Submitted:
10 December 2025
Posted:
26 December 2025
You are already at the latest version
Abstract
In order to clarify the control ability of Labidura riparia and Sycanus croceovittatus on Ostrinia furnacalis larvae, the predation ability and preference of L. riparia and S. croceovittatus on the 3rd instar larvae of O. furnacalis were studied in this study. The results showed that the predation ability of the two species to the 3rd instar larvae of O.furnacalis conformed to the Holling II functional response. The combination of L. riparia and S. croceovittatus had the best predation effect on the 3rd instar larvae of O.furnacalis, which was significantly higher than that of L. riparia or S. croceovittatus alone. The L. riparia showed a positive preference for the 1-3 instar larvae and a negative preference for the 4-5 instar larvae. The S. croceovittatus showed a positive preference for the 3-5 instar larvae and a negative preference for the 1-2 instar larvae. In summary, compared with the use of a natural enemy insect alone, the combined use of L. riparia and S. croceovittatus has a more significant effect on the prevention and control of O.furnacalis larvae, and can be used for biological control of O.furnacalis.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Test Insects and Instruments
2.2. Observation on the Predation Behavior of L. riparia and S. croceovittatus on the 3rd Instar Larvae of O.furnacalis
2.3. Predatory Functional Response of L. riparia and Yellow-Belted Rhinoceros to the 3rd Instar Larvae of O.furnacalis
2.4. The Predation Ability of the Combination of L. riparia and S. croceovittatus on the 3rd Instar Larvae of O.furnacalis
2.5. Predatory Preference of L. riparia and S. croceovittatus on Stream Bank
2.6. Data Processing
3. Results and Analysis
3.1. Predatory Behavior of L. riparia and S. croceovittatus on the 3rd Instar Larvae of O.furnacalis
3.2. Predatory Functional Response of L. riparia and S. croceovittatus to the 3rd Instar Larvae of O.furnacalis
3.3. The Amount of O. furnacalis Predation by L. riparia and S. croceovittatus in Combination
3.4. Feeding Preference of L. riparia and S. croceovittatus to Different Instars of O. furnacalis
4. Conclusions and Discussion
References
- Ruan, H.Y.; Meng, J.Y.; Yang, C.L.; Zhou, L.; Zhang, C.Y. Identification of Six Small Heat Shock Protein Genes in Ostrinia furnacalis (Lepidoptera: Pyralidae) and Analysis of Their Expression Patterns in Response to Environmental Stressors.Journal Of Insect Science.2022,22(06).
- Bi, H.L.; Merchant, A.; Gu, J.W.; Li, X.W.;Zhou, X.G.; Zhang, Q. CRISPR/Cas9-Mediated Mutagenesis of Abdominal-A and Ultrabithorax in the Asian O. furnacalis, Ostrinia furnacalis . Insects. 2022, 13(4).
- Li, Y.Y.; Li, M.M.; Yang, Q.; Chen, L.H.; Li, B.L.; Fang, A.S.; He, K.H.; Dong, J.G.; Zhao, Y.W.; Yu, Z.H.; Hao, Y.C.; Wu, J.X. Effects of Ostrinia furnacalis (Guenée) on maize yield and treatment threshold. Plant Protection. 2022,48(01):82-89. (In Chinese).
- Wang, Y.; Wang, K.Q.; Wang, X.X.; Liu, X.L.; Wang, C. Trapping effects of different sex pheromone lures on Ostrinia furnacalis males in Harbin. Plant Protection. 2023,49(06):338-342+349. (In Chinese).
- Wei, X.; Chen, R.Z. Effects of host plants on the development and protective enzyme activity of Ostrinia furnacalis. Chinese Journal of Applied Entomology. 2020,57(02):355-362. (In Chinese).
- Crespo, A.L.B.; Spencer, T.A.; Alves, A.P.; Hellmich, R.L; Blankenship, E.E.; Magalhaes, L.C .; Siegfried, B.D. On-plant survival and inheritance of resistance to Cry1Ab toxin from Bacillus thuringiensis in a field-derived strain of European O. furnacalis, Ostrinia nubilalis. Pest Management Science. 2009, 65(10): 1071-1081.
- Crespo, A.L.B.; Rodrigo-Simón, A .; Siqueira, H.A.A.; Pereira, E.J.G.; Ferré, J.; Siegfried, B.D. Cross-resistance and mechanism of resistance to Cry1Ab toxin from Bacillus thuringiensis in a field-derived strain of European O. furnacalis, Ostrinia nubilalis. Journal of Invertebrate Pathology, 2011, 107(3): 185-192.
- Alam, A.; Abbas, S.; Ahmad, B.; Shakeel, M.; Liang, J.Y.; Khan, K.A.; Ghramh, H.A.; Dewer, Y.; Ali, J.; Tonga, A.; Li, Q.Y.; Zhao, C.R. Leveraging volatile organic compound-induced toxic and behavioural effects for potential sustainable management of Ostrinia furnacalis. Bulletin Of Entomological Research. 2025.
- Tian, C.H.; Zhang, J.Y.; Xu, C.Y.; Li, G.P.; Huang, J.R.; Liu, Y.; Wang, G.S.; Feng, H.Y.; Yin, X.M.; Feng, H.Q. Predation capacity of Labidura riparia Pallas against Helicoverpa armigera (Hübner). Plant Protection. 2023,49(01):157-163. (In Chinese).
- Tian, C.H.; Zhang, J.Y.; Li, G.P.; Huang, J.R.; Yin, X.M.; Feng, H.Q. Biological characteristics of riparian earwig Labidura riparia and its predatory capacity against fall armyworm Spodoptera frugiperda. Journal of Plant Protection. 2022,49(05):1499-1504. (In Chinese).
- Tian, C.H.; Cao, H.Y.; Zhang, J.Y; Liu, X.G.; Cai, T.; Li, G.P.; Huang, J.R.; Feng, H.Q. Behavioral and Functional Responses of Labidura riparia Pallas Preying on Spodoptera frugiperda. Chinese Journal of Biological Control.2021,37(06):1160-1165. (In Chinese).
- Tian, C.H.; Li, J.P.; Zhang, Y.; Zhang, J.Y.; Gao, X.J.; Yin, X.M.; Yang, L.R.; Feng, H.Q. Involvement Of Gonolabis Distincta In The Control Of Root Maggots In Garlic Fields. Life-Basel, 2025, 15(8).
- Yao, B.K.; Du, J.Y.; Gao, L.P.; Cao, F.; Li, Y.; Wang, S.H.; Wang, X.P. Study of the predation of Plutella xylostella by Sycanus bifidus. Journal of Biosafety. 2025,34(01):81-85+102. (In Chinese).
- Huang, Z.H.; Wu, J.F.; Zhang, Z.Q. A Preliminary Study on the Bionomics and Application of Sycanus croceovittatus (Hemiptera, Reduviidae). Forest Research. 1991, (01): 57-64. (In Chinese).
- Wang, Y.N.; Wu, Z.B.; Zhao, S.Y.; He, Y.Z.; Huang, J.R.; Tian, C.H.; Li, G.P.; Feng, H.Q. Morphological characteristics of Sycanus croceovittatus Dohrn. Plant Protection. 2021, 47 (05):230-235. (In Chinese).
- Du, H.; Zhi, J.Z.; Zhou, J.S.; Liu, X.M.; Zhang, J.C.; Yue, J.W. Predatory functional response of Sycanus croceovittatus (Hemiptera: Reduviidae) adults on Spodoptera litura (Lepidoptera: Noctuidae) larvae. Journal of Biosafety. 2021,30(04):287-291. (In Chinese).
- Li, G.Y.; Li, L.; Wang, Y.Q.; Yang, Y.L. Study on feeding technology of Sycanus croceovittatus Dohrn. Yunnan Agricultural Science and Technology. 2022,(02):6-8. (In Chinese).
- Institute of Plant Protection, Henan Academy of Agricultural Sciences. An efficient bait for earwig trapping and its preparation method. 202011121772.2. 2021-01-01. (In Chinese).
- Holling, C.S.Some Characteristics of Simple Types of Predation and Parasitism.Canadian Entomologist. 1959, 91(7):385-398.
- Zhou, J.Z.;Chen, C.M. Quantitative Measurement Of Selectivity Of Predator For Prey. Acta Ecologica Sinica. 1987, (01):50-56. (In Chinese).
- Ren, X.M.; Xu, Z.W.; Zhao, B.; Lu, J.F.; Sun, Y.E.; Zhan, M.K. Predatory Behaviors and Abilities of Sycanus croceouittatus Adults to Spodoptera frugiperda Larvae. Biological Disaster Science. 2022,45(01):48-52. (In Chinese).
- Ge, F.; Men, X.Y.; Li, Z.; Ju, Q.; Zhang, X.R.; Liang, X.Y. Main achievements and prospects in the ecological regulation and management of pests in China. Chinese Journal of Applied Entomology. 2025,62(03):549-558. (In Chinese).
- Yang, J.K. Three-dimensional control measures of main pests in facility cultivation of melon and fruit vegetables. Shanghai Vegetables. 2007,(06):82. (In Chinese).
- WANG, J.X. Study on host search mechanism of twonatural enemies of Aromia gungii usingkairomone and their joint bio-controleffect. Hebei University.2022. (In Chinese).
- Wu, L.L.; Wang, L.D.; Li, Q.C.; Zhou, C.; Dong, Y.; Yan, F.; Xu, Y.Y. Common Control Effect of Chrysoperla sinica and Harmonia axyridis on Greenhouse Aphids. Heilongjiang Agricultural Sciences. 2018,(10):79-81. (In Chinese).
- Gao, X.Y.; Shi, J.; Qiao, Z.H.; Lv, D.L.; Wu, H.C. Study on the synergistic predation of Harmonia axyridis and Propylaea japonica adults on Aphis gossypii. Agriculture and Technology. 2016,36(10):19-20. (In Chinese).
- Sourassou, N.F.; Hanna, R.; Negloh, K.; Breeuwer, J.A.J.; Sabelis, M.W. Females as intraguild predators of males in cross-pairing experiments with phytoseiid mites. Experimental And Applied Acarology. 2013,61(2):173-182.



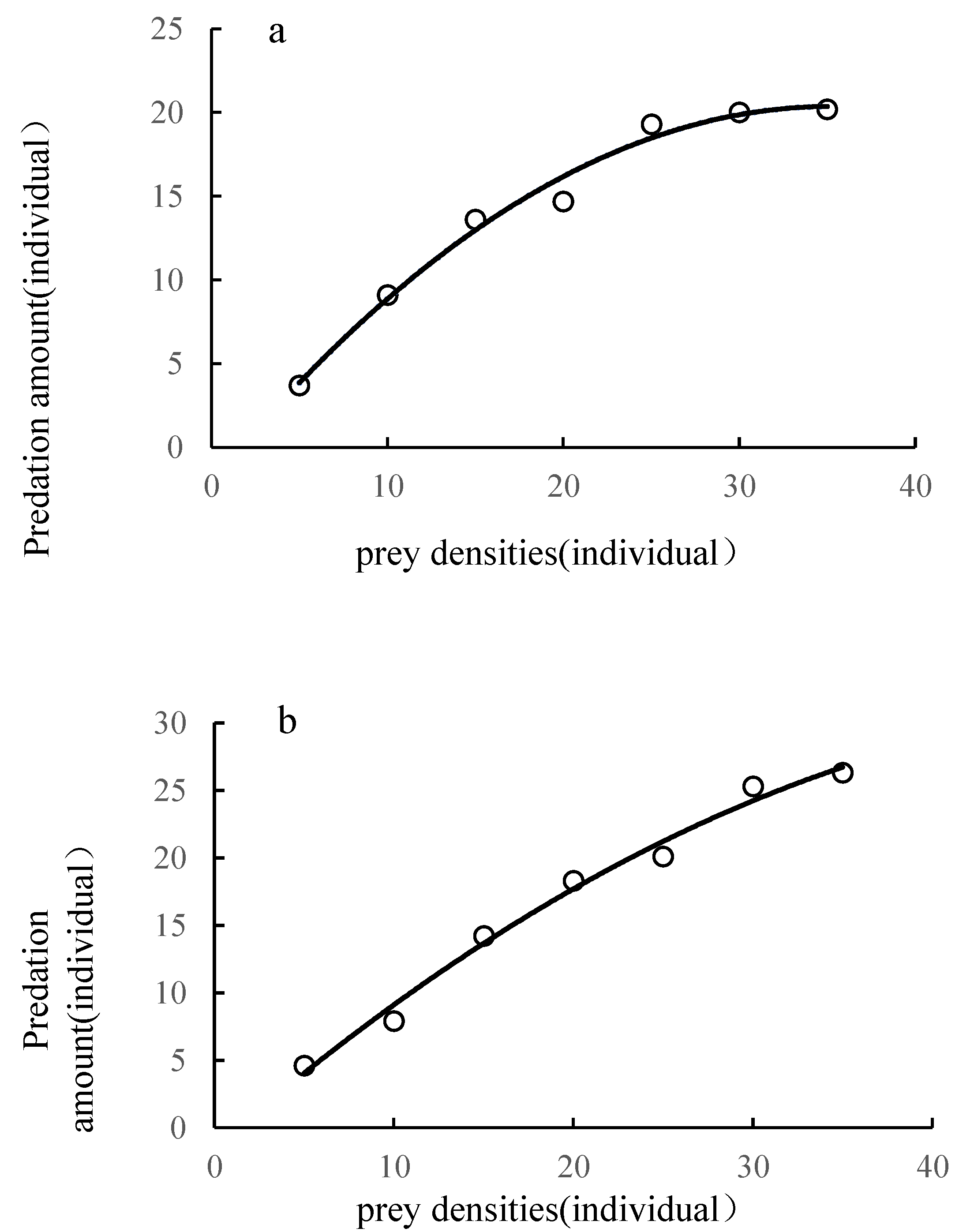
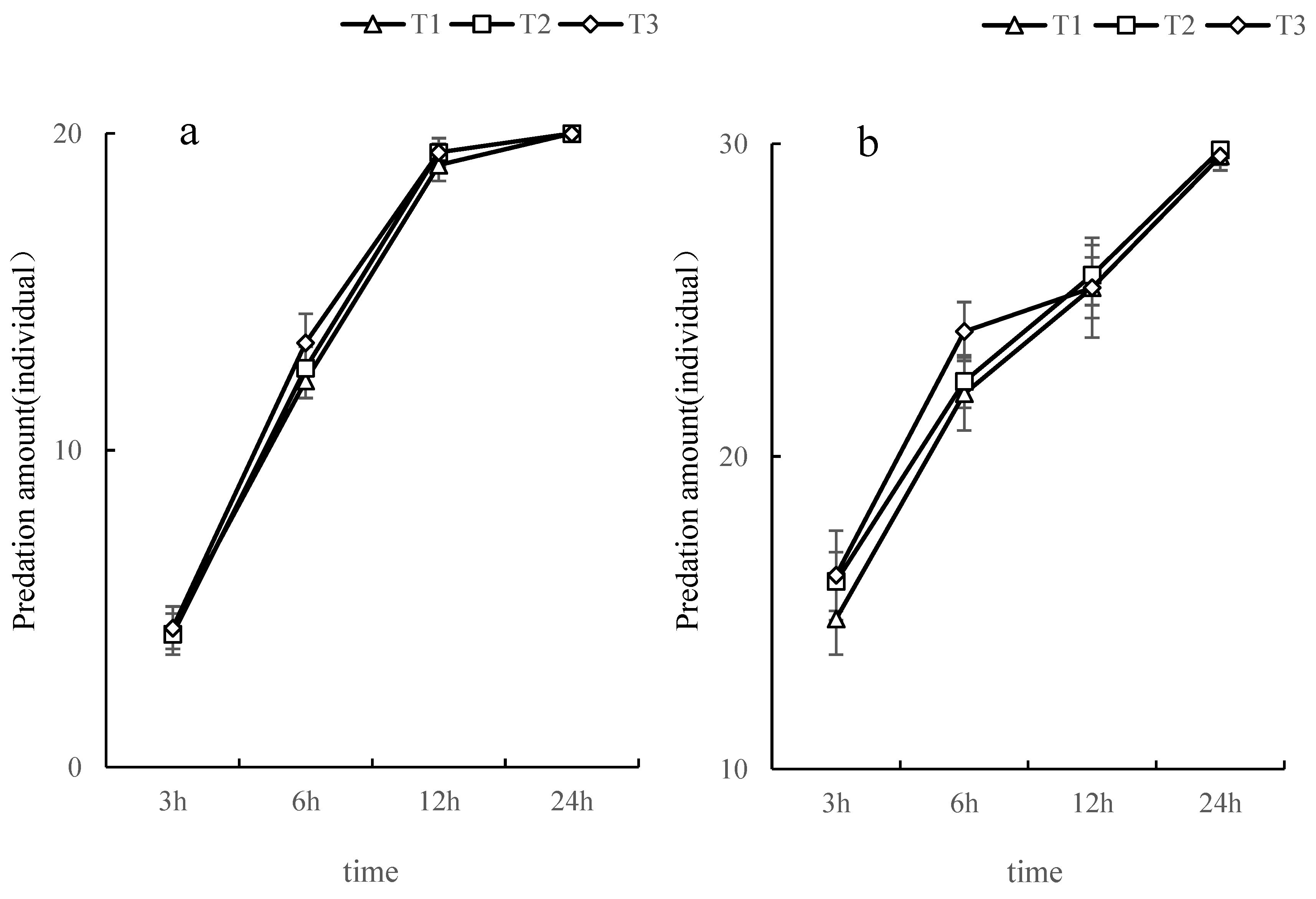
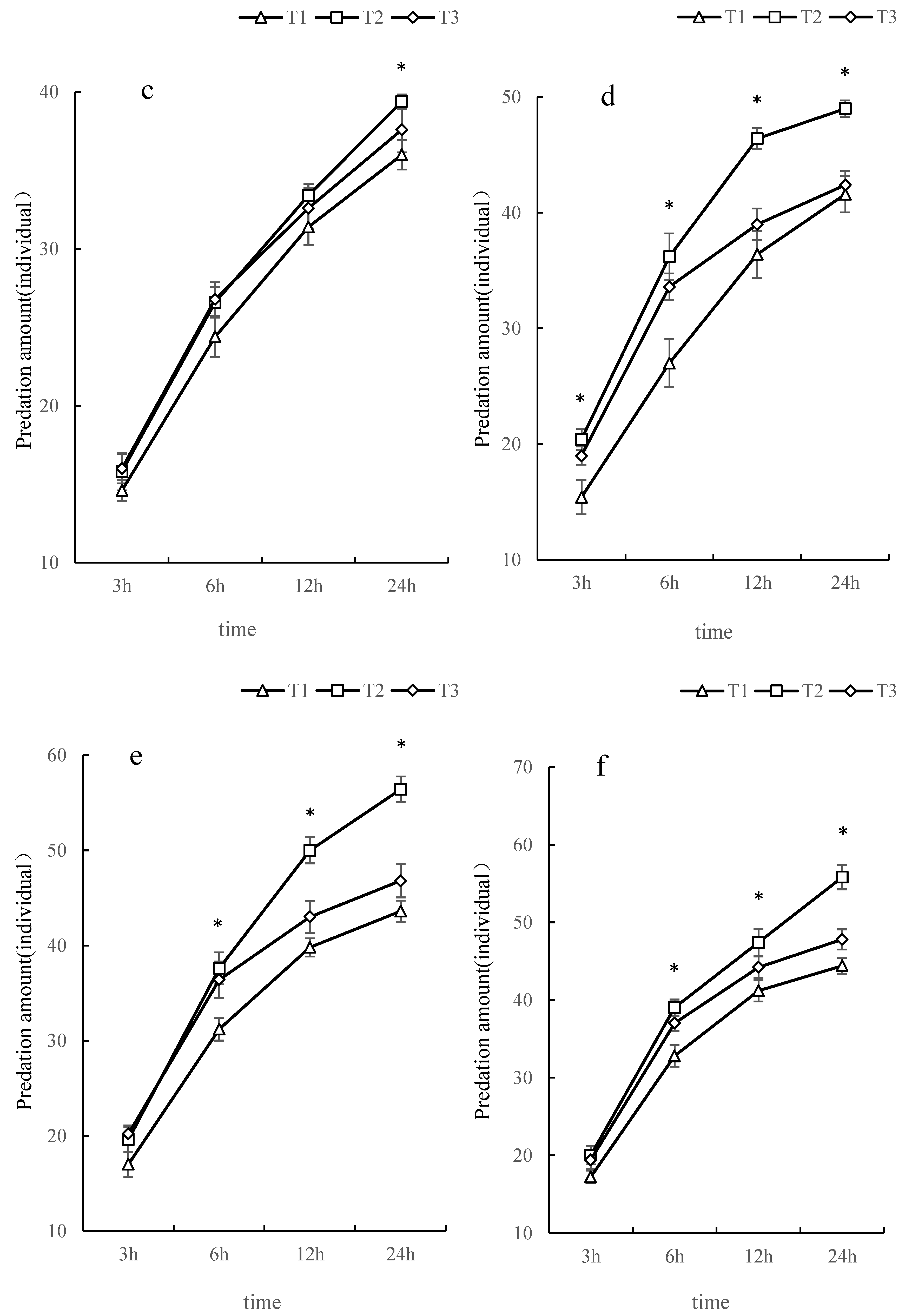
| Types of natural enemies | Holling Ⅱdisc equation | R2 | instantaneous attack rate | duration(d) | Predatory efficiency | daily maximum prey capacity (individual·d-1) |
| L. riparia | Na=1.181N/(1+0.027N) | 0.967 | 1.181 | 0.023 | 51.3 | 43.5 |
| S. croceovittatus | Na=1.025N/(1+0.009N) | 0.984 | 1.025 | 0.009 | 113.9 | 108.7 |
| O. furnacalis | L. riparia | S. croceovittatus | ||
| Predatoryquantity (individual) |
preference index (Ci) |
Predatoryquantity (individual) |
preference index (Ci) |
|
| 1st instar larva | 6.4±0.8 a | 0.23 | 1.4±0.4 c | -0.64 |
| 2nd instar larva | 6.0±0.6 a | 0.21 | 3.0±0.8 c | -0.37 |
| 3rd instar larva | 5.6±0.8 a | 0.16 | 7.2±0.7 b | 0.08 |
| 4th instar larva | 1.4±0.5 b | -0.53 | 9.4±0.5 a | 0.22 |
| 5th instar larva | 0.5±0.2 b | -0.75 | 9.2±0.4 a | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




