Submitted:
22 December 2025
Posted:
24 December 2025
You are already at the latest version
Abstract
2-Aryl-2-(3-indolyl)acetohydroxamic acids have emerged as promising antitumor agents; however, their poor pharmacokinetic profile remains a significant drawback. To address this limitation, we have synthesized homologs of such an acids—specifically 2-aryl-2-(3-indolyl)propionic acids—along with several other derivatives. The cytotoxicity of these compounds against glioblastoma cell lines was evaluated and compared to that of the parent acetohydroxamic acid derivatives.
Keywords:
1. Introduction
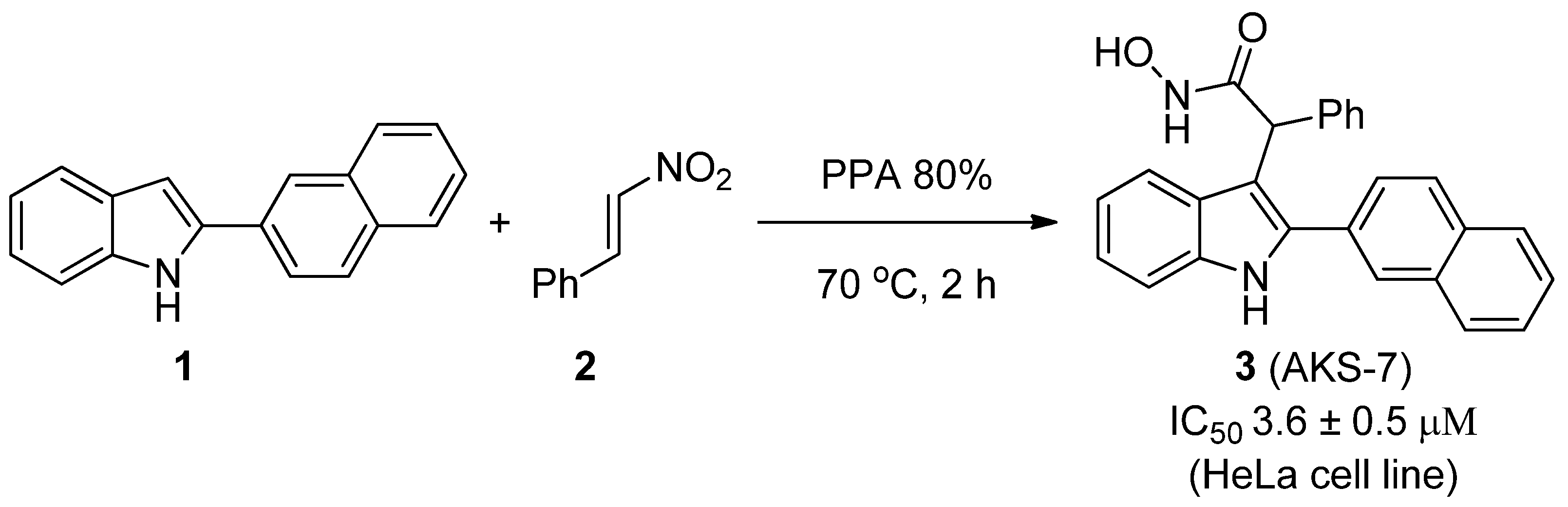
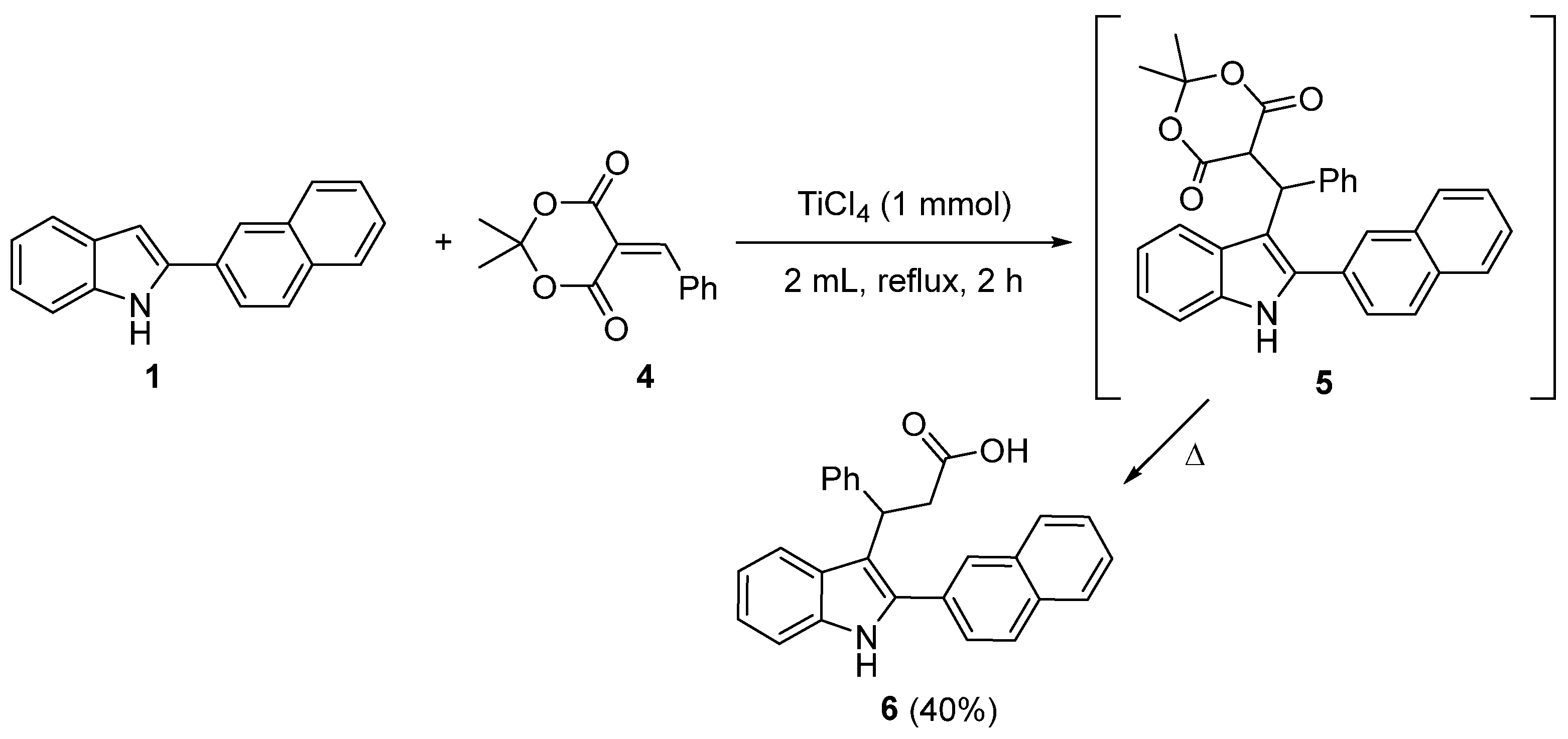
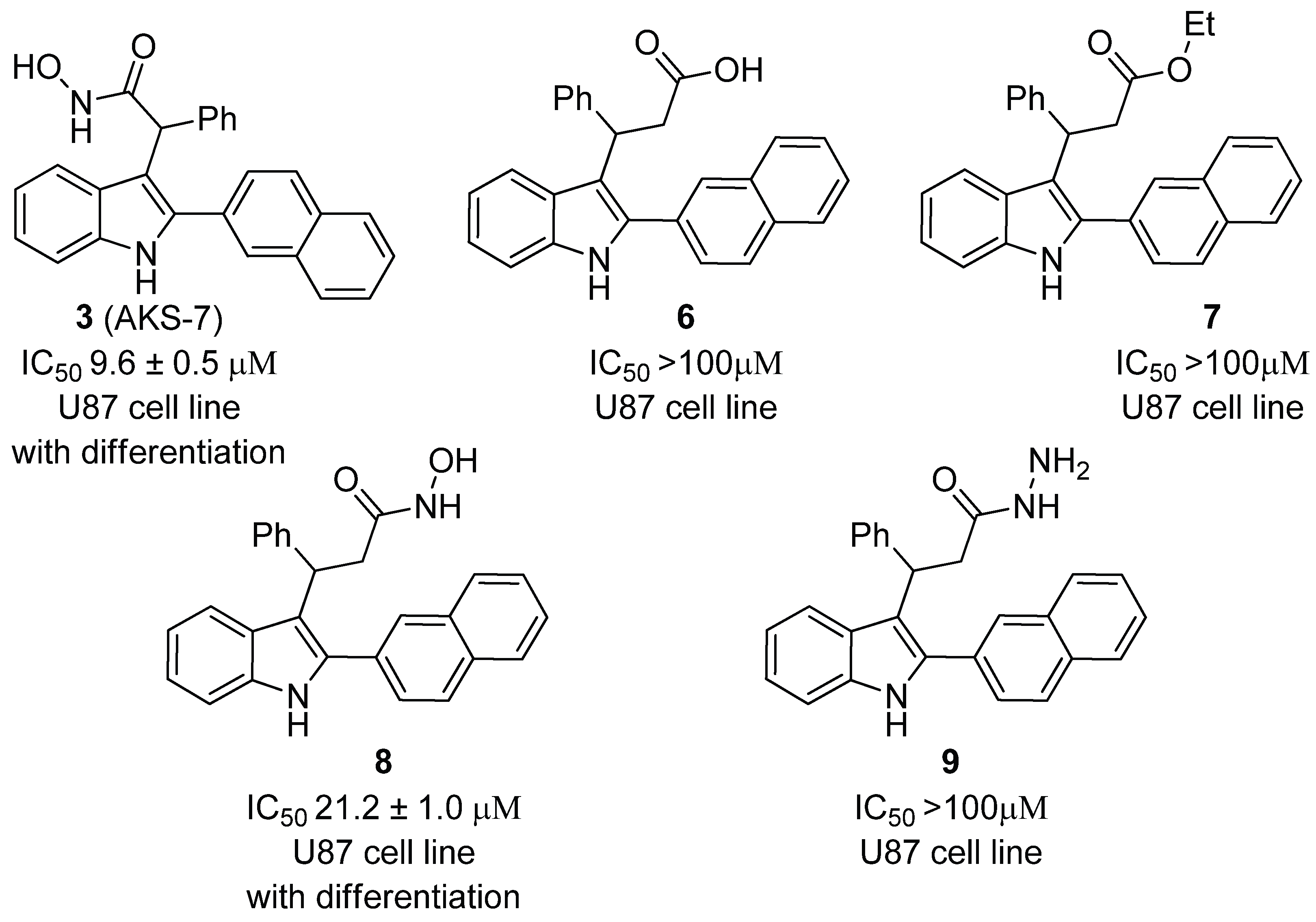
2. Results and Discussion
3. Materials and Methods
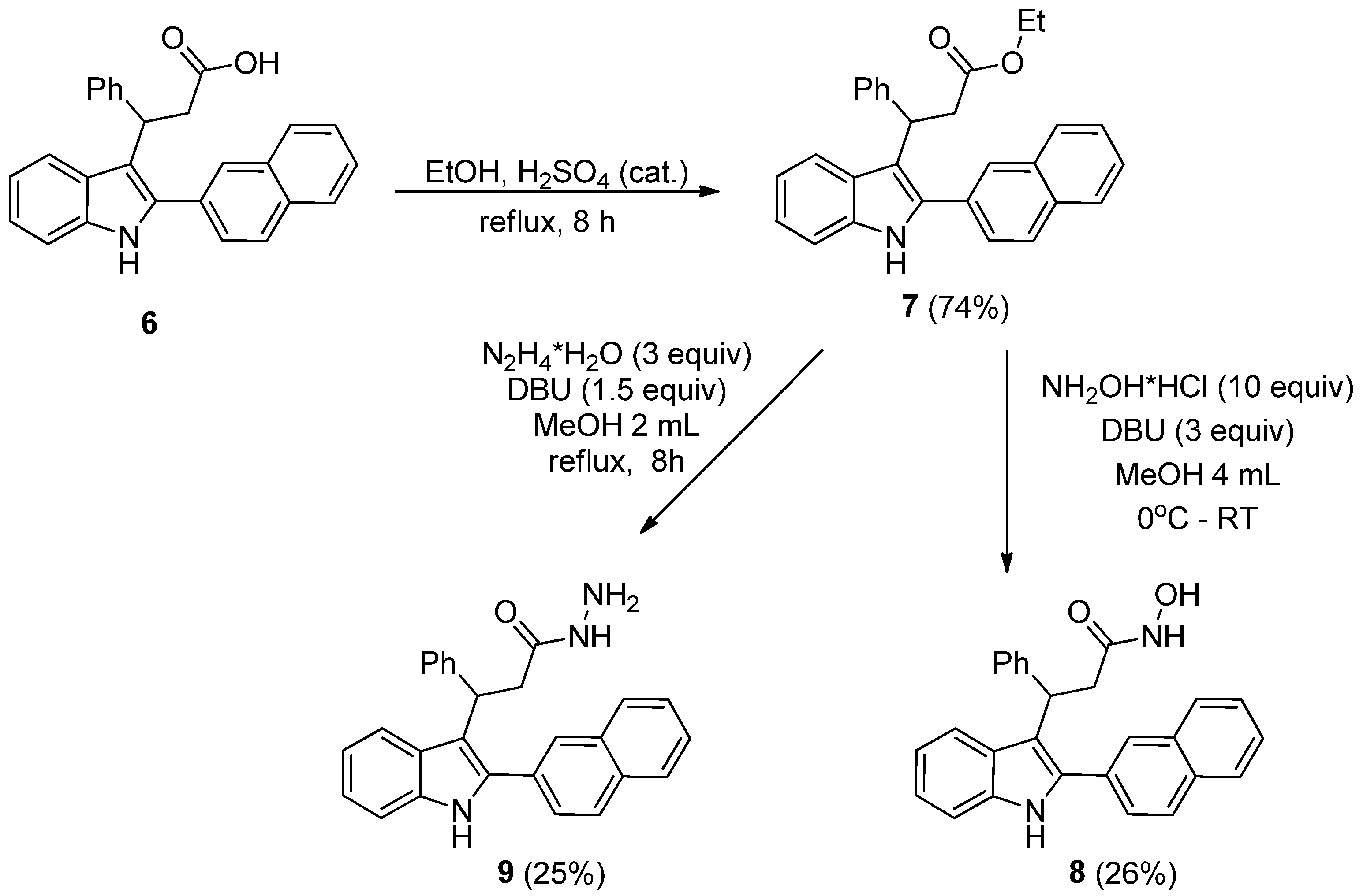
- 3-(2-(Naphthalen-2-yl)-1H-indol-3-yl)-3-phenylpropanoic acid (6). To a solution of titanium(IV) chloride (1.90 g, 1.0 eq.) in N,N-dimethylformamide (20.0 mL) was added 5-benzylidene-2,2-dimethyl-1,3-dioxane-4,6-dione (2.32 g, 1.0 eq.). Subsequently, 2-naphthylindole (2.43 g, 1.0 mmol) was added, and the reaction mixture was heated at reflux to 150 °C. The progress of the reaction was monitored by thin-layer chromatography (TLC). Upon completion, the mixture was quenched with water, and the resulting aqueous solution was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were concentrated under reduced pressure using a rotary evaporator. The crude residue was purified by silica gel column chromatography (eluent: petroleum ether/acetone, 5:1) to afford the target compound. The title compound was obtained as ligh-brown powder, mp 131−134 °C (benzene/petroleum ether). The yield was 1.57 g (4.0 mmol, 40%). Rf = 0.28 (petroleum ether/acetone 2:1). 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 11.41 (s, 1H), 8.14 (s, 1H), 8.06 (d, J = 8.3 Hz, 1H), 8.00 (d, J = 5.8 Hz, 1H), 7.93 (d, J = 6.2 Hz, 1H), 7.81 (d, J = 8.2 Hz, 1H), 7.62 – 7.52 (m, 3H), 7.40 (d, J = 7.9 Hz, 1H), 7.31 – 7.20 (m, 4H), 7.17 – 7.06 (m, 2H), 6.96 (t, J = 7.3 Hz, 1H), 5.00 (t, J = 7.2 Hz, 1H), 3.35 – 3.29 (m, 1H), 3.21 (dd, J = 15.5, 7.4 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 173.7, 144.9, 137.0, 135.5, 133.4, 132.7, 131.0, 128.8 (2C), 128.6, 128.4, 128.2, 128.0, 127.6 (2C), 127.4, 127.2, 127.1, 126.9, 126.3, 121.8, 120.6, 119.4, 114.2, 112.0, 41.8, 38.2. HRMS (ESI-TOF, m/z): Calculated for C27H21NNaO2 (M + Na)+ 414.1464; found 414.1469.
- Ethyl 3-(2-(naphthalen-2-yl)-1H-indol-3-yl)-3-phenylpropanoate (7). 3-Phenyl-3-(2-(naphthalen-2-yl)-1H-indol-3-yl)propionic acid (1.173 g, 1 mmol) was dissolved in ethanol (1.5 mL), followed by the addition of a catalytic amount of concentrated sulfuric acid (0.09 mL). The reaction mixture was heated at reflux for 8 h until the completion of the reaction, as monitored by thin-layer chromatography (TLC). Subsequently, water was added to the mixture, and the solution was neutralized with aqueous ammonia. The resulting mixture was extracted with ethyl acetate (3 × 30 mL). The organic layers were concentrated under reduced pressure using a rotary evaporator, and the crude residue was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate, 8:1) to afford the title compound. The title compound was obtained as ligh-yellow oil. The yield was 0.93 g (2.22 mmol, 74%). Rf = 0.38 (petroleum ether/ethyl acetate 4:1). 1H NMR (400 MHz, CDCl3) δ 8.20 (s, 1H), 8.00 – 7.95 (m, 1H), 7.93 – 7.86 (m, 2H), 7.85 – 7.80 (m, 1H), 7.70 – 7.62 (m, 2H), 7.57 – 7.50 (m, 2H), 7.39 (t, J = 7.4 Hz, 3H), 7.32 – 7.24 (m, 3H), 7.24 – 7.16 (m, 2H), 7.13 – 7.06 (m, 1H), 5.15 (t, J = 7.8 Hz, 1H), 3.93 (qq, J = 10.8, 7.1 Hz, 2H), 3.42 – 3.18 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 172.4, 144.2, 136.4, 135.7, 133.4, 132.9, 130.5, 128.60, 128.56 (2C), 128.3, 127.9, 127.9, 127.6 (2C), 126.7, 126.5, 126.5, 126.3, 122.3, 120.9, 119.9, 114.6, 111.2, 60.4, 40.5, 38.4, 29.8, 14.0. HRMS (ESI-TOF, m/z): Calculated for C29H25NNaO2 (M + Na)+ 442.1777; found 442.1778.
- N-Hydroxy-3-(2-(naphthalen-2-yl)-1H-indol-3-yl)-3-phenylpropanamide (8). To a stirred solution of ethyl 3-phenyl-3-(2-(naphthalen-2-yl)-1H-indol-3-yl)propionate (419 mg, 1.0 mmol) in methanol (4 mL) were added 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (456 mg, 3 mmol) and hydroxylamine hydrochloride (NH2OH*HCl) (700 mg, 10 mmol) at 0 °C. The reaction mixture was then allowed to stir at room temperature, with the progress monitored by thin-layer chromatography (TLC). Upon completion, the mixture was diluted with water and neutralized by the addition of acetic acid. The resulting solution was extracted with ethyl acetate (3 × 30 mL). The combined organic phases were concentrated under reduced pressure, and the crude residue was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate, 5:1) to yield the target hydroxamic acid. The title compound was obtained as dark-gray solid, mp 128−130 °C (benzene/petroleum ether). The yield was 0.053 g (0.26 mmol, 26%). Rf = 0.4 (petroleum ether/ethyl acetate 5:1). 1H NMR (400 MHz, DMSO-d6) δ 11.38 (s, 1H), 10.48 (s, 1H), 8.74 (s, 1H), 8.20 (s, 1H), 8.06 (d, J = 8.5 Hz, 1H), 7.99 (d, J = 7.8 Hz, 1H), 7.94 (d, J = 6.6 Hz, 1H), 7.87 (d, J = 8.4 Hz, 1H), 7.61 – 7.55 (m, 3H), 7.39 (d, J = 7.5 Hz, 1H), 7.24 – 7.18 (m, 4H), 7.12 – 7.07 (m, 2H), 7.00 – 6.94 (m, 1H), 5.09 (t, J = 7.5 Hz, 1H), 3.17 (dd, J = 14.6, 8.2 Hz, 1H), 2.94 (dd, J = 14.5, 7.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 167.7, 144.7, 136.6, 135.0, 133.0, 132.3, 130.5, 128.2 (2C), 128.1, 128.0, 127.7, 127.6, 127.3 (2C), 127.1, 126.8, 126.7, 126.4, 125.8, 121.3, 120.3, 118.8, 114.2, 111.6, 37.5, 37.4. HRMS (ESI-TOF, m/z): Calculated for C27H22N2NaO2 (M + Na)+ 429.1573; found 429.1576.
- 3-(2-(Naphthalen-2-yl)-1H-indol-3-yl)-3-phenylpropanehydrazide (9). To a stirred solution of ethyl 3-phenyl-3-(2-naphthyl-1H-indol-3-yl)propionate (419 mg, 1.0 mmol) in MeOH (2 mL), DBU (228 mg, 1.5 mmol) and hydrazine hydrate (N2H4*H2O) (150 mg) were added. The resulting mixture was heated at reflux for 8 h until the reaction reached completion, as monitored by TLC. Upon completion, the mixture was diluted with water and acidified with acetic acid to achieve a neutral pH. The aqueous phase was then extracted with ethyl acetate (3 × 10 mL). The organic solvent was removed under reduced pressure using a rotary evaporator, and the crude residue was purified by column chromatography (petroleum ether: ethyl acetate, 4:1). The title compound was obtained as light-brown solid, mp 140−142 °C (benzene/petroleum ether). The yield was 0.051 g (0.25 mmol, 25%). Rf = 0.2 (petroleum ether/ethyl acetate 2:1). 1H NMR (400 MHz, DMSO-d6) δ 11.37 (s, 1H), 9.09 (s, 1H), 8.19 (s, 1H), 8.06 (d, J = 8.5 Hz, 1H), 8.01 – 7.97 (m, 1H), 7.96 – 7.92 (m, 1H), 7.87 (d, J = 8.4 Hz, 1H), 7.60 – 7.55 (m, 3H), 7.39 (d, J = 8.0 Hz, 1H), 7.26 – 7.19 (m, 4H), 7.12 – 7.07 (m, 2H), 6.96 (t, J = 7.4 Hz, 1H), 5.10 (t, J = 7.5 Hz, 1H), 4.33 (br.s, 2H), 3.27 (dd, J = 14.8, 8.5 Hz, 1H), 2.96 (dd, J = 14.7, 6.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 170.2, 144.8, 136.6, 134.8, 132.9, 132.2, 130.5, 128.2 (2C), 128.1, 128.0, 127.7, 127.6, 127.3 (2C), 127.1, 126.8, 126.6, 126.4, 125.7, 121.3, 120.3, 118.8, 114.4, 111.5, 79.2, 37.5. HRMS (ESI-TOF, m/z): Calculated for C27H23N3NaO (M + Na)+ 428.1733; found 428.1729.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, P.; Chen, Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Antitumor Agents Based on Metal–Organic Frameworks. Angew. Chemie. 2021, 133, 16901–16914. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Sharma, V.; Verma, A.; Venugopal, S.; Mittal, A.; Singh, G.; Kaur, B. Indole Derived Anticancer Agents. ChemistrySelect. 2022, 7. [Google Scholar] [CrossRef]
- Russo, E.; Grondona, C.; Brullo, C.; Spallarossa, A.; Villa, C.; Tasso, B. Indole Antitumor Agents in Nanotechnology Formulations: An Overview. Pharmaceutics. 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Salerno, S.; Barresi, E.; Baglini, E.; Poggetti, V.; Da Settimo, F.; Taliani, S. Target-Based Anticancer Indole Derivatives for the Development of Anti-Glioblastoma Agents. Molecules. 2023, 28, 2587. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xu, Y.; Jin, X.; Zhang, Q.; Ouyang, F.; Han, L.; Zhan, M.; Li, X.; Liang, B.; Huang, X. Structure Modification and Biological Evaluation of Indole-Chalcone Derivatives as Anti-Tumor Agents through Dual Targeting Tubulin and TrxR. Eur. J. Med. Chem. 2022, 227, 113897. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Kaur, K.; Biharee, A.; Jaitak, V. Recent Development in Indole Derivatives as Anticancer Agent: A Mechanistic Approach. Anticancer. Agents Med. Chem. 2021, 21, 1802–1824. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, A. V.; Smirnov, A.N.; Magedov, I. V.; Reisenauer, M.R.; Aksenov, N.A.; Aksenova, I. V.; Pendleton, A.L.; Nguyen, G.; Johnston, R.K.; Rubin, M.; et al. Activity of 2-Aryl-2-(3-Indolyl)Acetohydroxamates against Drug-Resistant Cancer Cells. J. Med. Chem. 2015, 58, 2206–2220. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, D.A.; Aksenov, A. V.; Prityko, L.A.; Aksenov, N.A.; Frolova, L. V.; Rubin, M. Methylation of 2-Aryl-2-(3-Indolyl)Acetohydroxamic Acids and Evaluation of Cytotoxic Activity of the Products. Molbank. 2021, 2022, M1307. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




