.L.; Dunham-Snary, K.J.; Bentley, R.; Alizadeh, E.; Weir, E.K. Hypoxic Pulmonary Vasoconstriction: An Important Component of the Homeostatic Oxygen Sensing System. Physiol Res 2024, 73, S493-S510. [CrossRef]
- Wu, D.; Dasgupta, A.; Read, A.D.; Bentley, R.E.T.; Motamed, M.; Chen, K.H.; Al-Qazazi, R.; Mewburn, J.D.; Dunham-Snary, K.J.; Alizadeh, E.; et al. Oxygen sensing, mitochondrial biology and experimental therapeutics for pulmonary hypertension and cancer. Free Radic Biol Med 2021, 170, 150-178. [CrossRef]
- Weir, E.K.; Archer, S.L. The role of redox changes in oxygen sensing. Respir Physiol Neurobiol 2010, 174, 182-191. [CrossRef]
- Genova, M.L.; Pich, M.M.; Bernacchia, A.; Bianchi, C.; Biondi, A.; Bovina, C.; Falasca, A.I.; Formiggini, G.; Castelli, G.P.; Lenaz, G. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann N Y Acad Sci 2004, 1011, 86-100. [CrossRef]
- Read, A.D.; Bentley, R.E.; Archer, S.L.; Dunham-Snary, K.J. Mitochondrial iron-sulfur clusters: Structure, function, and an emerging role in vascular biology. Redox Biol 2021, 47, 102164. [CrossRef]
- Zickermann, V.; Wirth, C.; Nasiri, H.; Siegmund, K.; Schwalbe, H.; Hunte, C.; Brandt, U. Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I. Science 2015, 347, 44-49. [CrossRef]
- Treberg, J.R.; Braun, K.; Selseleh, P. Mitochondria can act as energy-sensing regulators of hydrogen peroxide availability. Redox Biol 2019, 20, 483-488. [CrossRef]
- Cardoso, A.R.; Chausse, B.; da Cunha, F.M.; Luevano-Martinez, L.A.; Marazzi, T.B.; Pessoa, P.S.; Queliconi, B.B.; Kowaltowski, A.J. Mitochondrial compartmentalization of redox processes. Free Radic Biol Med 2012, 52, 2201-2208. [CrossRef]
- Secilmis, M.; Altun, H.Y.; Pilic, J.; Erdogan, Y.C.; Cokluk, Z.; Ata, B.N.; Sevimli, G.; Zaki, A.G.; Yigit, E.N.; Ozturk, G.; et al. A Co-Culture-Based Multiparametric Imaging Technique to Dissect Local H(2)O(2) Signals with Targeted HyPer7. Biosensors (Basel) 2021, 11. [CrossRef]
- Lim, J.B.; Huang, B.K.; Deen, W.M.; Sikes, H.D. Analysis of the lifetime and spatial localization of hydrogen peroxide generated in the cytosol using a reduced kinetic model. Free Radic Biol Med 2015, 89, 47-53. [CrossRef]
- Archer, S.L.; Reeve, H.L.; Michelakis, E.; Puttagunta, L.; Waite, R.; Nelson, D.P.; Dinauer, M.C.; Weir, E.K. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci U S A 1999, 96, 7944-7949. [CrossRef]
- Hilenski, L.L.; Clempus, R.E.; Quinn, M.T.; Lambeth, J.D.; Griendling, K.K. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2004, 24, 677-683. [CrossRef]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A 2010, 107, 15681-15686. [CrossRef]
- Oakley, F.D.; Abbott, D.; Li, Q.; Engelhardt, J.F. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal 2009, 11, 1313-1333. [CrossRef]
- Shaifta, Y.; Snetkov, V.A.; Prieto-Lloret, J.; Knock, G.A.; Smirnov, S.V.; Aaronson, P.I.; Ward, J.P. Sphingosylphosphorylcholine potentiates vasoreactivity and voltage-gated Ca2+ entry via NOX1 and reactive oxygen species. Cardiovasc Res 2015, 106, 121-130. [CrossRef]
- Miyano, K.; Ueno, N.; Takeya, R.; Sumimoto, H. Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem 2006, 281, 21857-21868. [CrossRef]
- Schroder, K.; Weissmann, N.; Brandes, R.P. Organizers and activators: Cytosolic Nox proteins impacting on vascular function. Free Radic Biol Med 2017, 109, 22-32. [CrossRef]
- Streeter, J.; Schickling, B.M.; Jiang, S.; Stanic, B.; Thiel, W.H.; Gakhar, L.; Houtman, J.C.; Miller, F.J., Jr. Phosphorylation of Nox1 regulates association with NoxA1 activation domain. Circ Res 2014, 115, 911-918. [CrossRef]
- Niu, X.L.; Madamanchi, N.R.; Vendrov, A.E.; Tchivilev, I.; Rojas, M.; Madamanchi, C.; Brandes, R.P.; Krause, K.H.; Humphries, J.; Smith, A.; et al. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation 2010, 121, 549-559. [CrossRef]
- Zhang, F.; Jin, S.; Yi, F.; Xia, M.; Dewey, W.L.; Li, P.L. Local production of O2- by NAD(P)H oxidase in the sarcoplasmic reticulum of coronary arterial myocytes: cADPR-mediated Ca2+ regulation. Cell Signal 2008, 20, 637-644. [CrossRef]
- Vendrov, A.E.; Vendrov, K.C.; Smith, A.; Yuan, J.; Sumida, A.; Robidoux, J.; Runge, M.S.; Madamanchi, N.R. NOX4 NADPH Oxidase-Dependent Mitochondrial Oxidative Stress in Aging-Associated Cardiovascular Disease. Antioxid Redox Signal 2015, 23, 1389-1409. [CrossRef]
- Anilkumar, N.; San Jose, G.; Sawyer, I.; Santos, C.X.; Sand, C.; Brewer, A.C.; Warren, D.; Shah, A.M. A 28-kDa splice variant of NADPH oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arterioscler Thromb Vasc Biol 2013, 33, e104-112. [CrossRef]
- Kracun, D.; Lopes, L.R.; Cifuentes-Pagano, E.; Pagano, P.J. NADPH oxidases: redox regulation of cell homeostasis and disease. Physiol Rev 2025, 105, 1291-1428. [CrossRef]
- Sutliff, R.L.; Hilenski, L.L.; Amanso, A.M.; Parastatidis, I.; Dikalova, A.E.; Hansen, L.; Datla, S.R.; Long, J.S.; El-Ali, A.M.; Joseph, G.; et al. Polymerase delta interacting protein 2 sustains vascular structure and function. Arterioscler Thromb Vasc Biol 2013, 33, 2154-2161. [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 2020, 21, 363-383. [CrossRef]
- Stone, J.R.; Yang, S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 2006, 8, 243-270. [CrossRef]
- Oshino, N.; Chance, B.; Sies, H.; Bucher, T. The role of H 2 O 2 generation in perfused rat liver and the reaction of catalase compound I and hydrogen donors. Arch Biochem Biophys 1973, 154, 117-131. [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 1979, 59, 527-605. [CrossRef]
- Jones, D.P. Intracellular catalase function: analysis of the catalatic activity by product formation in isolated liver cells. Arch Biochem Biophys 1982, 214, 806-814. [CrossRef]
- Lyublinskaya, O.; Antunes, F. Measuring intracellular concentration of hydrogen peroxide with the use of genetically encoded H(2)O(2) biosensor HyPer. Redox Biol 2019, 24, 101200. [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta 2014, 1840, 1596-1604. [CrossRef]
- Antunes, F.; Cadenas, E. Estimation of H2O2 gradients across biomembranes. FEBS Lett 2000, 475, 121-126. [CrossRef]
- Makino, N.; Mochizuki, Y.; Bannai, S.; Sugita, Y. Kinetic studies on the removal of extracellular hydrogen peroxide by cultured fibroblasts. J Biol Chem 1994, 269, 1020-1025.
- Wagner, B.A.; Witmer, J.R.; van 't Erve, T.J.; Buettner, G.R. An Assay for the Rate of Removal of Extracellular Hydrogen Peroxide by Cells. Redox Biol 2013, 1, 210-217. [CrossRef]
- Huang, B.K.; Sikes, H.D. Quantifying intracellular hydrogen peroxide perturbations in terms of concentration. Redox Biol 2014, 2, 955-962. [CrossRef]
- Adimora, N.J.; Jones, D.P.; Kemp, M.L. A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses. Antioxid Redox Signal 2010, 13, 731-743. [CrossRef]
- Sobotta, M.C.; Barata, A.G.; Schmidt, U.; Mueller, S.; Millonig, G.; Dick, T.P. Exposing cells to H2O2: a quantitative comparison between continuous low-dose and one-time high-dose treatments. Free Radic Biol Med 2013, 60, 325-335. [CrossRef]
- Marinho, H.S.; Cyrne, L.; Cadenas, E.; Antunes, F. H2O2 delivery to cells: steady-state versus bolus addition. Methods Enzymol 2013, 526, 159-173. [CrossRef]
- Lim, J.B.; Langford, T.F.; Huang, B.K.; Deen, W.M.; Sikes, H.D. A reaction-diffusion model of cytosolic hydrogen peroxide. Free Radic Biol Med 2016, 90, 85-90. [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 2008, 295, C849-868. [CrossRef]
- Pardo-Pena, K.; Yanez-Hernandez, A.; Medina-Ceja, L.; Morales-Villagran, A. Ellagic acid and allopurinol decrease H(2)O(2) concentrations, epileptiform activity and astrogliosis after status epilepticus in the hippocampus of adult rats. Exp Brain Res 2022, 240, 1191-1203. [CrossRef]
- Pardo-Pena, K.; Sanchez-Lira, A.; Salazar-Sanchez, J.C.; Morales-Villagran, A. A novel online fluorescence method for in-vivo measurement of hydrogen peroxide during oxidative stress produced in a temporal lobe epilepsy model. Neuroreport 2018, 29, 621-630. [CrossRef]
- Pardo-Pena, K.; Lorea-Hernandez, J.J.; Camacho-Hernandez, N.P.; Ordaz, B.; Villasana-Salazar, B.; Morales-Villagran, A.; Pena-Ortega, F. Hydrogen peroxide extracellular concentration in the ventrolateral medulla and its increase in response to hypoxia in vitro: Possible role of microglia. Brain Res 2018, 1692, 87-99. [CrossRef]
- Puppulin, L.; Hosogi, S.; Sun, H.; Matsuo, K.; Inui, T.; Kumamoto, Y.; Suzaki, T.; Tanaka, H.; Marunaka, Y. Bioconjugation strategy for cell surface labelling with gold nanostructures designed for highly localized pH measurement. Nat Commun 2018, 9, 5278. [CrossRef]
- Hosogi, S.; Marunaka, Y.; Ashihara, E.; Yamada, T.; Sumino, A.; Tanaka, H.; Puppulin, L. Plasma membrane anchored nanosensor for quantifying endogenous production of H(2)O(2) in living cells. Biosens Bioelectron 2021, 179, 113077. [CrossRef]
- Forman, H.J.; Bernardo, A.; Davies, K.J. What is the concentration of hydrogen peroxide in blood and plasma? Arch Biochem Biophys 2016, 603, 48-53. [CrossRef]
- Sousa, T.; Gouveia, M.; Travasso, R.D.M.; Salvador, A. How abundant are superoxide and hydrogen peroxide in the vasculature lumen, how far can they reach? Redox Biol 2022, 58, 102527. [CrossRef]
- Bayer, S.B.; Maghzal, G.; Stocker, R.; Hampton, M.B.; Winterbourn, C.C. Neutrophil-mediated oxidation of erythrocyte peroxiredoxin 2 as a potential marker of oxidative stress in inflammation. FASEB J 2013, 27, 3315-3322. [CrossRef]
- Ezerina, D.; Morgan, B.; Dick, T.P. Imaging dynamic redox processes with genetically encoded probes. J Mol Cell Cardiol 2014, 73, 43-49. [CrossRef]
- Mishra, P.K.; Park, I.; Sharma, N.; Yoo, C.M.; Lee, H.Y.; Rhee, H.W. Enzymatic Recording of Local Hydrogen Peroxide Generation Using Genetically Encodable Enzyme. Anal Chem 2022, 94, 14869-14877. [CrossRef]
- Eid, M.; Barayeu, U.; Sulkova, K.; Aranda-Vallejo, C.; Dick, T.P. Using the heme peroxidase APEX2 to probe intracellular H(2)O(2) flux and diffusion. Nat Commun 2024, 15, 1239. [CrossRef]
- Yamdjeu, O.T.; Begerow, A.; Sommer, N.; Diener, M.; Weissmann, N.; Knoepp, F. H(2)O(2) Sensitivity of K(v) Channels in Hypoxic Pulmonary Vasoconstriction: Experimental Conditions Matter. Int J Mol Sci 2025, 26. [CrossRef]
- Mishina, N.M.; Tyurin-Kuzmin, P.A.; Markvicheva, K.N.; Vorotnikov, A.V.; Tkachuk, V.A.; Laketa, V.; Schultz, C.; Lukyanov, S.; Belousov, V.V. Does cellular hydrogen peroxide diffuse or act locally? Antioxid Redox Signal 2011, 14, 1-7. [CrossRef]
- Mishina, N.M.; Bogdanova, Y.A.; Ermakova, Y.G.; Panova, A.S.; Kotova, D.A.; Bilan, D.S.; Steinhorn, B.; Arner, E.S.J.; Michel, T.; Belousov, V.V. Which Antioxidant System Shapes Intracellular H(2)O(2) Gradients? Antioxid Redox Signal 2019, 31, 664-670. [CrossRef]
- Pak, V.V.; Ezerina, D.; Lyublinskaya, O.G.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Martinez Gache, S.A.; et al. Ultrasensitive Genetically Encoded Indicator for Hydrogen Peroxide Identifies Roles for the Oxidant in Cell Migration and Mitochondrial Function. Cell Metab 2020, 31, 642-653 e646. [CrossRef]
- Kritsiligkou, P.; Bosch, K.; Shen, T.K.; Meurer, M.; Knop, M.; Dick, T.P. Proteome-wide tagging with an H(2)O(2) biosensor reveals highly localized and dynamic redox microenvironments. Proc Natl Acad Sci U S A 2023, 120, e2314043120. [CrossRef]
- Potekhina, E.S.; Bass, D.I.; Ezerina, D.; Fleckenstein, D.D.; Chebotarev, A.S.; Sysoeva, V.A.; Maltsev, D.I.; Pak, V.V.; Moshchenko, A.A.; Sokolov, A.I.; et al. A color-tailored fluorogenic sensor for hydrogen peroxide. Nat Chem Biol 2025. [CrossRef]
- Booth, D.M.; Varnai, P.; Joseph, S.K.; Hajnoczky, G. Oxidative bursts of single mitochondria mediate retrograde signaling toward the ER. Mol Cell 2021, 81, 3866-3876 e3862. [CrossRef]
- Joseph, S.K.; Young, M.P.; Alzayady, K.; Yule, D.I.; Ali, M.; Booth, D.M.; Hajnoczky, G. Redox regulation of type-I inositol trisphosphate receptors in intact mammalian cells. J Biol Chem 2018, 293, 17464-17476. [CrossRef]
- King, M.P.; Attardi, G. Isolation of human cell lines lacking mitochondrial DNA. Methods Enzymol 1996, 264, 304-313. [CrossRef]
- Firth, A.L.; Gordienko, D.V.; Yuill, K.H.; Smirnov, S.V. Cellular localization of mitochondria contributes to Kv channel-mediated regulation of cellular excitability in pulmonary but not mesenteric circulation. Am J Physiol Lung Cell Mol Physiol 2009, 296, L347-360. [CrossRef]
- Archer, S.L.; Huang, J.; Henry, T.; Peterson, D.; Weir, E.K. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res 1993, 73, 1100-1112. [CrossRef]
- Weir, E.K. Does normoxic pulmonary vasodilatation rather than hypoxic vasoconstriction account for the pulmonary pressor response to hypoxia? Lancet 1978, 1, 476-477. [CrossRef]
- Kilfoil, P.J.; Tipparaju, S.M.; Barski, O.A.; Bhatnagar, A. Regulation of ion channels by pyridine nucleotides. Circ Res 2013, 112, 721-741. [CrossRef]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid Redox Signal 2015, 23, 734-746. [CrossRef]
- Dwenger, M.M.; Raph, S.M.; Reyzer, M.L.; Lisa Manier, M.; Riggs, D.W.; Wohl, Z.B.; Ohanyan, V.; Mack, G.; Pucci, T.; Moore, J.B.t.; et al. Pyridine nucleotide redox potential in coronary smooth muscle couples myocardial blood flow to cardiac metabolism. Nat Commun 2022, 13, 2051. [CrossRef]
- Liu, S.Q.; Jin, H.; Zacarias, A.; Srivastava, S.; Bhatnagar, A. Binding of pyridine coenzymes to the beta-subunit of the voltage sensitive potassium channels. Chem Biol Interact 2001, 130-132, 955-962. [CrossRef]
- Gutscher, M.; Pauleau, A.L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-time imaging of the intracellular glutathione redox potential. Nat Methods 2008, 5, 553-559. [CrossRef]
- Swain, L.; Kesemeyer, A.; Meyer-Roxlau, S.; Vettel, C.; Zieseniss, A.; Guntsch, A.; Jatho, A.; Becker, A.; Nanadikar, M.S.; Morgan, B.; et al. Redox Imaging Using Cardiac Myocyte-Specific Transgenic Biosensor Mice. Circ Res 2016, 119, 1004-1016. [CrossRef]
- Weir, E.K.; Lopez-Barneo, J.; Buckler, K.J.; Archer, S.L. Acute oxygen-sensing mechanisms. N Engl J Med 2005, 353, 2042-2055. [CrossRef]
- Weir, E.K.; Will, J.A.; Lundquist, L.J.; Eaton, J.W.; Chesler, E. Diamide inhibits pulmonary vasoconstriction induced by hypoxia or prostaglandin F2 alpha. Proc Soc Exp Biol Med 1983, 173, 96-103. [CrossRef]
- Burghuber, O.; Mathias, M.M.; McMurtry, I.F.; Reeves, J.T.; Voelkel, N.F. Lung edema due to hydrogen peroxide is independent of cyclooxygenase products. J Appl Physiol Respir Environ Exerc Physiol 1984, 56, 900-905. [CrossRef]
- Weir, E.K.; Eaton, J.W.; Chesler, E. Redox status and pulmonary vascular reactivity. Chest 1985, 88, 249S-252S. [CrossRef]
- Archer, S.L.; Will, J.A.; Weir, E.K. Redox status in the control of pulmonary vascular tone. Herz 1986, 11, 127-141.
- Archer, S.L.; Peterson, D.; Nelson, D.P.; DeMaster, E.G.; Kelly, B.; Eaton, J.W.; Weir, E.K. Oxygen radicals and antioxidant enzymes alter pulmonary vascular reactivity in the rat lung. J Appl Physiol (1985) 1989, 66, 102-111. [CrossRef]
- McMurtry, I.F. Angiotensin is not required for hypoxic constriction in salt solution-perfused rat lungs. J Appl Physiol Respir Environ Exerc Physiol 1984, 56, 375-380. [CrossRef]
- Archer, S.L.; Nelson, D.P.; Weir, E.K. Simultaneous measurement of O2 radicals and pulmonary vascular reactivity in rat lung. J Appl Physiol (1985) 1989, 67, 1903-1911. [CrossRef]
- Post, J.M.; Hume, J.R.; Archer, S.L.; Weir, E.K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol 1992, 262, C882-890. [CrossRef]
- Rounds, S.; McMurtry, I.F. Inhibitors of oxidative ATP production cause transient vasoconstriction and block subsequent pressor responses in rat lungs. Circ Res 1981, 48, 393-400. [CrossRef]
- Leach, R.M.; Sheehan, D.W.; Chacko, V.P.; Sylvester, J.T. Effects of hypoxia on energy state and pH in resting pulmonary and femoral arterial smooth muscles. Am J Physiol 1998, 275, L1051-1060. [CrossRef]
- Leach, R.M.; Sheehan, D.W.; Chacko, V.P.; Sylvester, J.T. Energy state, pH, and vasomotor tone during hypoxia in precontracted pulmonary and femoral arteries. Am J Physiol Lung Cell Mol Physiol 2000, 278, L294-304. [CrossRef]
- Buescher, P.C.; Pearse, D.B.; Pillai, R.P.; Litt, M.C.; Mitchell, M.C.; Sylvester, J.T. Energy state and vasomotor tone in hypoxic pig lungs. J Appl Physiol (1985) 1991, 70, 1874-1881. [CrossRef]
- Wu, W.; Platoshyn, O.; Firth, A.L.; Yuan, J.X. Hypoxia divergently regulates production of reactive oxygen species in human pulmonary and coronary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007, 293, L952-959. [CrossRef]
- Mehta, J.P.; Campian, J.L.; Guardiola, J.; Cabrera, J.A.; Weir, E.K.; Eaton, J.W. Generation of oxidants by hypoxic human pulmonary and coronary smooth-muscle cells. Chest 2008, 133, 1410-1414. [CrossRef]
- Divakaruni, A.S.; Jastroch, M. A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements. Nat Metab 2022, 4, 978-994. [CrossRef]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 2006, 3, 281-286. [CrossRef]
- Brandt, U. Energy converting NADH:quinone oxidoreductase (complex I). Annu Rev Biochem 2006, 75, 69-92. [CrossRef]
- Hameedi, M.A.; Grba, D.N.; Richardson, K.H.; Jones, A.J.Y.; Song, W.; Roessler, M.M.; Wright, J.J.; Hirst, J. A conserved arginine residue is critical for stabilizing the N2 FeS cluster in mitochondrial complex I. J Biol Chem 2021, 296, 100474. [CrossRef]
- Kashani-Poor, N.; Zwicker, K.; Kerscher, S.; Brandt, U. A central functional role for the 49-kDa subunit within the catalytic core of mitochondrial complex I. J Biol Chem 2001, 276, 24082-24087. [CrossRef]
- Prieur, I.; Lunardi, J.; Dupuis, A. Evidence for a quinone binding site close to the interface between NUOD and NUOB subunits of Complex I. Biochim Biophys Acta 2001, 1504, 173-178. [CrossRef]
- Read, A.D.; Bentley, R.E.T.; Martin, A.Y.; Mewburn, J.D.; Alizadeh, E.; Wu, D.; Lima, P.D.A.; Dunham-Snary, K.J.; Thebaud, B.; Sharp, W.; et al. Electron Leak From the Mitochondrial Electron Transport Chain Complex I at Site I(Q) Is Crucial for Oxygen Sensing in Rabbit and Human Ductus Arteriosus. J Am Heart Assoc 2023, 12, e029131. [CrossRef]
- Huttemann, M.; Sommer, N.; Weissmann, N.; Grossman, L.I. Letter by Huttemann et al Regarding Article, "Ndufs2, a Core Subunit of Mitochondrial Complex I, Is Essential for Acute Oxygen-Sensing and Hypoxic Pulmonary Vasoconstriction". Circ Res 2019, 125, e33-e34. [CrossRef]
- Dunham-Snary, K.J.; Archer, S.L. Response by Dunham-Snary and Archer to Letter Regarding Article, "Ndufs2, a Core Subunit of Mitochondrial Complex I, Is Essential for Acute Oxygen-Sensing and Hypoxic Pulmonary Vasoconstriction". Circ Res 2019, 125, e35-e36. [CrossRef]
- Onukwufor, J.O.; Farooqi, M.A.; Vodickova, A.; Koren, S.A.; Baldzizhar, A.; Berry, B.J.; Beutner, G.; Porter, G.A., Jr.; Belousov, V.; Grossfield, A.; et al. A reversible mitochondrial complex I thiol switch mediates hypoxic avoidance behavior in C. elegans. Nat Commun 2022, 13, 2403. [CrossRef]
- Thomas, G.; Ramwell, P. Induction of vascular relaxation by hydroperoxides. Biochem Biophys Res Commun 1986, 139, 102-108. [CrossRef]
- Morgan, B.; Van Laer, K.; Owusu, T.N.; Ezerina, D.; Pastor-Flores, D.; Amponsah, P.S.; Tursch, A.; Dick, T.P. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat Chem Biol 2016, 12, 437-443. [CrossRef]
- Bandara, A.B.; Drake, J.C.; James, C.C.; Smyth, J.W.; Brown, D.A. Complex I protein NDUFS2 is vital for growth, ROS generation, membrane integrity, apoptosis, and mitochondrial energetics. Mitochondrion 2021, 58, 160-168. [CrossRef]
- Sazanov, L.A. From the 'black box' to 'domino effect' mechanism: what have we learned from the structures of respiratory complex I. Biochem J 2023, 480, 319-333. [CrossRef]
- Pagniez-Mammeri, H.; Loublier, S.; Legrand, A.; Benit, P.; Rustin, P.; Slama, A. Mitochondrial complex I deficiency of nuclear origin I. Structural genes. Mol Genet Metab 2012, 105, 163-172. [CrossRef]
- Burska, D.; Stiburek, L.; Krizova, J.; Vanisova, M.; Martinek, V.; Sladkova, J.; Zamecnik, J.; Honzik, T.; Zeman, J.; Hansikova, H.; et al. Homozygous missense mutation in UQCRC2 associated with severe encephalomyopathy, mitochondrial complex III assembly defect and activation of mitochondrial protein quality control. Biochim Biophys Acta Mol Basis Dis 2021, 1867, 166147. [CrossRef]
- Iuso, A.; Scacco, S.; Piccoli, C.; Bellomo, F.; Petruzzella, V.; Trentadue, R.; Minuto, M.; Ripoli, M.; Capitanio, N.; Zeviani, M.; et al. Dysfunctions of cellular oxidative metabolism in patients with mutations in the NDUFS1 and NDUFS4 genes of complex I. J Biol Chem 2006, 281, 10374-10380. [CrossRef]
- McMurtry, I.F.; Davidson, A.B.; Reeves, J.T.; Grover, R.F. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res 1976, 38, 99-104. [CrossRef]
- Harder, D.R.; Madden, J.A.; Dawson, C. A membrane electrical mechanism for hypoxic vasoconstriction of small pulmonary arteries from cat. Chest 1985, 88, 233S-235S. [CrossRef]
- Hasunuma, K.; Rodman, D.M.; McMurtry, I.F. Effects of K+ channel blockers on vascular tone in the perfused rat lung. Am Rev Respir Dis 1991, 144, 884-887. [CrossRef]
- Yuan, X.J. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res 1995, 77, 370-378. [CrossRef]
- Yuan, X.J.; Goldman, W.F.; Tod, M.L.; Rubin, L.J.; Blaustein, M.P. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol 1993, 264, L116-123. [CrossRef]
- Archer, S.L.; Huang, J.M.; Reeve, H.L.; Hampl, V.; Tolarova, S.; Michelakis, E.; Weir, E.K. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res 1996, 78, 431-442. [CrossRef]
- Olschewski, A.; Hong, Z.; Nelson, D.P.; Weir, E.K. Graded response of K+ current, membrane potential, and [Ca2+]i to hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 2002, 283, L1143-1150. [CrossRef]
- Archer, S.L.; Souil, E.; Dinh-Xuan, A.T.; Schremmer, B.; Mercier, J.C.; El Yaagoubi, A.; Nguyen-Huu, L.; Reeve, H.L.; Hampl, V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 1998, 101, 2319-2330. [CrossRef]
- Archer, S.L.; London, B.; Hampl, V.; Wu, X.; Nsair, A.; Puttagunta, L.; Hashimoto, K.; Waite, R.E.; Michelakis, E.D. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J 2001, 15, 1801-1803. [CrossRef]
- Platoshyn, O.; Yu, Y.; Ko, E.A.; Remillard, C.V.; Yuan, J.X. Heterogeneity of hypoxia-mediated decrease in I(K(V)) and increase in [Ca2+](cyt) in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007, 293, L402-416. [CrossRef]
- Firth, A.L.; Platoshyn, O.; Brevnova, E.E.; Burg, E.D.; Powell, F.; Haddad, G.H.; Yuan, J.X. Hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Ann N Y Acad Sci 2009, 1177, 101-111. [CrossRef]
- Olschewski, A.; Li, Y.; Tang, B.; Hanze, J.; Eul, B.; Bohle, R.M.; Wilhelm, J.; Morty, R.E.; Brau, M.E.; Weir, E.K.; et al. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res 2006, 98, 1072-1080. [CrossRef]
- Nagaraj, C.; Tang, B.; Balint, Z.; Wygrecka, M.; Hrzenjak, A.; Kwapiszewska, G.; Stacher, E.; Lindenmann, J.; Weir, E.K.; Olschewski, H.; et al. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur Respir J 2013, 41, 85-95. [CrossRef]
- Fuchs, B.; Dietrich, A.; Gudermann, T.; Kalwa, H.; Grimminger, F.; Weissmann, N. The role of classical transient receptor potential channels in the regulation of hypoxic pulmonary vasoconstriction. Adv Exp Med Biol 2010, 661, 187-200. [CrossRef]
- Archer, S.L.; Michelakis, E.D.; Thebaud, B.; Bonnet, S.; Moudgil, R.; Wu, X.C.; Weir, E.K. A central role for oxygen-sensitive K+ channels and mitochondria in the specialized oxygen-sensing system. Novartis Found Symp 2006, 272, 157-171; discussion 171-155, 214-157.
- Olschewski, A.; Weir, E.K. Redox regulation of ion channels in the pulmonary circulation. Antioxid Redox Signal 2015, 22, 465-485. [CrossRef]
- Rocher, A.; Aaronson, P.I. The Thirty-Fifth Anniversary of K+ Channels in O2 sensing: What We Know and What We Don't Know. Oxygen 2024, 4, 53-89. [CrossRef]
- Firth, A.L.; Yuill, K.H.; Smirnov, S.V. Mitochondria-dependent regulation of Kv currents in rat pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2008, 295, L61-70. [CrossRef]
- Searle, G.J.; Hartness, M.E.; Hoareau, R.; Peers, C.; Kemp, P.J. Lack of contribution of mitochondrial electron transport to acute O(2) sensing in model airway chemoreceptors. Biochem Biophys Res Commun 2002, 291, 332-337. [CrossRef]
- Cogolludo, A.; Frazziano, G.; Cobeno, L.; Moreno, L.; Lodi, F.; Villamor, E.; Tamargo, J.; Perez-Vizcaino, F. Role of reactive oxygen species in Kv channel inhibition and vasoconstriction induced by TP receptor activation in rat pulmonary arteries. Ann N Y Acad Sci 2006, 1091, 41-51. [CrossRef]
- Frazziano, G.; Moreno, L.; Moral-Sanz, J.; Menendez, C.; Escolano, L.; Gonzalez, C.; Villamor, E.; Alvarez-Sala, J.L.; Cogolludo, A.L.; Perez-Vizcaino, F. Neutral sphingomyelinase, NADPH oxidase and reactive oxygen species. Role in acute hypoxic pulmonary vasoconstriction. J Cell Physiol 2011, 226, 2633-2640. [CrossRef]
- Post, J.M.; Gelband, C.H.; Hume, J.R. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res 1995, 77, 131-139. [CrossRef]
- Vandier, C.; Delpech, M.; Bonnet, P. Spontaneous transient outward currents and delayed rectifier K+ current: effects of hypoxia. Am J Physiol 1998, 275, L145-154. [CrossRef]
- Snetkov, V.A.; Smirnov, S.V.; Kua, J.; Aaronson, P.I.; Ward, J.P.; Knock, G.A. Superoxide differentially controls pulmonary and systemic vascular tone through multiple signalling pathways. Cardiovasc Res 2011, 89, 214-224. [CrossRef]
- Svoboda, L.K.; Reddie, K.G.; Zhang, L.; Vesely, E.D.; Williams, E.S.; Schumacher, S.M.; O'Connell, R.P.; Shaw, R.; Day, S.M.; Anumonwo, J.M.; et al. Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel Kv1.5. Circ Res 2012, 111, 842-853. [CrossRef]
- Raph, S.M.; Dwenger, M.M.; Hu, X.; Nystoriak, M.A. Basal NAD(H) redox state permits hydrogen peroxide-induced mesenteric artery dilatation. J Physiol 2023, 601, 2621-2634. [CrossRef]
- Moreno, L.; Frazziano, G.; Cogolludo, A.; Cobeno, L.; Tamargo, J.; Perez-Vizcaino, F. Role of protein kinase Czeta and its adaptor protein p62 in voltage-gated potassium channel modulation in pulmonary arteries. Mol Pharmacol 2007, 72, 1301-1309. [CrossRef]
- Schumacher, S.M.; McEwen, D.P.; Zhang, L.; Arendt, K.L.; Van Genderen, K.M.; Martens, J.R. Antiarrhythmic drug-induced internalization of the atrial-specific k+ channel kv1.5. Circ Res 2009, 104, 1390-1398. [CrossRef]
- Lee, S.; Park, M.; So, I.; Earm, Y.E. NADH and NAD modulates Ca(2+)-activated K+ channels in small pulmonary arterial smooth muscle cells of the rabbit. Pflugers Arch 1994, 427, 378-380. [CrossRef]
- Reeve, H.L.; Weir, E.K.; Nelson, D.P.; Peterson, D.A.; Archer, S.L. Opposing effects of oxidants and antioxidants on K+ channel activity and tone in rat vascular tissue. Exp Physiol 1995, 80, 825-834. [CrossRef]
- Park, M.K.; Lee, S.H.; Ho, W.K.; Earm, Y.E. Redox agents as a link between hypoxia and the responses of ionic channels in rabbit pulmonary vascular smooth muscle. Exp Physiol 1995, 80, 835-842. [CrossRef]
- Park, M.K.; Bae, Y.M.; Lee, S.H.; Ho, W.K.; Earm, Y.E. Modulation of voltage-dependent K+ channel by redox potential in pulmonary and ear arterial smooth muscle cells of the rabbit. Pflugers Arch 1997, 434, 764-771. [CrossRef]
- Olschewski, A.; Hong, Z.; Peterson, D.A.; Nelson, D.P.; Porter, V.A.; Weir, E.K. Opposite effects of redox status on membrane potential, cytosolic calcium, and tone in pulmonary arteries and ductus arteriosus. Am J Physiol Lung Cell Mol Physiol 2004, 286, L15-22. [CrossRef]
- Schach, C.; Xu, M.; Platoshyn, O.; Keller, S.H.; Yuan, J.X. Thiol oxidation causes pulmonary vasodilation by activating K+ channels and inhibiting store-operated Ca2+ channels. Am J Physiol Lung Cell Mol Physiol 2007, 292, L685-698. [CrossRef]
- Yuan, X.J.; Tod, M.L.; Rubin, L.J.; Blaustein, M.P. Deoxyglucose and reduced glutathione mimic effects of hypoxia on K+ and Ca2+ conductances in pulmonary artery cells. Am J Physiol 1994, 267, L52-63. [CrossRef]
- Weir, E.K.; Hong, Z.; Porter, V.A.; Reeve, H.L. Redox signaling in oxygen sensing by vessels. Respir Physiol Neurobiol 2002, 132, 121-130. [CrossRef]
- Marshall, C.; Mamary, A.J.; Verhoeven, A.J.; Marshall, B.E. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 1996, 15, 633-644. [CrossRef]
- Killilea, D.W.; Hester, R.; Balczon, R.; Babal, P.; Gillespie, M.N. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2000, 279, L408-412. [CrossRef]
- Abdalla, S.; Will, J.A. Potentiation of the hypoxic contraction of guinea-pig isolated pulmonary arteries by two inhibitors of superoxide dismutase. Gen Pharmacol 1995, 26, 785-792. [CrossRef]
- Duranteau, J.; Chandel, N.S.; Kulisz, A.; Shao, Z.; Schumacker, P.T. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 1998, 273, 11619-11624. [CrossRef]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 1998, 95, 11715-11720. [CrossRef]
- Turrens, J.F.; Alexandre, A.; Lehninger, A.L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 1985, 237, 408-414. [CrossRef]
- Heumuller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.; Busse, R.; Schroder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008, 51, 211-217. [CrossRef]
- Leach, R.M.; Hill, H.M.; Snetkov, V.A.; Robertson, T.P.; Ward, J.P. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol 2001, 536, 211-224. [CrossRef]
- Robb, E.L.; Hall, A.R.; Prime, T.A.; Eaton, S.; Szibor, M.; Viscomi, C.; James, A.M.; Murphy, M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J Biol Chem 2018, 293, 9869-9879. [CrossRef]
- Waypa, G.B.; Marks, J.D.; Mack, M.M.; Boriboun, C.; Mungai, P.T.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res 2002, 91, 719-726. [CrossRef]
- Weissmann, N.; Ebert, N.; Ahrens, M.; Ghofrani, H.A.; Schermuly, R.T.; Hanze, J.; Fink, L.; Rose, F.; Conzen, J.; Seeger, W.; et al. Effects of mitochondrial inhibitors and uncouplers on hypoxic vasoconstriction in rabbit lungs. Am J Respir Cell Mol Biol 2003, 29, 721-732. [CrossRef]
- Weissmann, N.; Tadic, A.; Hanze, J.; Rose, F.; Winterhalder, S.; Nollen, M.; Schermuly, R.T.; Ghofrani, H.A.; Seeger, W.; Grimminger, F. Hypoxic vasoconstriction in intact lungs: a role for NADPH oxidase-derived H(2)O(2)? Am J Physiol Lung Cell Mol Physiol 2000, 279, L683-690. [CrossRef]
- Weissmann, N.; Winterhalder, S.; Nollen, M.; Voswinckel, R.; Quanz, K.; Ghofrani, H.A.; Schermuly, R.T.; Seeger, W.; Grimminger, F. NO and reactive oxygen species are involved in biphasic hypoxic vasoconstriction of isolated rabbit lungs. Am J Physiol Lung Cell Mol Physiol 2001, 280, L638-645. [CrossRef]
- Weissmann, N.; Zeller, S.; Schafer, R.U.; Turowski, C.; Ay, M.; Quanz, K.; Ghofrani, H.A.; Schermuly, R.T.; Fink, L.; Seeger, W.; et al. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 2006, 34, 505-513. [CrossRef]
- Liu, J.Q.; Sham, J.S.; Shimoda, L.A.; Kuppusamy, P.; Sylvester, J.T. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 2003, 285, L322-333. [CrossRef]
- Dikalov, S.; Griendling, K.K.; Harrison, D.G. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 2007, 49, 717-727. [CrossRef]
- Guzy, R.D.; Hoyos, B.; Robin, E.; Chen, H.; Liu, L.; Mansfield, K.D.; Simon, M.C.; Hammerling, U.; Schumacker, P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 2005, 1, 401-408. [CrossRef]
- Waypa, G.B.; Guzy, R.; Mungai, P.T.; Mack, M.M.; Marks, J.D.; Roe, M.W.; Schumacker, P.T. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res 2006, 99, 970-978. [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001, 30, 1191-1212. [CrossRef]
- Sabharwal, S.S.; Waypa, G.B.; Marks, J.D.; Schumacker, P.T. Peroxiredoxin-5 targeted to the mitochondrial intermembrane space attenuates hypoxia-induced reactive oxygen species signalling. Biochem J 2013, 456, 337-346. [CrossRef]
- Wang, Q.S.; Zheng, Y.M.; Dong, L.; Ho, Y.S.; Guo, Z.; Wang, Y.X. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med 2007, 42, 642-653. [CrossRef]
- Yang, Z.; Zhuan, B.; Yan, Y.; Jiang, S.; Wang, T. Roles of different mitochondrial electron transport chain complexes in hypoxia-induced pulmonary vasoconstriction. Cell Biol Int 2016, 40, 188-195. [CrossRef]
- Waypa, G.B.; Marks, J.D.; Guzy, R.; Mungai, P.T.; Schriewer, J.; Dokic, D.; Schumacker, P.T. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 2010, 106, 526-535. [CrossRef]
- Meyer, A.J.; Dick, T.P. Fluorescent protein-based redox probes. Antioxid Redox Signal 2010, 13, 621-650. [CrossRef]
- Desireddi, J.R.; Farrow, K.N.; Marks, J.D.; Waypa, G.B.; Schumacker, P.T. Hypoxia increases ROS signaling and cytosolic Ca(2+) in pulmonary artery smooth muscle cells of mouse lungs slices. Antioxid Redox Signal 2010, 12, 595-602. [CrossRef]
- Doctrow, S.R.; Huffman, K.; Marcus, C.B.; Musleh, W.; Bruce, A.; Baudry, M.; Malfroy, B. Salen-manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv Pharmacol 1997, 38, 247-269. [CrossRef]
- Casteels, R.; Raeymaekers, L.; Suzuki, H.; Van Eldere, J. Tension response and 45Ca release in vascular smooth muscle incubated in Ca-free solution. Pflugers Arch 1981, 392, 139-145. [CrossRef]
- Paddenberg, R.; Konig, P.; Faulhammer, P.; Goldenberg, A.; Pfeil, U.; Kummer, W. Hypoxic vasoconstriction of partial muscular intra-acinar pulmonary arteries in murine precision cut lung slices. Respir Res 2006, 7, 93. [CrossRef]
- Sanderson, M.J.; Bai, Y.; Perez-Zoghbi, J. Ca(2+) oscillations regulate contraction of intrapulmonary smooth muscle cells. Adv Exp Med Biol 2010, 661, 77-96. [CrossRef]
- Truong, L.; Zheng, Y.M.; Wang, Y.X. Mitochondrial Rieske iron-sulfur protein in pulmonary artery smooth muscle: A key primary signaling molecule in pulmonary hypertension. Arch Biochem Biophys 2020, 683, 108234. [CrossRef]
- Song, T.; Zheng, Y.M.; Wang, Y.X. Cross Talk Between Mitochondrial Reactive Oxygen Species and Sarcoplasmic Reticulum Calcium in Pulmonary Arterial Smooth Muscle Cells. Adv Exp Med Biol 2017, 967, 289-298. [CrossRef]
- Yang, Z.; Song, T.; Truong, L.; Reyes-Garcia, J.; Wang, L.; Zheng, Y.M.; Wang, Y.X. Important Role of Sarcoplasmic Reticulum Ca(2+) Release via Ryanodine Receptor-2 Channel in Hypoxia-Induced Rieske Iron-Sulfur Protein-Mediated Mitochondrial Reactive Oxygen Species Generation in Pulmonary Artery Smooth Muscle Cells. Antioxid Redox Signal 2020, 32, 447-462. [CrossRef]
- Anso, E.; Weinberg, S.E.; Diebold, L.P.; Thompson, B.J.; Malinge, S.; Schumacker, P.T.; Liu, X.; Zhang, Y.; Shao, Z.; Steadman, M.; et al. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat Cell Biol 2017, 19, 614-625. [CrossRef]
- Hughes, B.G.; Hekimi, S. A mild impairment of mitochondrial electron transport has sex-specific effects on lifespan and aging in mice. PLoS One 2011, 6, e26116. [CrossRef]
- Waypa, G.B.; Smith, K.A.; Mungai, P.T.; Dudley, V.J.; Helmin, K.A.; Singer, B.D.; Peek, C.B.; Bass, J.; Nelson, L.; Shah, S.J.; et al. Mitochondria regulate proliferation in adult cardiac myocytes. J Clin Invest 2024, 134. [CrossRef]
- Horvath, A.; Horakova, E.; Dunajcikova, P.; Verner, Z.; Pravdova, E.; Slapetova, I.; Cuninkova, L.; Lukes, J. Downregulation of the nuclear-encoded subunits of the complexes III and IV disrupts their respective complexes but not complex I in procyclic Trypanosoma brucei. Mol Microbiol 2005, 58, 116-130. [CrossRef]
- Owens, K.M.; Kulawiec, M.; Desouki, M.M.; Vanniarajan, A.; Singh, K.K. Impaired OXPHOS complex III in breast cancer. PLoS One 2011, 6, e23846. [CrossRef]
- Kadenbach, B.; Huttemann, M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion 2015, 24, 64-76. [CrossRef]
- Zhou, T.; Chien, M.S.; Kaleem, S.; Matsunami, H. Single cell transcriptome analysis of mouse carotid body glomus cells. J Physiol 2016, 594, 4225-4251. [CrossRef]
- Gao, L.; Bonilla-Henao, V.; Garcia-Flores, P.; Arias-Mayenco, I.; Ortega-Saenz, P.; Lopez-Barneo, J. Gene expression analyses reveal metabolic specifications in acute O(2) -sensing chemoreceptor cells. J Physiol 2017, 595, 6091-6120. [CrossRef]
- Pak, O.; Scheibe, S.; Esfandiary, A.; Gierhardt, M.; Sydykov, A.; Logan, A.; Fysikopoulos, A.; Veit, F.; Hecker, M.; Kroschel, F.; et al. Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension. Eur Respir J 2018. [CrossRef]
- Weissmann, N.; Kuzkaya, N.; Fuchs, B.; Tiyerili, V.; Schafer, R.U.; Schutte, H.; Ghofrani, H.A.; Schermuly, R.T.; Schudt, C.; Sydykov, A.; et al. Detection of reactive oxygen species in isolated, perfused lungs by electron spin resonance spectroscopy. Respir Res 2005, 6, 86. [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat Metab 2022, 4, 651-662. [CrossRef]
- Dry, I.B.; Moore, A.L.; Day, D.A.; Wiskich, J.T. Regulation of alternative pathway activity in plant mitochondria: nonlinear relationship between electron flux and the redox poise of the quinone pool. Arch Biochem Biophys 1989, 273, 148-157. [CrossRef]
- El-Khoury, R.; Dufour, E.; Rak, M.; Ramanantsoa, N.; Grandchamp, N.; Csaba, Z.; Duvillie, B.; Benit, P.; Gallego, J.; Gressens, P.; et al. Alternative oxidase expression in the mouse enables bypassing cytochrome c oxidase blockade and limits mitochondrial ROS overproduction. PLoS Genet 2013, 9, e1003182. [CrossRef]
- Maxwell, D.P.; Wang, Y.; McIntosh, L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci U S A 1999, 96, 8271-8276. [CrossRef]
- Moreno-Dominguez, A.; Ortega-Saenz, P.; Gao, L.; Colinas, O.; Garcia-Flores, P.; Bonilla-Henao, V.; Aragones, J.; Huttemann, M.; Grossman, L.I.; Weissmann, N.; et al. Acute O2 sensing through HIF2alpha-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci Signal 2020, 13. [CrossRef]
- Tanaka, H.; Nishimaru, K.; Aikawa, T.; Hirayama, W.; Tanaka, Y.; Shigenobu, K. Effect of SEA0400, a novel inhibitor of sodium-calcium exchanger, on myocardial ionic currents. Br J Pharmacol 2002, 135, 1096-1100. [CrossRef]
- Ruiz, A.; Alberdi, E.; Matute, C. CGP37157, an inhibitor of the mitochondrial Na+/Ca2+ exchanger, protects neurons from excitotoxicity by blocking voltage-gated Ca2+ channels. Cell Death Dis 2014, 5, e1156. [CrossRef]
- Friedman, S.M. The effects of external sodium substitution on cell sodium and potassium in vascular smooth muscle. J Physiol 1977, 270, 195-208. [CrossRef]
- Nita, II; Hershfinkel, M.; Lewis, E.C.; Sekler, I. A crosstalk between Na(+) channels, Na(+)/K(+) pump and mitochondrial Na(+) transporters controls glucose-dependent cytosolic and mitochondrial Na(+) signals. Cell Calcium 2015, 57, 69-75. [CrossRef]
- Majander, A.; Finel, M.; Wikstrom, M. Diphenyleneiodonium inhibits reduction of iron-sulfur clusters in the mitochondrial NADH-ubiquinone oxidoreductase (Complex I). J Biol Chem 1994, 269, 21037-21042.
- Mohazzab, K.M.; Fayngersh, R.P.; Kaminski, P.M.; Wolin, M.S. Potential role of NADH oxidoreductase-derived reactive O2 species in calf pulmonary arterial PO2-elicited responses. Am J Physiol 1995, 269, L637-644. [CrossRef]
- Grimminger, F.; Weissmann, N.; Spriestersbach, R.; Becker, E.; Rosseau, S.; Seeger, W. Effects of NADPH oxidase inhibitors on hypoxic vasoconstriction in buffer-perfused rabbit lungs. Am J Physiol 1995, 268, L747-752. [CrossRef]
- Diatchuk, V.; Lotan, O.; Koshkin, V.; Wikstroem, P.; Pick, E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem 1997, 272, 13292-13301. [CrossRef]
- Lahiri, S.; Ehleben, W.; Acker, H. Chemoreceptor discharges and cytochrome redox changes of the rat carotid body: role of heme ligands. Proc Natl Acad Sci U S A 1999, 96, 9427-9432. [CrossRef]
- Rathore, R.; Zheng, Y.M.; Li, X.Q.; Wang, Q.S.; Liu, Q.H.; Ginnan, R.; Singer, H.A.; Ho, Y.S.; Wang, Y.X. Mitochondrial ROS-PKCepsilon signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun 2006, 351, 784-790. [CrossRef]
- Cogolludo, A.; Moreno, L.; Frazziano, G.; Moral-Sanz, J.; Menendez, C.; Castaneda, J.; Gonzalez, C.; Villamor, E.; Perez-Vizcaino, F. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc Res 2009, 82, 296-302. [CrossRef]
- Moral-Sanz, J.; Gonzalez, T.; Menendez, C.; David, M.; Moreno, L.; Macias, A.; Cortijo, J.; Valenzuela, C.; Perez-Vizcaino, F.; Cogolludo, A. Ceramide inhibits Kv currents and contributes to TP-receptor-induced vasoconstriction in rat and human pulmonary arteries. Am J Physiol Cell Physiol 2011, 301, C186-194. [CrossRef]
- Wu-Zhang, A.X.; Newton, A.C. Protein kinase C pharmacology: refining the toolbox. Biochem J 2013, 452, 195-209. [CrossRef]
- Veith, C.; Kraut, S.; Wilhelm, J.; Sommer, N.; Quanz, K.; Seeger, W.; Brandes, R.P.; Weissmann, N.; Schroder, K. NADPH oxidase 4 is not involved in hypoxia-induced pulmonary hypertension. Pulm Circ 2016, 6, 397-400. [CrossRef]
- Murtaza, G.; Paddenberg, R.; Pfeil, U.; Goldenberg, A.; Mermer, P.; Kummer, W. Hypoxia-induced pulmonary vasoconstriction of intra-acinar arteries is impaired in NADPH oxidase 4 gene-deficient mice. Pulm Circ 2018, 8, 2045894018808240. [CrossRef]
- Hales, C.A. The site and mechanism of oxygen sensing for the pulmonary vessels. Chest 1985, 88, 235S-240S. [CrossRef]
- Nagaraj, C.; Tabeling, C.; Nagy, B.M.; Jain, P.P.; Marsh, L.M.; Papp, R.; Pienn, M.; Witzenrath, M.; Ghanim, B.; Klepetko, W.; et al. Hypoxic vascular response and ventilation/perfusion matching in end-stage COPD may depend on p22phox. Eur Respir J 2017, 50. [CrossRef]
- Littler, C.M.; Morris, K.G., Jr.; Fagan, K.A.; McMurtry, I.F.; Messing, R.O.; Dempsey, E.C. Protein kinase C-epsilon-null mice have decreased hypoxic pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol 2003, 284, H1321-1331. [CrossRef]
- Robertson, T.P.; Aaronson, P.I.; Ward, J.P. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitization. Am J Physiol 1995, 268, H301-307. [CrossRef]
- Stuart, J.A.; Fonseca, J.; Moradi, F.; Cunningham, C.; Seliman, B.; Worsfold, C.R.; Dolan, S.; Abando, J.; Maddalena, L.A. How Supraphysiological Oxygen Levels in Standard Cell Culture Affect Oxygen-Consuming Reactions. Oxid Med Cell Longev 2018, 2018, 8238459. [CrossRef]
- Wang, X.T.; McCullough, K.D.; Wang, X.J.; Carpenter, G.; Holbrook, N.J. Oxidative stress-induced phospholipase C-gamma 1 activation enhances cell survival. J Biol Chem 2001, 276, 28364-28371. [CrossRef]
- Strielkov, I.V.; Kizub, I.V.; Khromov, A.S.; Soloviev, A.I. Evidence for the role of phosphatidylcholine-specific phospholipase C in sustained hypoxic pulmonary vasoconstriction. Vascul Pharmacol 2013, 58, 292-298. [CrossRef]
- Liao, B.; Zheng, Y.M.; Yadav, V.R.; Korde, A.S.; Wang, Y.X. Hypoxia induces intracellular Ca2+ release by causing reactive oxygen species-mediated dissociation of FK506-binding protein 12.6 from ryanodine receptor 2 in pulmonary artery myocytes. Antioxid Redox Signal 2011, 14, 37-47. [CrossRef]
- Chen, T.X.; Xu, X.Y.; Zhao, Z.; Zhao, F.Y.; Gao, Y.M.; Yan, X.H.; Wan, Y. Hydrogen peroxide is a critical regulator of the hypoxia-induced alterations of store-operated Ca(2+) entry into rat pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2017, 312, L477-L487. [CrossRef]
- Lin, M.J.; Yang, X.R.; Cao, Y.N.; Sham, J.S. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007, 292, L1598-1608. [CrossRef]
- Park, J.M.; Do, V.Q.; Seo, Y.S.; Kim, H.J.; Nam, J.H.; Yin, M.Z.; Kim, H.J.; Kim, S.J.; Griendling, K.K.; Lee, M.Y. NADPH Oxidase 1 Mediates Acute Blood Pressure Response to Angiotensin II by Contributing to Calcium Influx in Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol 2022, 42, e117-e130. [CrossRef]
- Maier, T.; Follmann, M.; Hessler, G.; Kleemann, H.W.; Hachtel, S.; Fuchs, B.; Weissmann, N.; Linz, W.; Schmidt, T.; Lohn, M.; et al. Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br J Pharmacol 2015, 172, 3650-3660. [CrossRef]
- Knock, G.A.; Ward, J.P. Redox regulation of protein kinases as a modulator of vascular function. Antioxid Redox Signal 2011, 15, 1531-1547. [CrossRef]
- Knock, G.A.; Snetkov, V.A.; Shaifta, Y.; Drndarski, S.; Ward, J.P.; Aaronson, P.I. Role of src-family kinases in hypoxic vasoconstriction of rat pulmonary artery. Cardiovasc Res 2008, 80, 453-462. [CrossRef]
- Knock, G.A.; Snetkov, V.A.; Shaifta, Y.; Connolly, M.; Drndarski, S.; Noah, A.; Pourmahram, G.E.; Becker, S.; Aaronson, P.I.; Ward, J.P. Superoxide constricts rat pulmonary arteries via Rho-kinase-mediated Ca(2+) sensitization. Free Radic Biol Med 2009, 46, 633-642. [CrossRef]
- MacKay, C.E.; Shaifta, Y.; Snetkov, V.V.; Francois, A.A.; Ward, J.P.T.; Knock, G.A. ROS-dependent activation of RhoA/Rho-kinase in pulmonary artery: Role of Src-family kinases and ARHGEF1. Free Radic Biol Med 2017, 110, 316-331. [CrossRef]
- Forman, H.J.; Ursini, F.; Maiorino, M. An overview of mechanisms of redox signaling. J Mol Cell Cardiol 2014, 73, 2-9. [CrossRef]
- Jernigan, N.L.; Resta, T.C.; Gonzalez Bosc, L.V. Altered Redox Balance in the Development of Chronic Hypoxia-induced Pulmonary Hypertension. Adv Exp Med Biol 2017, 967, 83-103. [CrossRef]
- Pourmahram, G.E.; Snetkov, V.A.; Shaifta, Y.; Drndarski, S.; Knock, G.A.; Aaronson, P.I.; Ward, J.P. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med 2008, 45, 1468-1476. [CrossRef]
- Genet, N.; Billaud, M.; Rossignol, R.; Dubois, M.; Gillibert-Duplantier, J.; Isakson, B.E.; Marthan, R.; Savineau, J.P.; Guibert, C. Signaling Pathways Linked to Serotonin-Induced Superoxide Anion Production: A Physiological Role for Mitochondria in Pulmonary Arteries. Front Physiol 2017, 8, 76. [CrossRef]
- Poyton, R.O.; Castello, P.R.; Ball, K.A.; Woo, D.K.; Pan, N. Mitochondria and hypoxic signaling: a new view. Ann N Y Acad Sci 2009, 1177, 48-56. [CrossRef]
- Viner, R.I.; Williams, T.D.; Schoneich, C. Peroxynitrite modification of protein thiols: oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry 1999, 38, 12408-12415. [CrossRef]
- Martinez-Ruiz, A.; Cadenas, S.; Lamas, S. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic Biol Med 2011, 51, 17-29. [CrossRef]
- Andre, F.R.; dos Santos, P.F.; Rando, D.G. Theoretical studies of the role of C-terminal cysteines in the process of S-nitrosylation of human Src kinases. J Mol Model 2016, 22, 23. [CrossRef]
- Minetti, M.; Mallozzi, C.; Di Stasi, A.M. Peroxynitrite activates kinases of the src family and upregulates tyrosine phosphorylation signaling. Free Radic Biol Med 2002, 33, 744-754. [CrossRef]
- Lock, J.T.; Sinkins, W.G.; Schilling, W.P. Effect of protein S-glutathionylation on Ca2+ homeostasis in cultured aortic endothelial cells. Am J Physiol Heart Circ Physiol 2011, 300, H493-506. [CrossRef]
- Sun, J.; Yamaguchi, N.; Xu, L.; Eu, J.P.; Stamler, J.S.; Meissner, G. Regulation of the cardiac muscle ryanodine receptor by O(2) tension and S-nitrosoglutathione. Biochemistry 2008, 47, 13985-13990. [CrossRef]
- Flores-Tamez, V.; Escalante, B.; Rios, A. Peroxynitrite-Induced Intracellular Ca2+ Depression in Cardiac Myocytes: Role of Sarco/Endoplasmic Reticulum Ca2+ Pump. Folia Biol (Praha) 2019, 65, 237-245. [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Peroxiredoxin 2: An Important Element of the Antioxidant Defense of the Erythrocyte. Antioxidants (Basel) 2023, 12. [CrossRef]
- Emerling, B.M.; Weinberg, F.; Snyder, C.; Burgess, Z.; Mutlu, G.M.; Viollet, B.; Budinger, G.R.; Chandel, N.S. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med 2009, 46, 1386-1391. [CrossRef]
- Gusarova, G.A.; Trejo, H.E.; Dada, L.A.; Briva, A.; Welch, L.C.; Hamanaka, R.B.; Mutlu, G.M.; Chandel, N.S.; Prakriya, M.; Sznajder, J.I. Hypoxia leads to Na,K-ATPase downregulation via Ca(2+) release-activated Ca(2+) channels and AMPK activation. Mol Cell Biol 2011, 31, 3546-3556. [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 2010, 11, 554-565. [CrossRef]
- Choi, S.L.; Kim, S.J.; Lee, K.T.; Kim, J.; Mu, J.; Birnbaum, M.J.; Soo Kim, S.; Ha, J. The regulation of AMP-activated protein kinase by H(2)O(2). Biochem Biophys Res Commun 2001, 287, 92-97. [CrossRef]
- Prieto-Lloret, J.; Aaronson, P.I. Hydrogen Sulfide as an O2 Sensor: A Critical Analysis. Adv Exp Med Biol 2017, 967, 261-276. [CrossRef]
- Prieto-Lloret, J.; Shaifta, Y.; Ward, J.P.; Aaronson, P.I. Hypoxic pulmonary vasoconstriction in isolated rat pulmonary arteries is not inhibited by antagonists of H2 S-synthesizing pathways. J Physiol 2015, 593, 385-401. [CrossRef]
- Iciek, M.; Bilska-Wilkosz, A.; Gorny, M. Sulfane sulfur - new findings on an old topic. Acta Biochim Pol 2019, 66, 533-544. [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Markel, T.A.; Drucker, N.; Boone, D.; Whiteman, M.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; et al. Extended hypoxia-mediated H(2) S production provides for long-term oxygen sensing. Acta Physiol (Oxf) 2020, 228, e13368. [CrossRef]
- Goubern, M.; Andriamihaja, M.; Nubel, T.; Blachier, F.; Bouillaud, F. Sulfide, the first inorganic substrate for human cells. FASEB J 2007, 21, 1699-1706. [CrossRef]
- Skovgaard, N.; Olson, K.R. Hydrogen sulfide mediates hypoxic vasoconstriction through a production of mitochondrial ROS in trout gills. Am J Physiol Regul Integr Comp Physiol 2012, 303, R487-494. [CrossRef]
- Gupte, R.S.; Rawat, D.K.; Chettimada, S.; Cioffi, D.L.; Wolin, M.S.; Gerthoffer, W.T.; McMurtry, I.F.; Gupte, S.A. Activation of glucose-6-phosphate dehydrogenase promotes acute hypoxic pulmonary artery contraction. J Biol Chem 2010, 285, 19561-19571. [CrossRef]
- Gupte, S.A.; Okada, T.; McMurtry, I.F.; Oka, M. Role of pentose phosphate pathway-derived NADPH in hypoxic pulmonary vasoconstriction. Pulm Pharmacol Ther 2006, 19, 303-309. [CrossRef]
- Houtkooper, R.H.; Canto, C.; Wanders, R.J.; Auwerx, J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev 2010, 31, 194-223. [CrossRef]
- Wilson, H.L.; Dipp, M.; Thomas, J.M.; Lad, C.; Galione, A.; Evans, A.M. Adp-ribosyl cyclase and cyclic ADP-ribose hydrolase act as a redox sensor. a primary role for cyclic ADP-ribose in hypoxic pulmonary vasoconstriction. J Biol Chem 2001, 276, 11180-11188. [CrossRef]
- Moreno-Dominguez, A.; Colinas, O.; Arias-Mayenco, I.; Cabeza, J.M.; Lopez-Ogayar, J.L.; Chandel, N.S.; Weissmann, N.; Sommer, N.; Pascual, A.; Lopez-Barneo, J. Hif1alpha-dependent mitochondrial acute O(2) sensing and signaling to myocyte Ca(2+) channels mediate arterial hypoxic vasodilation. Nat Commun 2024, 15, 6649. [CrossRef]
- Arias-Mayenco, I.; Gonzalez-Rodriguez, P.; Torres-Torrelo, H.; Gao, L.; Fernandez-Aguera, M.C.; Bonilla-Henao, V.; Ortega-Saenz, P.; Lopez-Barneo, J. Acute O(2) Sensing: Role of Coenzyme QH(2)/Q Ratio and Mitochondrial ROS Compartmentalization. Cell Metab 2018, 28, 145-158 e144. [CrossRef]
- Dwenger, M.M.; Ohanyan, V.; Navedo, M.F.; Nystoriak, M.A. Coronary microvascular Kv1 channels as regulatory sensors of intracellular pyridine nucleotide redox potential. Microcirculation 2018, 25. [CrossRef]
- Torres-Torrelo, H.; Ortega-Saenz, P.; Gao, L.; Lopez-Barneo, J. Lactate sensing mechanisms in arterial chemoreceptor cells. Nat Commun 2021, 12, 4166. [CrossRef]
- Michelakis, E.D.; Rebeyka, I.; Wu, X.; Nsair, A.; Thebaud, B.; Hashimoto, K.; Dyck, J.R.; Haromy, A.; Harry, G.; Barr, A.; et al. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res 2002, 91, 478-486. [CrossRef]
- Hirst, J.; King, M.S.; Pryde, K.R. The production of reactive oxygen species by complex I. Biochem Soc Trans 2008, 36, 976-980. [CrossRef]
- Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Orr, A.L.; Brand, M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol 2013, 1, 304-312. [CrossRef]
- Reeve, H.L.; Tolarova, S.; Nelson, D.P.; Archer, S.; Weir, E.K. Redox control of oxygen sensing in the rabbit ductus arteriosus. J Physiol 2001, 533, 253-261. [CrossRef]
- Bonnet, S.; Michelakis, E.D.; Porter, C.J.; Andrade-Navarro, M.A.; Thebaud, B.; Bonnet, S.; Haromy, A.; Harry, G.; Moudgil, R.; McMurtry, M.S.; et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 2006, 113, 2630-2641. [CrossRef]
- Ahmad, M.; Kelly, M.R.; Zhao, X.; Kandhi, S.; Wolin, M.S. Roles for Nox4 in the contractile response of bovine pulmonary arteries to hypoxia. Am J Physiol Heart Circ Physiol 2010, 298, H1879-1888. [CrossRef]
- Paky, A.; Michael, J.R.; Burke-Wolin, T.M.; Wolin, M.S.; Gurtner, G.H. Endogenous production of superoxide by rabbit lungs: effects of hypoxia or metabolic inhibitors. J Appl Physiol (1985) 1993, 74, 2868-2874. [CrossRef]
- Wang, Y.X.; Zheng, Y.M.; Abdullaev, I.; Kotlikoff, M.I. Metabolic inhibition with cyanide induces calcium release in pulmonary artery myocytes and Xenopus oocytes. Am J Physiol Cell Physiol 2003, 284, C378-388. [CrossRef]
- Young, T.A.; Cunningham, C.C.; Bailey, S.M. Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch Biochem Biophys 2002, 405, 65-72. [CrossRef]
- Starkov, A.A.; Fiskum, G. Myxothiazol induces H(2)O(2) production from mitochondrial respiratory chain. Biochem Biophys Res Commun 2001, 281, 645-650. [CrossRef]
- Watson, M.A.; Wong, H.S.; Brand, M.D. Use of S1QELs and S3QELs to link mitochondrial sites of superoxide and hydrogen peroxide generation to physiological and pathological outcomes. Biochem Soc Trans 2019, 47, 1461-1469. [CrossRef]
- Kilbride, S.M.; Telford, J.E.; Davey, G.P. Complex I Controls Mitochondrial and Plasma Membrane Potentials in Nerve Terminals. Neurochem Res 2021, 46, 100-107. [CrossRef]
- Cheung, J.Y.; Wang, J.; Zhang, X.Q.; Song, J.; Davidyock, J.M.; Prado, F.J.; Shanmughapriya, S.; Worth, A.M.; Madesh, M.; Judenherc-Haouzi, A.; et al. Methylene Blue Counteracts H(2)S-Induced Cardiac Ion Channel Dysfunction and ATP Reduction. Cardiovasc Toxicol 2018, 18, 407-419. [CrossRef]
- Cheranov, S.Y.; Jaggar, J.H. Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries. J Physiol 2004, 556, 755-771. [CrossRef]
- McCarron, J.G.; Olson, M.L.; Wilson, C.; Sandison, M.E.; Chalmers, S. Examining the role of mitochondria in Ca(2)(+) signaling in native vascular smooth muscle. Microcirculation 2013, 20, 317-329. [CrossRef]
- Sward, K.; Dreja, K.; Lindqvist, A.; Persson, E.; Hellstrand, P. Influence of mitochondrial inhibition on global and local [Ca(2+)](I) in rat tail artery. Circ Res 2002, 90, 792-799. [CrossRef]
- Doege, K.; Heine, S.; Jensen, I.; Jelkmann, W.; Metzen, E. Inhibition of mitochondrial respiration elevates oxygen concentration but leaves regulation of hypoxia-inducible factor (HIF) intact. Blood 2005, 106, 2311-2317. [CrossRef]
- Potter, M.; Badder, L.; Hoade, Y.; Johnston, I.G.; Morten, K.J. Monitoring Intracellular Oxygen Concentration: Implications for Hypoxia Studies and Real-Time Oxygen Monitoring. Adv Exp Med Biol 2016, 876, 257-263. [CrossRef]
- Hagen, T.; Taylor, C.T.; Lam, F.; Moncada, S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science 2003, 302, 1975-1978. [CrossRef]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 2008, 60, 418-469. [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J 2015, 29, 4766-4771. [CrossRef]
- Pedre, B.; Barayeu, U.; Ezerina, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H(2)S and sulfane sulfur species. Pharmacol Ther 2021, 228, 107916. [CrossRef]
- Barbosa, N.V.; Nogueira, C.W.; Nogara, P.A.; de Bem, A.F.; Aschner, M.; Rocha, J.B.T. Organoselenium compounds as mimics of selenoproteins and thiol modifier agents. Metallomics 2017, 9, 1703-1734. [CrossRef]
- Olson, K.R.; Gao, Y.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; Markel, T.A.; Boone, D.; Stahelin, R.V.; Batinic-Haberle, I.; Straubg, K.D. Effects of Manganese Porphyrins on Cellular Sulfur Metabolism. Molecules 2020, 25. [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins--From superoxide dismutation to H2O2-driven pathways. Redox Biol 2015, 5, 43-65. [CrossRef]
- Silveira, L.R.; Pereira-Da-Silva, L.; Juel, C.; Hellsten, Y. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med 2003, 35, 455-464. [CrossRef]
- Messent, M.; Griffiths, M.J.; Quinlan, G.J.; Gutteridge, J.M.; Evans, T.W. Ischaemia--reperfusion injury in the rat is modulated by superoxide generation and leads to an augmentation of the hypoxic pulmonary vascular response. Clin Sci (Lond) 1996, 90, 47-54. [CrossRef]
- Shen, C.Y.; Lee, J.F.; Su, C.L.; Wang, D.; Chen, C.F. Hypoxia and reoxygenation of the lung tissues induced mRNA expressions of superoxide dismutase and catalase and interventions from different antioxidants. Transplant Proc 2008, 40, 2182-2184. [CrossRef]
- Francis, B.N.; Wilkins, M.R.; Zhao, L. Tetrahydrobiopterin and the regulation of hypoxic pulmonary vasoconstriction. Eur Respir J 2010, 36, 323-330. [CrossRef]
- Ahmad, M.; Zhao, X.; Kelly, M.R.; Kandhi, S.; Perez, O.; Abraham, N.G.; Wolin, M.S. Heme oxygenase-1 induction modulates hypoxic pulmonary vasoconstriction through upregulation of ecSOD. Am J Physiol Heart Circ Physiol 2009, 297, H1453-1461. [CrossRef]
- Patel, D.; Alhawaj, R.; Wolin, M.S. Exposure of mice to chronic hypoxia attenuates pulmonary arterial contractile responses to acute hypoxia by increases in extracellular hydrogen peroxide. Am J Physiol Regul Integr Comp Physiol 2014, 307, R426-433. [CrossRef]
- Bopp, C.; Auger, C.; Mebazaa, A.; Joshi, G.P.; Schini-Kerth, V.B.; Diemunsch, P. Urapidil, but not dihydropyridine calcium channel inhibitors, preserves the hypoxic pulmonary vasoconstriction: an experimental study in pig arteries. Fundam Clin Pharmacol 2019, 33, 527-534. [CrossRef]
- Demiryurek, A.T.; Wadsworth, R.M.; Kane, K.A. Pharmacological evidence for the role of mediators in hypoxia-induced vasoconstriction in sheep isolated intrapulmonary artery rings. Eur J Pharmacol 1991, 203, 1-8. [CrossRef]
- Weissmann, N.; Grimminger, F.; Voswinckel, R.; Conzen, J.; Seeger, W. Nitro blue tetrazolium inhibits but does not mimic hypoxic vasoconstriction in isolated rabbit lungs. Am J Physiol 1998, 274, L721-727. [CrossRef]
- Mohazzab, K.M.; Wolin, M.S. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery PO2 sensor. Am J Physiol 1994, 267, L823-831. [CrossRef]
- Du, W.; Frazier, M.; McMahon, T.J.; Eu, J.P. Redox activation of intracellular calcium release channels (ryanodine receptors) in the sustained phase of hypoxia-induced pulmonary vasoconstriction. Chest 2005, 128, 556S-558S. [CrossRef]
- Mansoori, S.; Moosavi, S.M.S.; Ketabchi, F. The Interaction between Trolox and 4,4'-diisothiocyanatostilbene-2,2'-disulfonic Acid on Hypoxic Pulmonary Vasoconstriction in the Isolated Rabbit Lung. Iran J Med Sci 2017, 42, 284-291.
- Hodyc, D.; Snorek, M.; Brtnicky, T.; Herget, J. Superoxide dismutase mimetic tempol inhibits hypoxic pulmonary vasoconstriction in rats independently of nitric oxide production. Exp Physiol 2007, 92, 945-951. [CrossRef]
- Pak, O.; Scheibe, S.; Esfandiary, A.; Gierhardt, M.; Sydykov, A.; Logan, A.; Fysikopoulos, A.; Veit, F.; Hecker, M.; Kroschel, F.; et al. Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension. Eur Respir J 2018, 51. [CrossRef]
- Go, Y.M.; Chandler, J.D.; Jones, D.P. The cysteine proteome. Free Radic Biol Med 2015, 84, 227-245. [CrossRef]
- Meyrick, B.; Reid, L. Hypoxia-induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am J Pathol 1980, 100, 151-178.
- Burke, T.M.; Wolin, M.S. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol 1987, 252, H721-732. [CrossRef]
- Sheehan, D.W.; Giese, E.C.; Gugino, S.F.; Russell, J.A. Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am J Physiol 1993, 264, H1542-1547. [CrossRef]
- Jin, N.; Rhoades, R.A. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am J Physiol 1997, 272, H2686-2692. [CrossRef]
- Jones, R.D.; Thompson, J.S.; Morice, A.H. The effect of hydrogen peroxide on hypoxia, prostaglandin F2 alpha and potassium chloride induced contractions in isolated rat pulmonary arteries. Pulm Pharmacol Ther 1997, 10, 37-42. [CrossRef]
- Pelaez, N.J.; Braun, T.R.; Paul, R.J.; Meiss, R.A.; Packer, C.S. H(2)O(2) mediates Ca(2+)- and MLC(20) phosphorylation-independent contraction in intact and permeabilized vascular muscle. Am J Physiol Heart Circ Physiol 2000, 279, H1185-1193. [CrossRef]
- Kerkar, S.; Speyer, C.; Tyburski, J.; Steffes, C. Reactive oxygen metabolites induce a biphasic contractile response in microvascular lung pericytes. J Trauma 2001, 51, 440-445. [CrossRef]
- Nagi, M.N.; Mansour, M.A.; Al-Shabanah, O.A.; El-Kashef, H.A. Melatonin inhibits the contractile effect of vanadate in the isolated pulmonary arterial rings of rats: possible role of hydrogen peroxide. J Biochem Mol Toxicol 2002, 16, 273-278. [CrossRef]
- Oeckler, R.A.; Arcuino, E.; Ahmad, M.; Olson, S.C.; Wolin, M.S. Cytosolic NADH redox and thiol oxidation regulate pulmonary arterial force through ERK MAP kinase. Am J Physiol Lung Cell Mol Physiol 2005, 288, L1017-1025. [CrossRef]
- Maki, J.; Hirano, M.; Hoka, S.; Kanaide, H.; Hirano, K. Involvement of reactive oxygen species in thrombin-induced pulmonary vasoconstriction. Am J Respir Crit Care Med 2010, 182, 1435-1444. [CrossRef]
- Neo, B.H.; Kandhi, S.; Wolin, M.S. Roles for soluble guanylate cyclase and a thiol oxidation-elicited subunit dimerization of protein kinase G in pulmonary artery relaxation to hydrogen peroxide. Am J Physiol Heart Circ Physiol 2010, 299, H1235-1241. [CrossRef]
- Zaloudikova, M.; Herget, J.; Vizek, M. The contractile response of isolated small pulmonary arteries induced by activated macrophages. Physiol Res 2014, 63, 267-270. [CrossRef]
- Lucchesi, P.A.; Belmadani, S.; Matrougui, K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens 2005, 23, 571-579. [CrossRef]
- Thakali, K.; Davenport, L.; Fink, G.D.; Watts, S.W. Pleiotropic effects of hydrogen peroxide in arteries and veins from normotensive and hypertensive rats. Hypertension 2006, 47, 482-487. [CrossRef]
- Neo, B.H.; Kandhi, S.; Wolin, M.S. Roles for redox mechanisms controlling protein kinase G in pulmonary and coronary artery responses to hypoxia. Am J Physiol Heart Circ Physiol 2011, 301, H2295-2304. [CrossRef]
- Zhang, D.X.; Borbouse, L.; Gebremedhin, D.; Mendoza, S.A.; Zinkevich, N.S.; Li, R.; Gutterman, D.D. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res 2012, 110, 471-480. [CrossRef]
- Nishijima, Y.; Cao, S.; Chabowski, D.S.; Korishettar, A.; Ge, A.; Zheng, X.; Sparapani, R.; Gutterman, D.D.; Zhang, D.X. Contribution of K(V)1.5 Channel to Hydrogen Peroxide-Induced Human Arteriolar Dilation and Its Modulation by Coronary Artery Disease. Circ Res 2017, 120, 658-669. [CrossRef]
- Gutterman, D.D.; Miura, H.; Liu, Y. Redox modulation of vascular tone: focus of potassium channel mechanisms of dilation. Arterioscler Thromb Vasc Biol 2005, 25, 671-678. [CrossRef]
- Matoba, T.; Shimokawa, H.; Kubota, H.; Morikawa, K.; Fujiki, T.; Kunihiro, I.; Mukai, Y.; Hirakawa, Y.; Takeshita, A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 2002, 290, 909-913. [CrossRef]
- Yang, W.; Block, E.R. Effect of hypoxia and reoxygenation on the formation and release of reactive oxygen species by porcine pulmonary artery endothelial cells. J Cell Physiol 1995, 164, 414-423. [CrossRef]
- Wojciak-Stothard, B.; Tsang, L.Y.; Haworth, S.G. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 2005, 288, L749-760. [CrossRef]
- Munzel, T.; Afanas'ev, I.B.; Kleschyov, A.L.; Harrison, D.G. Detection of superoxide in vascular tissue. Arterioscler Thromb Vasc Biol 2002, 22, 1761-1768. [CrossRef]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med 2007, 43, 995-1022. [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., 2nd; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 2012, 52, 1-6. [CrossRef]
- Zielonka, J.; Kalyanaraman, B. Small-molecule luminescent probes for the detection of cellular oxidizing and nitrating species. Free Radic Biol Med 2018, 128, 3-22. [CrossRef]
- Li, Y.; Zhu, H.; Trush, M.A. Detection of mitochondria-derived reactive oxygen species production by the chemilumigenic probes lucigenin and luminol. Biochim Biophys Acta 1999, 1428, 1-12. [CrossRef]
- Rembish, S.J.; Trush, M.A. Further evidence that lucigenin-derived chemiluminescence monitors mitochondrial superoxide generation in rat alveolar macrophages. Free Radic Biol Med 1994, 17, 117-126. [CrossRef]
- Janiszewski, M.; Souza, H.P.; Liu, X.; Pedro, M.A.; Zweier, J.L.; Laurindo, F.R. Overestimation of NADH-driven vascular oxidase activity due to lucigenin artifacts. Free Radic Biol Med 2002, 32, 446-453. [CrossRef]
- Budd, S.L.; Castilho, R.F.; Nicholls, D.G. Mitochondrial membrane potential and hydroethidine-monitored superoxide generation in cultured cerebellar granule cells. FEBS Lett 1997, 415, 21-24. [CrossRef]
- Adesina, S.E.; Kang, B.Y.; Bijli, K.M.; Ma, J.; Cheng, J.; Murphy, T.C.; Michael Hart, C.; Sutliff, R.L. Targeting mitochondrial reactive oxygen species to modulate hypoxia-induced pulmonary hypertension. Free Radic Biol Med 2015, 87, 36-47. [CrossRef]
- Sen, B.; Benoit, B.; Brand, M.D. Hypoxia decreases mitochondrial ROS production in cells. Free Radic Biol Med 2024, 224, 1-8. [CrossRef]
- Fang, J.; Wong, H.S.; Brand, M.D. Production of superoxide and hydrogen peroxide in the mitochondrial matrix is dominated by site I(Q) of complex I in diverse cell lines. Redox Biol 2020, 37, 101722. [CrossRef]
- Dooley, C.T.; Dore, T.M.; Hanson, G.T.; Jackson, W.C.; Remington, S.J.; Tsien, R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 2004, 279, 22284-22293. [CrossRef]
- Lukyanov, K.A.; Belousov, V.V. Genetically encoded fluorescent redox sensors. Biochim Biophys Acta 2014, 1840, 745-756. [CrossRef]
- Pouvreau, S. Genetically encoded reactive oxygen species (ROS) and redox indicators. Biotechnol J 2014, 9, 282-293. [CrossRef]
- Nash, K.M.; Rockenbauer, A.; Villamena, F.A. Reactive nitrogen species reactivities with nitrones: theoretical and experimental studies. Chem Res Toxicol 2012, 25, 1581-1597. [CrossRef]
- Dikalov, S.I.; Polienko, Y.F.; Kirilyuk, I. Electron Paramagnetic Resonance Measurements of Reactive Oxygen Species by Cyclic Hydroxylamine Spin Probes. Antioxid Redox Signal 2018, 28, 1433-1443. [CrossRef]
- Gonzalez, C.; Sanz-Alfayate, G.; Obeso, A.; Agapito, M.T. Role of glutathione redox state in oxygen sensing by carotid body chemoreceptor cells. Methods Enzymol 2004, 381, 40-71. [CrossRef]
- Dikalov, S.I.; Kirilyuk, I.A.; Voinov, M.; Grigor'ev, I.A. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res 2011, 45, 417-430. [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Dabritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457-470 e413. [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 1997, 416, 15-18. [CrossRef]
- Brand, M.D. Riding the tiger - physiological and pathological effects of superoxide and hydrogen peroxide generated in the mitochondrial matrix. Crit Rev Biochem Mol Biol 2020, 55, 592-661. [CrossRef]
- Bell, E.L.; Klimova, T.A.; Eisenbart, J.; Moraes, C.T.; Murphy, M.P.; Budinger, G.R.; Chandel, N.S. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol 2007, 177, 1029-1036. [CrossRef]
- Bell, E.L.; Klimova, T.A.; Eisenbart, J.; Schumacker, P.T.; Chandel, N.S. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol 2007, 27, 5737-5745. [CrossRef]
- Chandel, N.S.; Schumacker, P.T. Cellular oxygen sensing by mitochondria: old questions, new insight. J Appl Physiol (1985) 2000, 88, 1880-1889. [CrossRef]
- Dada, L.A.; Chandel, N.S.; Ridge, K.M.; Pedemonte, C.; Bertorello, A.M.; Sznajder, J.I. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest 2003, 111, 1057-1064. [CrossRef]
- Hernansanz-Agustin, P.; Izquierdo-Alvarez, A.; Sanchez-Gomez, F.J.; Ramos, E.; Villa-Pina, T.; Lamas, S.; Bogdanova, A.; Martinez-Ruiz, A. Acute hypoxia produces a superoxide burst in cells. Free Radic Biol Med 2014, 71, 146-156. [CrossRef]
- Loor, G.; Kondapalli, J.; Iwase, H.; Chandel, N.S.; Waypa, G.B.; Guzy, R.D.; Vanden Hoek, T.L.; Schumacker, P.T. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta 2011, 1813, 1382-1394. [CrossRef]
- Mansfield, K.D.; Guzy, R.D.; Pan, Y.; Young, R.M.; Cash, T.P.; Schumacker, P.T.; Simon, M.C. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 2005, 1, 393-399. [CrossRef]
- Mironov, S.L.; Langohr, K. Modulation of synaptic and channel activities in the respiratory network of the mice by NO/cGMP signalling pathways. Brain Res 2007, 1130, 73-82. [CrossRef]
- Na, N.; Chandel, N.S.; Litvan, J.; Ridge, K.M. Mitochondrial reactive oxygen species are required for hypoxia-induced degradation of keratin intermediate filaments. FASEB J 2010, 24, 799-809. [CrossRef]
- Schafer, M.; Schafer, C.; Ewald, N.; Piper, H.M.; Noll, T. Role of redox signaling in the autonomous proliferative response of endothelial cells to hypoxia. Circ Res 2003, 92, 1010-1015. [CrossRef]
- Debski, D.; Smulik, R.; Zielonka, J.; Michalowski, B.; Jakubowska, M.; Debowska, K.; Adamus, J.; Marcinek, A.; Kalyanaraman, B.; Sikora, A. Mechanism of oxidative conversion of Amplex(R) Red to resorufin: Pulse radiolysis and enzymatic studies. Free Radic Biol Med 2016, 95, 323-332. [CrossRef]
- Ferrer-Sueta, G.; Radi, R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol 2009, 4, 161-177. [CrossRef]
- Eid, M.; Barayeu, U.; Dick, T.P. Chemogenetic detection and quantitation of H(2)O(2) in living cells. Nat Protoc 2025. [CrossRef]
- Gupte, S.A.; Li, K.X.; Okada, T.; Sato, K.; Oka, M. Inhibitors of pentose phosphate pathway cause vasodilation: involvement of voltage-gated potassium channels. J Pharmacol Exp Ther 2002, 301, 299-305. [CrossRef]
- Burke-Wolin, T.; Wolin, M.S. H2O2 and cGMP may function as an O2 sensor in the pulmonary artery. J Appl Physiol (1985) 1989, 66, 167-170. [CrossRef]
- Burke-Wolin, T.M.; Wolin, M.S. Inhibition of cGMP-associated pulmonary arterial relaxation to H2O2 and O2 by ethanol. Am J Physiol 1990, 258, H1267-1273. [CrossRef]
- Omar, H.A.; Wolin, M.S. Endothelium-dependent and independent cGMP mechanisms appear to mediate O2 responses in calf pulmonary resistance arteries. Am J Physiol 1992, 262, L560-565. [CrossRef]
- Mohazzab, K.M.; Wolin, M.S. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. Am J Physiol 1994, 267, L815-822. [CrossRef]
- Monaco, J.A.; Burke-Wolin, T. NO and H2O2 mechanisms of guanylate cyclase activation in oxygen-dependent responses of rat pulmonary circulation. Am J Physiol 1995, 268, L546-550. [CrossRef]
- Wolin, M.S.; Burke-Wolin, T.M.; Mohazzab, H.K. Roles for NAD(P)H oxidases and reactive oxygen species in vascular oxygen sensing mechanisms. Respir Physiol 1999, 115, 229-238. [CrossRef]
- Cross, A.R.; Jones, O.T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta 1991, 1057, 281-298. [CrossRef]
- Cross, A.R.; Henderson, L.; Jones, O.T.; Delpiano, M.A.; Hentschel, J.; Acker, H. Involvement of an NAD(P)H oxidase as a pO2 sensor protein in the rat carotid body. Biochem J 1990, 272, 743-747. [CrossRef]
- Griendling, K.K.; Ushio-Fukai, M. NADH/NADPH Oxidase and Vascular Function. Trends Cardiovasc Med 1997, 7, 301-307. [CrossRef]
- Lambeth, J.D.; Cheng, G.; Arnold, R.S.; Edens, W.A. Novel homologs of gp91phox. Trends Biochem Sci 2000, 25, 459-461. [CrossRef]
- Gupte, S.A.; Kaminski, P.M.; Floyd, B.; Agarwal, R.; Ali, N.; Ahmad, M.; Edwards, J.; Wolin, M.S. Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol 2005, 288, H13-21. [CrossRef]
- Gupte, S.A.; Rupawalla, T.; Phillibert, D., Jr.; Wolin, M.S. NADPH and heme redox modulate pulmonary artery relaxation and guanylate cyclase activation by NO. Am J Physiol 1999, 277, L1124-1132. [CrossRef]
- Gupte, S.A.; Arshad, M.; Viola, S.; Kaminski, P.M.; Ungvari, Z.; Rabbani, G.; Koller, A.; Wolin, M.S. Pentose phosphate pathway coordinates multiple redox-controlled relaxing mechanisms in bovine coronary arteries. Am J Physiol Heart Circ Physiol 2003, 285, H2316-2326. [CrossRef]
- Mohazzab, H.K.; Kaminski, P.M.; Fayngersh, R.P.; Wolin, M.S. Oxygen-elicited responses in calf coronary arteries: role of H2O2 production via NADH-derived superoxide. Am J Physiol 1996, 270, H1044-1053. [CrossRef]
- Gupte, S.A.; Wolin, M.S. Hypoxia promotes relaxation of bovine coronary arteries through lowering cytosolic NADPH. Am J Physiol Heart Circ Physiol 2006, 290, H2228-2238. [CrossRef]
- Mallet, R.T.; Olivencia-Yurvati, A.H.; Bunger, R. Pyruvate enhancement of cardiac performance: Cellular mechanisms and clinical application. Exp Biol Med (Maywood) 2018, 243, 198-210. [CrossRef]
- Wolin, M.S.; Gupte, S.A.; Mingone, C.J.; Neo, B.H.; Gao, Q.; Ahmad, M. Redox regulation of responses to hypoxia and NO-cGMP signaling in pulmonary vascular pathophysiology. Ann N Y Acad Sci 2010, 1203, 126-132. [CrossRef]
- Mingone, C.J.; Gupte, S.A.; Ali, N.; Oeckler, R.A.; Wolin, M.S. Thiol oxidation inhibits nitric oxide-mediated pulmonary artery relaxation and guanylate cyclase stimulation. Am J Physiol Lung Cell Mol Physiol 2006, 290, L549-557. [CrossRef]
- Wolin, M.S.; Ahmad, M.; Gupte, S.A. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol 2005, 289, L159-173. [CrossRef]
- Wolin, M.S. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 2009, 296, H539-549. [CrossRef]
- Pandolfi, P.P.; Sonati, F.; Rivi, R.; Mason, P.; Grosveld, F.; Luzzatto, L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J 1995, 14, 5209-5215. [CrossRef]
- Gupte, R.S.; Ata, H.; Rawat, D.; Abe, M.; Taylor, M.S.; Ochi, R.; Gupte, S.A. Glucose-6-phosphate dehydrogenase is a regulator of vascular smooth muscle contraction. Antioxid Redox Signal 2011, 14, 543-558. [CrossRef]
- Neo, B.H.; Kandhi, S.; Ahmad, M.; Wolin, M.S. Redox regulation of guanylate cyclase and protein kinase G in vascular responses to hypoxia. Respir Physiol Neurobiol 2010, 174, 259-264. [CrossRef]
- Wolin, M.S.; Burke, T.M. Hydrogen peroxide elicits activation of bovine pulmonary arterial soluble guanylate cyclase by a mechanism associated with its metabolism by catalase. Biochem Biophys Res Commun 1987, 143, 20-25. [CrossRef]
- Mingone, C.J.; Gupte, S.A.; Iesaki, T.; Wolin, M.S. Hypoxia enhances a cGMP-independent nitric oxide relaxing mechanism in pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 2003, 285, L296-304. [CrossRef]
- Adachi, T.; Weisbrod, R.M.; Pimentel, D.R.; Ying, J.; Sharov, V.S.; Schoneich, C.; Cohen, R.A. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 2004, 10, 1200-1207. [CrossRef]
- Burgoyne, J.R.; Oka, S.; Ale-Agha, N.; Eaton, P. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxid Redox Signal 2013, 18, 1042-1052. [CrossRef]
- Meurer, S.; Pioch, S.; Pabst, T.; Opitz, N.; Schmidt, P.M.; Beckhaus, T.; Wagner, K.; Matt, S.; Gegenbauer, K.; Geschka, S.; et al. Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Circ Res 2009, 105, 33-41. [CrossRef]
- Neo, B.H.; Patel, D.; Kandhi, S.; Wolin, M.S. Roles for cytosolic NADPH redox in regulating pulmonary artery relaxation by thiol oxidation-elicited subunit dimerization of protein kinase G1alpha. Am J Physiol Heart Circ Physiol 2013, 305, H330-343. [CrossRef]
- Chettimada, S.; Rawat, D.K.; Dey, N.; Kobelja, R.; Simms, Z.; Wolin, M.S.; Lincoln, T.M.; Gupte, S.A. Glc-6-PD and PKG contribute to hypoxia-induced decrease in smooth muscle cell contractile phenotype proteins in pulmonary artery. Am J Physiol Lung Cell Mol Physiol 2012, 303, L64-74. [CrossRef]
- Daiber, A.; Oelze, M.; Steven, S.; Kroller-Schon, S.; Munzel, T. Taking up the cudgels for the traditional reactive oxygen and nitrogen species detection assays and their use in the cardiovascular system. Redox Biol 2017, 12, 35-49. [CrossRef]
- Hyman, A.L.; Lippton, H.L.; Kadowitz, P.J. Methylene blue prevents hypoxic pulmonary vasoconstriction in cats. Am J Physiol 1991, 260, H586-592. [CrossRef]
- Cremona, G.; Wood, A.M.; Hall, L.W.; Bower, E.A.; Higenbottam, T. Effect of inhibitors of nitric oxide release and action on vascular tone in isolated lungs of pig, sheep, dog and man. J Physiol 1994, 481 ( Pt 1), 185-195. [CrossRef]
- Dumas, J.P.; Dumas, M.; Sgro, C.; Advenier, C.; Giudicelli, J.F. Effects of two K+ channel openers, aprikalim and pinacidil, on hypoxic pulmonary vasoconstriction. Eur J Pharmacol 1994, 263, 17-23. [CrossRef]
- Mazmanian, G.M.; Baudet, B.; Brink, C.; Cerrina, J.; Kirkiacharian, S.; Weiss, M. Methylene blue potentiates vascular reactivity in isolated rat lungs. J Appl Physiol (1985) 1989, 66, 1040-1045. [CrossRef]
- Fouty, B.; Komalavilas, P.; Muramatsu, M.; Cohen, A.; McMurtry, I.F.; Lincoln, T.M.; Rodman, D.M. Protein kinase G is not essential to NO-cGMP modulation of basal tone in rat pulmonary circulation. Am J Physiol 1998, 274, H672-678. [CrossRef]
- Rodman, D.M.; Yamaguchi, T.; O'Brien, R.F.; McMurtry, I.F. Methylene blue enhances hypoxic contraction in isolated rat pulmonary arteries. Chest 1988, 93, 93S-94S. [CrossRef]
- Vermeersch, P.; Buys, E.; Pokreisz, P.; Marsboom, G.; Ichinose, F.; Sips, P.; Pellens, M.; Gillijns, H.; Swinnen, M.; Graveline, A.; et al. Soluble guanylate cyclase-alpha1 deficiency selectively inhibits the pulmonary vasodilator response to nitric oxide and increases the pulmonary vascular remodeling response to chronic hypoxia. Circulation 2007, 116, 936-943. [CrossRef]
- Spohr, F.; Busch, C.J.; Teschendorf, P.; Weimann, J. Selective inhibition of guanylate cyclase prevents impairment of hypoxic pulmonary vasoconstriction in endotoxemic mice. J Physiol Pharmacol 2009, 60, 107-112.
- Rodman, D.M.; Yamaguchi, T.; Hasunuma, K.; O'Brien, R.F.; McMurtry, I.F. Effects of hypoxia on endothelium-dependent relaxation of rat pulmonary artery. Am J Physiol 1990, 258, L207-214. [CrossRef]
- Ye, L.; Liu, J.; Liu, H.; Ying, L.; Dou, D.; Chen, Z.; Xu, X.; Raj, J.U.; Gao, Y. Sulfhydryl-dependent dimerization of soluble guanylyl cyclase modulates the relaxation of porcine pulmonary arteries to nitric oxide. Pflugers Arch 2013, 465, 333-341. [CrossRef]
- Harteneck, C.; Koesling, D.; Soling, A.; Schultz, G.; Bohme, E. Expression of soluble guanylyl cyclase. Catalytic activity requires two enzyme subunits. FEBS Lett 1990, 272, 221-223. [CrossRef]
- Le Cras, T.D.; McMurtry, I.F. Nitric oxide production in the hypoxic lung. Am J Physiol Lung Cell Mol Physiol 2001, 280, L575-582. [CrossRef]
- Manoury, B.; Idres, S.; Leblais, V.; Fischmeister, R. Ion channels as effectors of cyclic nucleotide pathways: Functional relevance for arterial tone regulation. Pharmacol Ther 2020, 209, 107499. [CrossRef]
- Rudyk, O.; Prysyazhna, O.; Burgoyne, J.R.; Eaton, P. Nitroglycerin fails to lower blood pressure in redox-dead Cys42Ser PKG1alpha knock-in mouse. Circulation 2012, 126, 287-295. [CrossRef]
- Prysyazhna, O.; Rudyk, O.; Eaton, P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med 2012, 18, 286-290. [CrossRef]
- Rudyk, O.; Rowan, A.; Prysyazhna, O.; Krasemann, S.; Hartmann, K.; Zhang, M.; Shah, A.M.; Ruppert, C.; Weiss, A.; Schermuly, R.T.; et al. Oxidation of PKGIalpha mediates an endogenous adaptation to pulmonary hypertension. Proc Natl Acad Sci U S A 2019, 116, 13016-13025. [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Gruning, N.M.; Kruger, A.; Tauqeer Alam, M.; et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc 2015, 90, 927-963. [CrossRef]
- Mullarky, E.; Cantley, L.C. Diverting Glycolysis to Combat Oxidative Stress. In Innovative Medicine: Basic Research and Development, Nakao, K., Minato, N., Uemoto, S., Eds.; Tokyo, 2015; pp. 3-23.
- Gupte, R.; Dhagia, V.; Rocic, P.; Ochi, R.; Gupte, S.A. Glucose-6-phosphate dehydrogenase increases Ca(2+) currents by interacting with Ca(v)1.2 and reducing intrinsic inactivation of the L-type calcium channel. Am J Physiol Heart Circ Physiol 2020, 319, H144-H158. [CrossRef]
- Chang, H.H.; Bennett, A.M.; Cameron, W.D.; Floro, E.; Au, A.; McFaul, C.M.; Yip, C.M.; Rocheleau, J.V. Targeting Apollo-NADP(+) to Image NADPH Generation in Pancreatic Beta-Cell Organelles. ACS Sens 2022, 7, 3308-3317. [CrossRef]
- Han, H.H.; Ge, P.X.; Li, W.J.; Hu, X.L.; He, X.P. Recent Advancement in Fluorescent Probes for Peroxynitrite (ONOO(-)). Sensors (Basel) 2025, 25. [CrossRef]
- Hung, Y.P.; Albeck, J.G.; Tantama, M.; Yellen, G. Imaging cytosolic NADH-NAD(+) redox state with a genetically encoded fluorescent biosensor. Cell Metab 2011, 14, 545-554. [CrossRef]
- Zanetti, C.; Gaspar, R.D.L.; Zhdanov, A.V.; Maguire, N.M.; Joyce, S.A.; Collins, S.G.; Maguire, A.R.; Papkovsky, D.B. Heterosubstituted Derivatives of PtPFPP for O(2) Sensing and Cell Analysis: Structure-Activity Relationships. Bioconjug Chem 2022, 33, 2161-2169. [CrossRef]
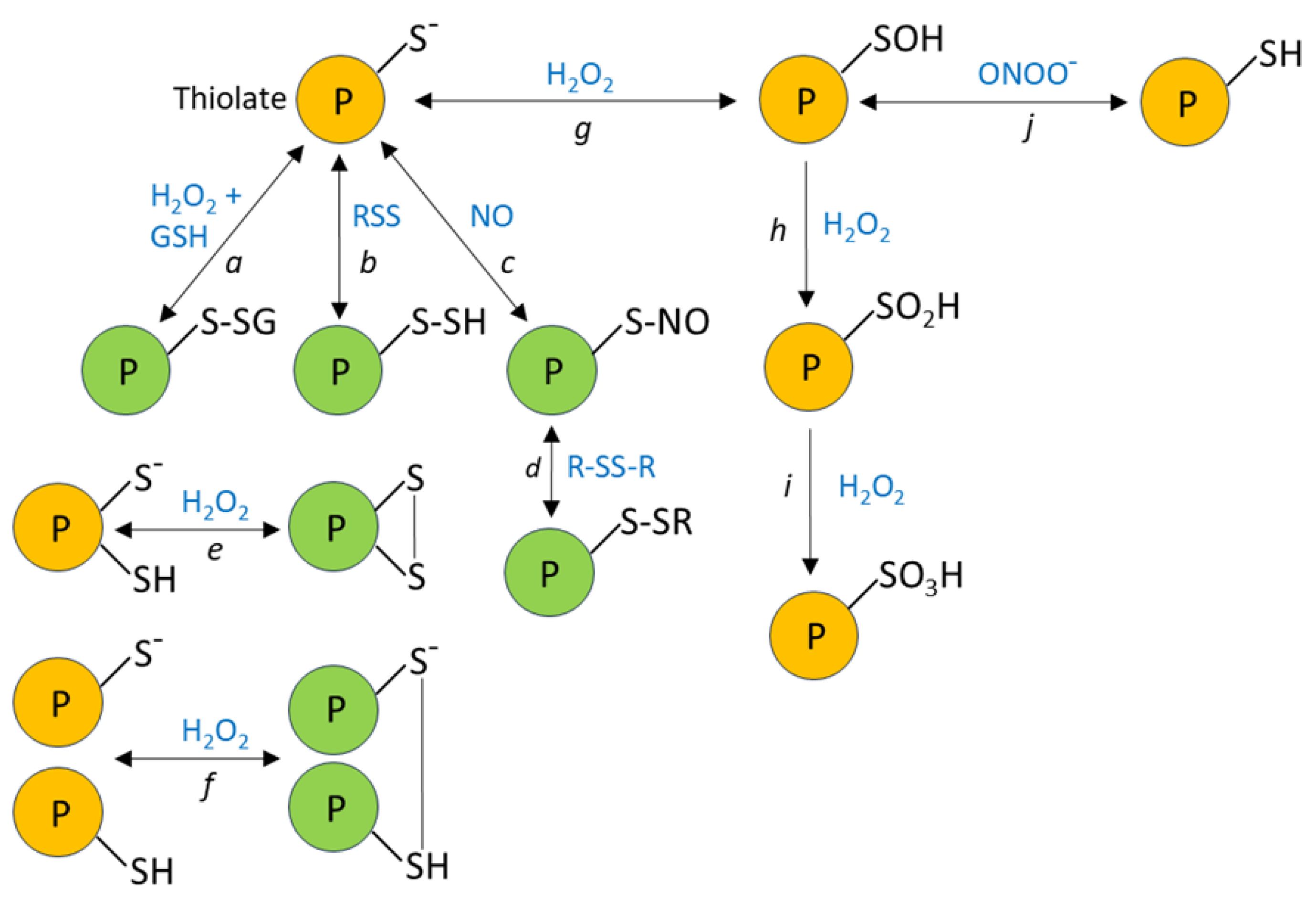









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




