1. Introduction
Aortic coarctation (CoA) is a congenital narrowing of the thoracic aorta, most commonly at the isthmus just distal to the left subclavian artery, accounting for ~5–8% of congenital heart disease [
1,
2]. While often diagnosed in childhood, many patients present in adulthood with hypertension, arm-leg blood pressure differentials, or incidental findings on imaging [
1,
2,
3]. Adult CoA may be native or recurrent after prior repair, carrying significant risks of left ventricular hypertrophy, aortic aneurysm, premature coronary disease, and cerebrovascular events if unmanaged [
2,
4,
5]. Transthoracic echocardiography (TTE) provides initial screening, but its acoustic limitations in adults make cross-sectional imaging—computed tomography (CT) and cardiovascular magnetic resonance (CMR)—essential for definitive diagnosis, treatment planning, and surveillance [
2,
3,
6]. These modalities are complementary, not competitive. This review details a modern, integrated imaging strategy, framing CoA as a systemic disease requiring a personalized, lifelong imaging approach to optimize outcomes.
1.1. Aortic Coarctation and Associated Cardiovascular Malformations
In adult patients, CoA is seldom an isolated lesion.
CoA may also be associated with other lesions including bicuspid aortic valve, sub or supra-aortic stenosis, mitral valve stenosis, anomalous origin of right subclavian artery or may be a cardiac manifestation in some syndromes [
4].
The most frequent association is with a BAV, present in over 50% of cases [
2,
7,
8]. This association necessitates vigilant, lifelong surveillance of the aortic root and ascending aorta due to the concomitant risks of progressive aortopathy and valvular dysfunction [
9]. Furthermore, the evaluation of aortic regurgitation in BAV can be challenging due to eccentric jets, underscoring the need for a multimodality imaging approach [
9].
Additional congenital cardiac anomalies are common and include ventricular septal defects, persistent ductus arteriosus, and arch hypoplasia [
2,
4,
7]. CoA is also a key component of complex syndromes such as Shone's complex and hypoplastic left heart syndrome.
Moreover, there is a well-documented association with specific genetic syndromes. These include Turner syndrome, where CoA and BAV are prevalent [
10], and Loeys-Dietz syndrome, caused by mutations in the TGF-β pathway [
11]. Anomalies of the coronary arteries, such as anomalous origin or course, have also been variably reported in patients with CoA and BAV [
12].
The chronic upstream hypertension induced by CoA provokes profound systemic arterial remodeling, leading to the development of an extensive collateral circulation network via intercostal, internal mammary, and thoracic wall arteries. The presence and degree of these collaterals are pathognomonic on cross-sectional imaging and serve as an important marker of hemodynamic significance, correlating with the chronicity and severity of the stenosis [
3,
13]. The persistent hypertensive state accelerates vascular aging, promoting aortic dilation both proximal and distal to the coarctation and leading to left ventricular concentric hypertrophy. Crucially, these sequelae may endure despite anatomically successful repair, underscoring the necessity for serial assessment of ventricular mass and function to guide ongoing risk stratification and medical therapy [
2,
5,
14,
15,
16].
1.2. How It Occurs in Adulthood
Clinical adulthood manifestations depend on the severity of CoA and range from asymptomatic patients, as rarely incidental finding, to hypertension, that is the most common presentation [
7]. CoA should be suspected of secondary hypertension in young hypertensive adults before the age of 40 years [
17]. Typical symptoms may be caused by pre-stenotic elevated blood pressure such as headache, dizziness, epistaxis and tinnitus, and/or post-stenotic hypoperfusion like claudication, cold feet and abdominal angina, due to pressure gradient between upper and lower extremities. Non treated hypertension may also lead to long term complications like hypertension-mediated organ damage (HMOD), especially left ventricular hypertrophy and/or dysfunction, aortic and intracranial aneurysm, that may result in aneurysmal ruptures, hemorrhages or dissections [
18]. CoA may also be associated with other lesions including bicuspid aortic valve (50-85%), sub or supra-aortic stenosis, mitral valve stenosis, anomalous origin of right subclavian artery or may be a cardiac manifestation in Turner Syndrome [
4].
1.3. How to Diagnose Aortic Coarctation in Adults
On physical examination, systolic blood pressure is higher in the upper extremities compared with lower ones (significant gradient > 20 mmhg). If coarctation is proximal to the left subclavian artery, systolic blood pressure is also higher in the right arm compared with the left arm. Femoral pulses are weaker than brachial and radial ones. Prominent pulsations and thrill are evident in suprasternal notch. Along the left sternal border and between the scapulae a systolic-diastolic murmur is present, due to collateral circulation [
19]. Chest X-ray findings may be the double contouring of the descending aorta due to pre and post CoA aortic dilatation and rib notching from fourth to eight ribs due to collateral circulation [
20]. Electrocardiography (ECG) may show left ventricular (LV) hypertrophy signs. However the primary and initial imaging modality for CoA diagnosis is transthoracic echocardiography (TTE) that allows anatomical and hemodynamic assessment for diagnosis and post-intervention follow up.
1.4. How to Treat
Systemic secondary hypertension due to CoA with arm-leg systolic pressure difference > 20 mmHg or invasively peak-to-peak gradient ≥20 mmHg (CW mean gradient ≥22 mmHg), re-coarctation of CoA with ≥20 mmHg and presence of signs and symptoms like LV hypertrophy, heart failure, claudication due to CoA are class I indications for intervention in adults. Significant collateral vessels and lower-limb hypoperfusion despite a low gradient (due to possible underestimation) are class II indications for intervention [
4] Endovascular stenting is first-line treatment in adult and balloon angioplasty is reserved only for re-coarctation [
8]; covered stent are preferred to prevent complications (lesions of the aortic wall) in the presence of risk factors such as tortuous aorta, presence of aneurysms or pseudoaneurysms, genetically determined aortopathies leading to greater fragility of the wall and higher risk of dissection [
21]. Surgical approach including resection or extended end-to-end anastomosis, patch aortoplasty or extra-anatomic graft is indicated when percutaneous approach is not feasible (extensive arch hypoplasia, long segment disease) or in presence of concomitant surgical diseases like aortic arch/ ascending aorta aneurism or associated significant aortic valve stenosis or regurgitation [
8].
2. Diagnosis of Aortic Coarctation: Usefulness and Limitations of Echocardiography
2.1. Usefulness and Limitations
Usefulness: TTE is a highly available, low-cost, radiation and contrast-free primary diagnostic and follow-up tool [
9]. It allows anatomical definition and functional evaluation for diagnosis and follow up after intervention. It provides information about LV size, diastolic and systolic function and initial myocardial damage by speckle tracking measures. It’s the safest method as it does not require either radiation or contrast exposure.
Limitation: The main limitations are the acoustic window with limited visualization of aortic arch and descending thoracic aorta, operator-dependency, doppler gradient inaccuracies due to the presence of collateral circulation and the absence of extracardiac evaluation of collateral circulation (
Table 1).
2.2. Anatomical Detail
Suprasternal view allows evaluation of aortic arch and isthmus to calculate isthmic diameter/ascending aortic ratio < 0,7. From the parasternal long and short axis it’s possible to assess any associated anomalies like BAV, aortic aneurysm and secondary remodelling like LV hypertrophy.
2.3. Functional Data
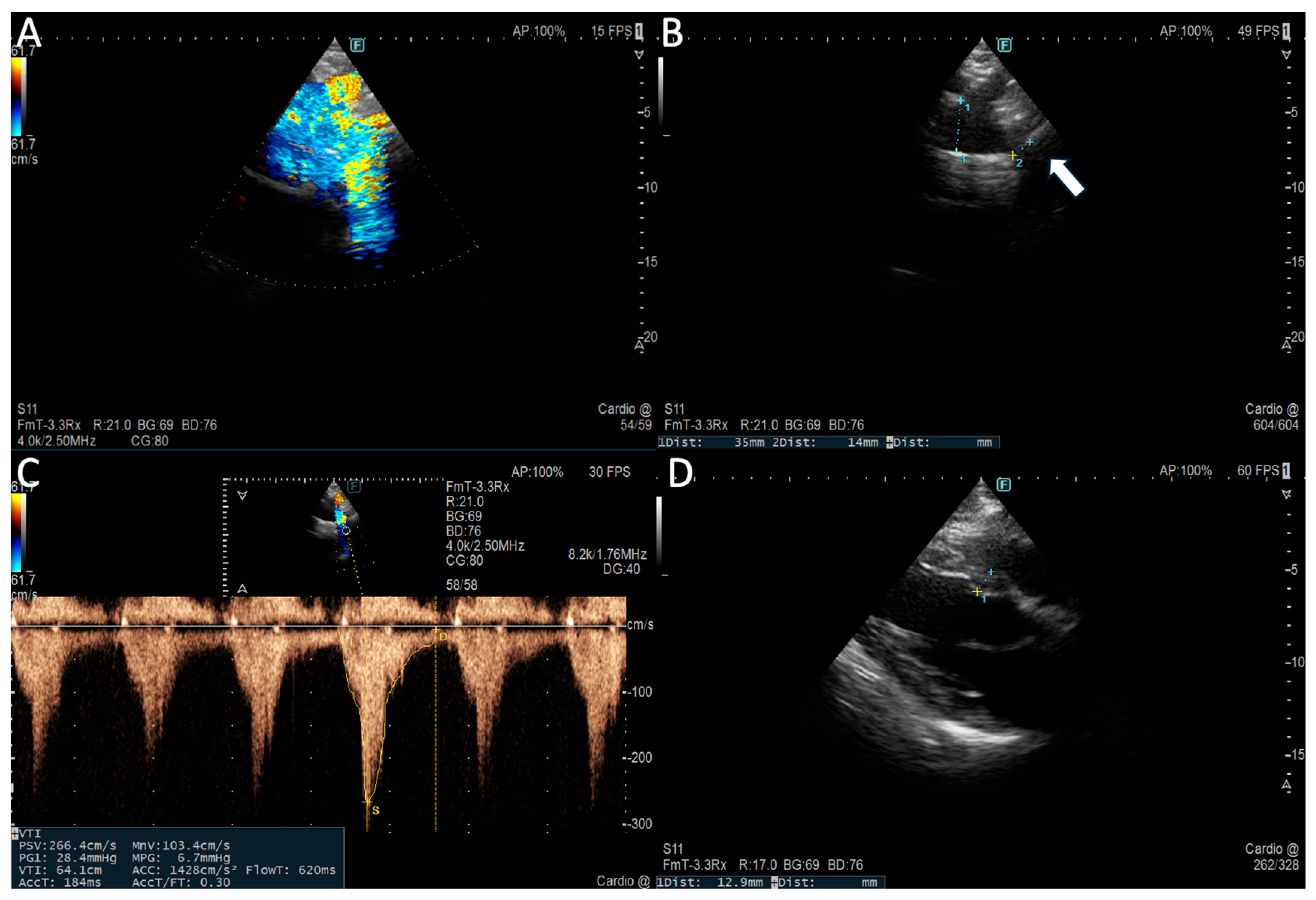
Suprasternal view allows functional evaluation with continous-wave doppler (CW) to assess peak systolic velocity (>2 m/s) and peak pressure gradient (> 20 mmHg) across the CoA-site and pulsed-wave doppler demonstrating prolonged diastolic flow (diastolic tail) with high velocity (> 1 m/s) due to downstream obstruction [
23] Subcostal view shows delayed (> 50 ms from ECG R wave) and reduced (< 55 cm/s) systolic upstroke velocity and persistence of anterograde diastolic flow in distal thoracic and upper abdominal aorta [
22] (
Figure 1)
CoA indirect findings may be elevated LV afterload with or without ejection fraction reduction [
7]. CoA is associated with higher prevalence of LV diastolic dysfunction with normal ejection fraction because of pressure overload that leads to concentric remodelling [
23]. Effective repair and systolic pressure control may lead to diastolic function improvement [
24].
Speckle-tracking echocardiography is an accurate measure of LV systolic function and subclinical myocardial damage. Early intervention may have positive effects on preservation of LV systolic function and could improve impaired global longitudinal strain (GLS) [
25]. Furthermore, Vivian Wing-yi Li et al. demonstrate that even altered arterial stiffness assessed by TTE is associated to mechanical alterations of the LV and left atrium (LA) strain parameters with an anomalous artery-LV-LA coupling that supports the use of strain indices for the functional surveillance of patients undergoing CoA repair intervention [
26]
3. Diagnosis of Aortic Coarctation: Usefulness and Limitations of CT Scan
Computed tomography angiography (CTA) is a fundamental tool in the management of adult CoA. Its high spatial resolution provides detailed, three-dimensional visualization of the thoracic aorta, its branch vessels, and the collateral circulation that develops in response to chronic obstruction [
1,
2,
3].
Technical Protocol and Optimization
The diagnostic quality of a CTA study depends on a carefully optimized protocol. To fully evaluate the coarctation and the frequently extensive collateral network, the scan must cover from the supra-aortic vessels to the diaphragm [
3,
13]. Technically, thin slices (≤0.625 mm) are required for high-quality multi-planar reconstructions. ECG gating is crucial to eliminate motion in the aortic root and ascending aorta, which is especially important for assessing the associated aortopathy often seen with a bicuspid aortic valve [
5,
27]. A controlled injection of contrast (typically 1-1.5 mL/kg at 4-6 mL/s), timed from the ascending aorta, is necessary to ensure uniform opacification across the narrowed segment [
27,
28]. Given that these patients are young and require lifelong imaging, strict dose-reduction techniques are mandatory. These include using the lowest possible tube voltage (e.g., 80-100 kVp for normal-weight patients), automatic tube current modulation, and iterative reconstruction algorithms and, when available, dual-energy techniques improve contrast-to-noise and reduce blooming from calcification or metallic implants [
5,
27,
28]. The analysis should include advanced post-processing: centerline analysis for accurate measurements perpendicular to the vessel lumen, curved planar reformats to visualize the entire arch in a single image, and 3D models that are invaluable for planning procedures and communicating with the heart team [
3,
28].
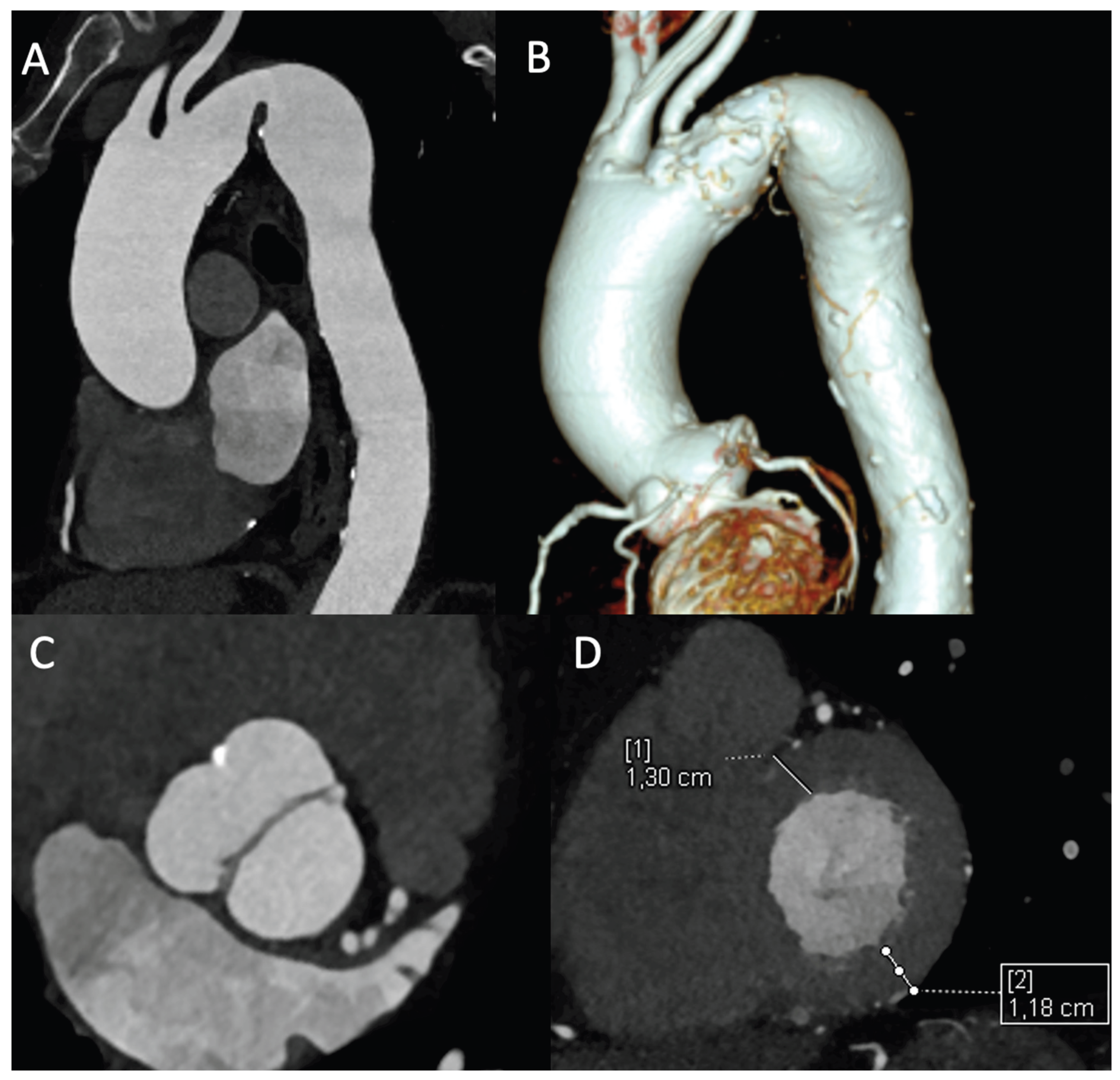
3.1. Comprehensive Anatomic Assessment and Clinical Implications
This anatomic precision directly impacts clinical decision-making. CTA precisely shows the location and type of the narrowing (discrete or long-segment), measures its severity, and identifies post-stenotic dilation [
2,
3]. The hemodynamic significance can be estimated by calculating the coarctation index (the ratio of the narrowest diameter to the aorta at the diaphragm), with a value below 0.6 suggesting severe disease [
2,
3]. Beyond the primary lesion, CTA clearly shows arch hypoplasia, anatomic variations (e.g., bovine arch or aberrant subclavian arteries), and the relationship of the lesion to branch vessels — all information that influences treatment decisions [
3]. A major strength is its ability to map the collateral blood vessels, which is a clear sign of a long-standing, severe blockage [
3,
13].
Additionally, the examination allows for the assessment of secondary cardiac changes. In the context of chronic pressure overload caused by CoA, the evaluation of left ventricular wall thickness and the presence of concentric hypertrophy are essential. While CMR is the gold standard for volumetric and functional quantification, ECG-gated CTA provides excellent morphological assessment of the ventricles. The detection of significant hypertrophy reinforces the diagnosis of a hemodynamically significant and long-standing coarctation, contributing to the overall risk stratification of the patient [
5] (
Figure 2).
Emerging technologies like CT-derived fractional flow reserve (CT-FFR) show promise for bridging the gap between pure anatomic and functional hemodynamic assessment in coarctation. This technique, by simulating blood flow and pressure gradients from a standard anatomic CTA dataset, could potentially provide a non-invasive estimate of the physiological significance of the stenosis, thereby adding a crucial functional dimension to the exquisite anatomic detail offered by CTA [
29].
CTA is particularly valuable for identifying complications that directly affect management decisions. The identification of heavy circumferential calcification at the coarctation site is a critical finding that may contraindicate plain balloon angioplasty due to the risk of aortic rupture, instead favoring primary stenting or surgical repair [
3,
28]. Similarly, the detection of a calcified patent ductus arteriosus may alter the surgical approach. For aneurysm surveillance after repair, CTA provides the precise measurements needed to determine the timing of intervention [
5,
27]. The modality is also excellent for characterizing the aortic wall, identifying features like penetrating atherosclerotic ulcers or intramural hematoma that may require specific management [
3,
5,
27]. For patients with a bicuspid aortic valve, an ECG-gated CTA can simultaneously provide accurate measurements of the aortic root and ascending aorta for aortopathy monitoring, making it a comprehensive single-modality assessment for many patients [
5,
7].
3.2. Integration into Therapeutic Decision-Making
The primary clinical value of CTA lies in its ability to guide specific therapeutic choices. In transcatheter stenting planning, CTA provides more than just anatomic measurements; it offers prognostic information that directly influences device selection and procedure strategy. The precise characterization of landing zones helps select appropriate stent sizes to prevent complications like stent migration or aortic wall injury [
1,
3,
28]. The relationship of the coarctation to the left subclavian artery determines whether coverage is feasible or if surgical alternatives should be considered.
For surgical planning, especially in re-operations, CTA provides essential information for risk stratification. The identification of large, fragile collateral vessels alerts the surgical team to potential bleeding risks and may influence the choice of surgical approach [
2,
3]. The relationship between the sternum and major vascular structures is crucial for planning safe re-entry in re-do operations. In patients with arch hypoplasia, CTA measurements help determine whether a simple resection or extended arch repair is necessary [
2,
3].
After intervention, CTA is indispensable for surveillance. The use of sharp reconstruction kernels minimizes blooming artifacts, allowing accurate assessment of stent apposition and detecting in-stent restenosis or pseudoaneurysm formation at surgical anastomoses [
1,
3,
27].
3.3. Usefulness and Limitations
Usefulness: The primary strength of CTA lies in its unparalleled anatomic definition. It is essential for confirming the diagnosis, planning interventions (both transcatheter and surgical), and for long-term surveillance, especially in stented patients where it produces minimal artifacts [
27]. Its ability to precisely characterize the coarctation (location, length, severity, calcification), map the collateral circulation, and evaluate the entire thoracic aorta and branch vessels makes it the cornerstone of pre-procedural planning [
2,
3,
13]. Furthermore, ECG-gated CTA allows for concurrent assessment of the aortic root for associated aortopathy and provides a morphological evaluation of left ventricular hypertrophy, adding functional context to the anatomic data [
5,
7]. Its wide availability and rapid acquisition also make it the preferred test in urgent settings or when CMR is contraindicated.
Limitations: The principal limitation of CTA is its inability to directly quantify hemodynamics; it cannot measure pressure gradients across the stenosis, which requires Doppler ultrasound, CMR, or catheterization [
1,
2,
3]. Furthermore, the cumulative lifetime risk from ionizing radiation must be actively managed with strict dose-reduction protocols [
5,
28]. The necessity for iodinated contrast media carries a risk of nephrotoxicity and allergic reactions, mandating careful patient selection, pre-procedural hydration in at-risk individuals, or consideration of alternative imaging in cases of severe renal impairment [
5,
27] (
Table 2).
4. Diagnosis of Aortic Coarctation: Usefulness and Limitations of MRI
CMR is a pivotal non-invasive modality for the comprehensive management of adults with CoA, uniquely providing a
one-stop-shop evaluation that integrates detailed anatomy, hemodynamic significance, and end-organ (ventricular) consequences—all without ionizing radiation [
1,
2,
5,
7,
9].
A tailored CMR protocol for adult CoA is systematic, addressing the multifocal nature of the disease through five core components designed to answer specific clinical questions [
1,
7,
9,
30]:
Cine Imaging for Anatomy and Function: Balanced steady-state free precession (bSSFP) sequences are fundamental. Beyond standard long- and short-axis planes for the quantitative assessment of LV volumes, ejection fraction, and mass—the non-invasive gold standard—specific cine images aligned along the double-oblique axis of the aortic arch are crucial. These "aortic cines" dynamically visualize the jet across the coarctation and any associated arch hypoplasia [
9,
30]. Furthermore, high-resolution cine images acquired in the short-axis plane of the aortic valve allow for a precise anatomical assessment of the valve morphology, enabling the differentiation between tricuspid and BAV. The same views provide a functional assessment, revealing restricted leaflet motion suggestive of stenosis or a central coaptation defect indicative of regurgitation. This evaluation is a critical part of the examination, given the high prevalence of BAV and concomitant valvulopathy in the CoA population.
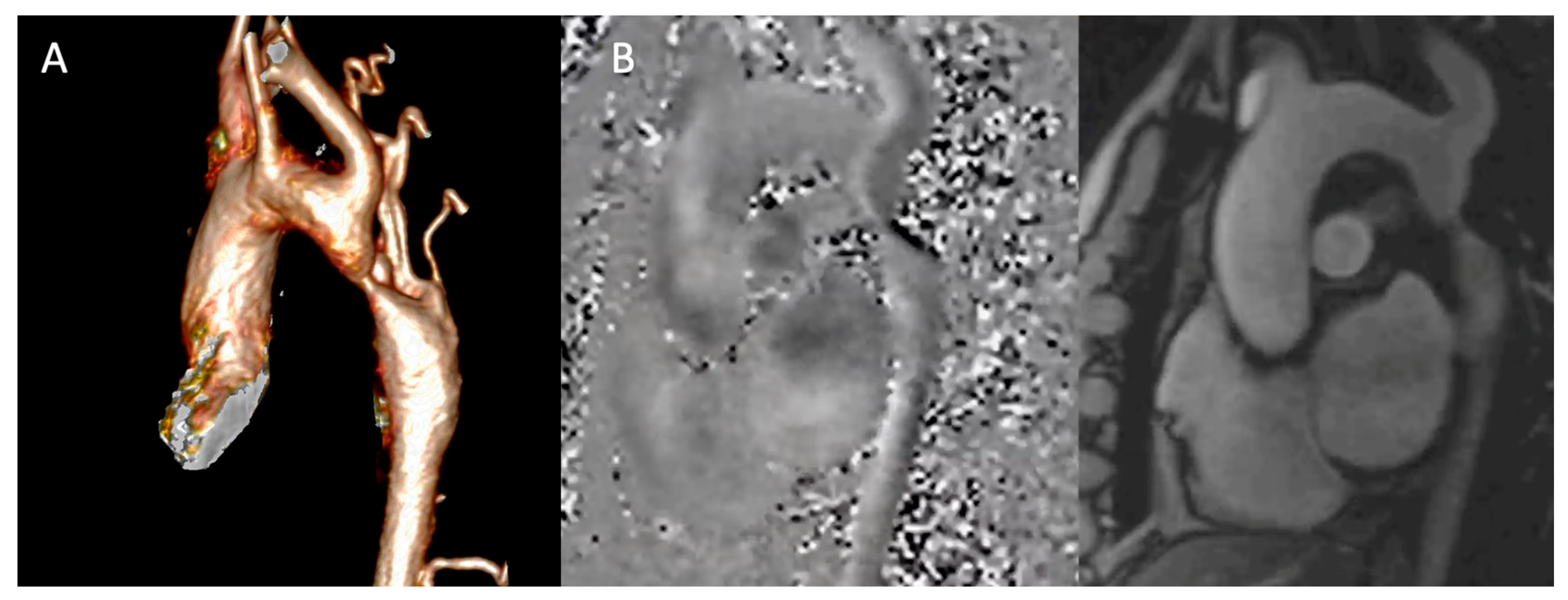
Angiography for Roadmapping: Contrast-enhanced magnetic resonance angiography (CE-MRA) provides high-resolution 3D anatomy (
Figure 3A). Acquired with isotropic voxels (≤1.5 mm), these datasets allow for exquisite multi-planar reformations, precisely defining the coarctation's location, length, and minimal diameter. For patients where gadolinium is contraindicated, non-contrast 3D acquisitions (e.g., respiratory-navigated, ECG-gated bSSFP) offer a reliable alternative [
3,
7,
30]. Furthermore, modern non-contrast techniques like 3D Dixon-based water-fat separation provide robust luminal and wall characterization, simultaneously visualizing the aortic lumen and periaortic fat, which can be valuable in post-surgical cases.
Phase-Contrast MRI (Figure 3B): Through-plane phase-contrast sequences, with the imaging slice positioned perpendicular to the aorta at the stenosis, allow estimation of the peak instantaneous gradient (ΔP ≈ 4V²max). Careful slice placement is critical, as misalignment can significantly underestimate the velocity. Crucially, flow measurements in the ascending aorta and at the diaphragm enable calculation of the collateral flow index: (Distal Aortic Flow – Ascending Aortic Flow) / Ascending Aortic Flow. An index >10-20% provides objective, quantitative evidence of hemodynamically significant stenosis, which is critical for advocating for intervention in asymptomatic patients [
14,
30].
Myocardial Tissue Characterization: Late gadolinium enhancement (LGE) imaging can identify focal myocardial fibrosis. Native T1 mapping and extracellular volume (ECV) fraction offer a quantitative assessment of diffuse interstitial fibrosis, providing a sensitive marker of preclinical myocardial disease that may influence medical therapy [
7,
9]. While still a research tool in this specific population, the presence of elevated ECV may identify a subgroup of patients with more advanced hypertensive heart disease who warrant closer monitoring [
31].
4D Flow: Emerging 4D flow MRI techniques enable comprehensive, three-dimensional visualization of blood flow patterns, wall shear stress, and energy loss throughout the thoracic aorta. This allows for the quantification of regional wall shear stress in the ascending aorta, which may help stratify the risk of progressive aortopathy in patients with an associated bicuspid aortic valve, moving beyond simple diameter measurements for risk assessment [
32].
4.1. Usefulness and Limitations
Usefulness: CMR is the preferred modality for initial diagnosis and serial follow-up in young, non-stented patients due to its lack of radiation. Its ability to provide a synergistic assessment of anatomy, ventricular impact, and hemodynamics aligns perfectly with the understanding of CoA as a complex systemic disease and provides robust risk stratification. A key advantage is its capacity for quantitative hemodynamic assessment. The collateral flow index, in particular, is a unique strength, offering a direct measure of hemodynamic significance that is often more sensitive than anatomic measurements alone and is crucial for risk stratification [
5,
8,
14]. The clinical utility of CMR is profound at every stage. In the newly diagnosed adult, it serves as a definitive baseline examination. This dataset is indispensable for deciding if and when intervention is warranted [
1,
2,
9]; for example, a patient with a discrete narrowing but a high collateral flow index and significant LV hypertrophy has a clear physiological indication for repair, even in the absence of severe symptoms [
14]. In post-intervention surveillance, CMR is the modality of choice. It effectively monitors for re-coarctation and, most importantly, tracks the regression of LV mass—a key marker of successful treatment and reverse remodeling. The persistence of LV hypertrophy despite a seemingly adequate anatomical repair is a poor prognostic sign and should prompt aggressive medical management [
15,
16]. Looking ahead, the integration of artificial intelligence for automated quantification of ventricular parameters and aortic dimensions will further enhance the reproducibility and efficiency of CMR. Furthermore, the ongoing validation of 4D flow-derived biomarkers, such as energy loss and wall shear stress, promises a future where CMR can not only diagnose but also better predict the individual patient's risk of disease progression.
Limitations: Acknowledging CMR's limitations is essential for its appropriate application. Metallic implants, particularly older stents, can cause significant susceptibility artifacts, obscuring the lumen. In such cases CTA is the complementary modality of choice for definitive anatomic evaluation [
3,
5,
27]. Turbulent flow can lead to signal loss and potential underestimation of peak velocities; careful technique is required. It is also important to recognize that in the presence of significant arrhythmia, obtaining diagnostic image quality can be challenging, though newer arrhythmia-rejection algorithms are mitigating this limitation [
33]. The examination has a longer acquisition time and is not as widely available as CTA. It is also contraindicated in patients with certain non-MRI compatible implants and severe claustrophobia (
Table 3).
5. Conclusions
Adult CoA is a lifelong systemic disease, not merely a focal aortic stenosis. Optimal long-term outcomes, therefore, depend on an integrated, multimodal imaging strategy that is dynamically tailored to the individual patient. This approach strategically leverages the specific strengths of each technique: echocardiography serves as the essential first-line tool for initial screening, functional valvular assessment, and basic ventricular evaluation; CTA provides unmatched spatial resolution for definitive anatomic diagnosis, procedural planning of interventions, and surveillance of stents or complex anatomy; and CMR offers a comprehensive, radiation-free "one-stop-shop" for precise quantification of hemodynamic significance, collateral flow, and serial monitoring of ventricular remodeling.
This synergistic, patient-centered strategy allows for sophisticated risk stratification—identifying patients with persistent collateral flow or significant LV hypertrophy who may benefit from intensified therapy—and enables the design of personalized surveillance intervals. Rigorous blood pressure control combined with this standardized, expert-center imaging protocol is essential to mitigate late complications. Future integration of advanced techniques like 4D flow MRI and artificial intelligence for automated measurements promises to further refine risk prediction and personalize long-term management, translating anatomic correction into durable cardiovascular health across the adult lifespan.
The novel perspective of this review lies in moving beyond a simple comparison of imaging techniques to frame this dynamic, integrated strategy for lifelong care. The clinical implications are clear: imaging must be comprehensive, reflecting the complex nature of CoA as a disease of the aorta and myocardium; a tailored approach leveraging the complementary strengths of echocardiography, CTA, and CMR is essential to guide intervention and optimize outcomes; and this multimodal framework facilitates personalized surveillance to mitigate late risks such as aneurysm formation, recoarctation, and hypertensive heart disease.
Author Contributions
Conceptualization: LLM, LM, FI,MF, ML, SD, CS, CI, RI; Writing – Original Draft Preparation: LLM, LM, FI, RI; Writing – Review & Editing: LLM, LM, FI,MF, ML, SD, CS, CI, RI. All the authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BAV |
Bicuspid Aortic Valve |
| CoA |
Aortic coarctation |
| TTE |
Transthoracic echocardiography |
| CT |
computed tomography |
| CMR |
cardiovascular magnetic resonance |
| HMOD |
hypertension-mediated organ damage |
| ECG |
Electrocardiography |
| LV |
Left Ventricle |
| GLS |
global longitudinal strain |
| LA |
left atrium |
| CTA |
Computed tomography angiography |
| CT-FFR |
CT-derived fractional flow reserve |
| CE-MRA |
Contrast-enhanced magnetic resonance angiography |
| LGE |
Late gadolinium enhancement |
| ECV |
extracellular volume |
References
- Thakkar, AN; Chinnadurai, P; Lin, CH. Imaging adult patients with coarctation of the aorta. Curr Opin Cardiol. 2017, 32(5), 503–512. [Google Scholar] [CrossRef]
- Dijkema, EJ; Leiner, T; Grotenhuis, HB. Diagnosis, imaging and clinical management of aortic coarctation. Heart 2017, 103(15), 1148–1155. [Google Scholar] [CrossRef]
- Karaosmanoglu, AD; Khawaja, RD; Onur, MR; Kalra, MK. CT and MRI of Aortic Coarctation: Pre- and Postsurgical Findings. AJR Am J Roentgenol. 2015, 204(3), W224–W233. [Google Scholar] [CrossRef]
- Baumgartner, H; de Backer, J; Babu-Narayan, S V.; Budts, W; Chessa, M; Diller, GP; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. In European Heart Journal; Oxford University Press, 2021; Vol. 42, pp. 563–645. [Google Scholar]
- Isselbacher, EM; Preventza, O; Black, JH, 3rd; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. Circulation 2022, 146(24), e334–e482. [Google Scholar] [CrossRef]
- Salciccioli, KB; Zachariah, JP. Coarctation of the Aorta: Modern Paradigms Across the Lifespan. Hypertension 2023, 80(10), 1970–1979. [Google Scholar] [CrossRef]
- Leo, I; Vinco, G; Camera, M; et al. Non-Invasive Imaging Assessment in Patients with Aortic Coarctation. J Clin Med. 2024, 13(1), 28. [Google Scholar] [CrossRef]
- Agasthi, P; Shah, S; Kovelamudi, S; et al. Management of adults with coarctation of aorta. World J Cardiol. 2020, 12(7), 259–272. [Google Scholar] [CrossRef] [PubMed]
- La Mura, L; Lembo, M; Musella, F; D'Amato, M; D'Andrea, A; Izzo, R; Esposito, G. Aortic Regurgitation in Bicuspid Aortic Valve: The Role of Multimodality Imaging. J Clin Med. 2024, 13, 3924. [Google Scholar] [CrossRef] [PubMed]
- Miller, MJ; Geffner, ME; Lippe, B; et al. Echocardiography reveals a high incidence of bicuspid aortic valve in Turner syndrome. J Pediatr. 1983, 102(1), 47–50. [Google Scholar] [CrossRef] [PubMed]
- Patel, ND; Crawford, T; Magruder, JT; et al. Cardiovascular operations for Loeys-Dietz syndrome: Intermediate-term results. J Thorac Cardiovasc Surg. 2017, 153(2), 406–412. [Google Scholar] [CrossRef]
- Doty, DB. Anomalous origin of the left circumflex coronary artery associated with bicuspid aortic valve. J Thorac Cardiovasc Surg. 2001, 122(4), 842–843. [Google Scholar] [CrossRef]
- Riquelme, C; Mellado, P; Morán, S; et al. Coarctation of the aorta and its collateral supply: MR imaging. RadioGraphics 1999, 19(1), 107–124. [Google Scholar]
- Azarine, A; Garçon, P; Stansal, A; et al. Four-Dimensional Flow MRI: Principles and Cardiovascular Applications. RadioGraphics 2019, 39(4), 1202–1220. [Google Scholar] [CrossRef] [PubMed]
- Egbe, AC; Anderson, JH; Ammash, NM; Taggart, NW. Left Ventricular Remodeling After Transcatheter Versus Surgical Therapy in Adults With Coarctation of Aorta. JACC Cardiovasc Imaging 2020, 13(9), 1863–1872. [Google Scholar] [CrossRef]
- Egbe, AC; Miranda, WR; Connolly, HM. Predictors of left ventricular reverse remodelling after coarctation of aorta intervention. Eur Heart J Cardiovasc Imaging 2021, 22(10), 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, CP; Bruno, RM; Brouwers, S; Canavan, MD; Ceconi, C; Christodorescu, RM; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. In European Heart Journal; Oxford University Press, 2024; Vol. 45, pp. 3912–4018. [Google Scholar]
- Mazzolai, L; Teixido-Tura, G; Lanzi, S; Boc, V; Bossone, E; Brodmann, M; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases : Developed by the task force on the management of peripheral arterial and aortic diseases of the European Society of Cardiology (ESC Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS, the European Reference Network on Rare Multisystemic Vascular Diseases (VASCERN, and the European Society of Vascular Medicine (ESVM). Eur Heart J 2024, 45(36), 3538–700. [Google Scholar]
- Prisant, LM; Mawulawde, K; Kapoor, D; Joe, C. Coarctation of the aorta: a secondary cause of hypertension. J Clin Hypertens (Greenwich) 2004, 6(6). [Google Scholar] [CrossRef]
- Alkashkari, W; Albugami, S; Hijazi, ZM. Management of Coarctation of The Aorta in Adult Patients: State of The Art. Korean Circ J 2019, 49(4), 298–313. [Google Scholar] [CrossRef]
- Pan, M; Pericet, C; González-Manzanares, R; Díaz, MA; Suárez de Lezo, J; Hidalgo, F; Alvarado, M; Dueñas, G; Gómez, E; Espejo, S; Perea, J; Romero, M; Ojeda, S. Very long-term follow-up after aortic stenting for coarctation of the aorta. Rev Esp Cardiol (Engl Ed) 2024, 77(4), 332–341. [Google Scholar] [CrossRef]
- Salahuddin, Ayesha; Chan, Alice; Zaidi, Ali N. ‘The Adult with Coarctation of the Aorta’. Congenital Heart Disease. IntechOpen 2018. [Google Scholar] [CrossRef]
- Egbe, AC; Miranda, WR; Connolly, HM. Increased prevalence of left ventricular diastolic dysfunction in adults with repaired coarctation of aorta. Int J Cardiol Heart Vasc. Erratum in: Int J Cardiol Heart Vasc. 2020 Dec 19;32:100698. 2020, 28, 100530. [Google Scholar] [CrossRef]
- Lam, YY; Kaya, MG; Li, W; Mahadevan, VS; Khan, AA; Henein, MY; Mullen, M. Effect of endovascular stenting of aortic coarctation on biventricular function in adults. Heart 2007, 93(11), 1441–7. [Google Scholar] [CrossRef]
- Aragão, NFDV; Borgo, JNV; Jesus, CA; Davoglio, T; Armstrong, ADC; Barretto, RBM; Le Bihan, D; Assef, JE; Pedra, CAC; Pedra, SRFF. Myocardial strain pattern progress in patients with Coarctation of the Aorta undergoing aortic stenting. Echocardiography 2021, 38(1), 64–71. [Google Scholar] [CrossRef]
- Li, VW; Cheung, YF. Arterial-left ventricular-left atrial coupling late after repair of aortic coarctation and interruption. Eur Heart J Cardiovasc Imaging 2015, 16(7), 771–80. [Google Scholar] [CrossRef]
- Nance, JW; Ringel, RE; Fishman, EK. Coarctation of the aorta in adolescents and adults: A review of clinical features and CT imaging. J Cardiovasc Comput Tomogr 2016, 10(1), 1–12. [Google Scholar] [CrossRef]
- Hager, A; Kaemmerer, H; Leppert, A; Prokop, M; Blücher, S; Stern, H; Hess, J. Follow-up of adults with coarctation of the aorta: comparison of helical CT and MRI, and impact on assessing diameter changes. Chest 2004, 126(4), 1169–76. [Google Scholar] [PubMed]
- Bigelow, M. M.; Elsayed, M.; Zafar, M. A.; et al. Computed tomography–derived fractional flow reserve in aortic coarctation: a comparison with invasive measurements. Cardiology in the Young 2024, 34(1), 148–154. [Google Scholar]
- Nielsen, JC; Powell, AJ; Gauvreau, K; et al. Magnetic resonance imaging predictors of coarctation severity. Circulation 2005, 111(5), 622–628. [Google Scholar] [CrossRef]
- Treiber, G.; Gotschy, A.; Wójta, J.; et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is associated with impaired exercise capacity in adult patients with aortic coarctation. Sci Rep 2021, 11, 15544. [Google Scholar]
- Guala, A; Evangelista, A; Teixido-Tura, G; et al. Leaflet fusion length is associated with aortic dilation and flow alterations in non-dysfunctional bicuspid aortic valve. Eur Radiol. 2021, 31(12), 9262–9272. [Google Scholar] [PubMed]
- Schuster, A.; Hor, K. N.; Kowallick, J. T.; Beerbaum, P.; Kutty, S. Cardiovascular Magnetic Resonance Myocardial Feature Tracking: Concepts and Clinical Applications. Circulation: Cardiovascular Imaging 2016, 9(4), e004077. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).