Submitted:
22 December 2025
Posted:
24 December 2025
You are already at the latest version
Abstract
Background: Oral food challenges (OFCs) are still the reference standard for confirming food allergy, yet the influence of previous anaphylaxis on challenge outcomes remains uncertain. Patients with a history of anaphylaxis are often considered at higher risk, which may affect clinical decision-making process. In our study, we sought to identify predictors of OFC failure stratified by previous history of anaphylaxis. Methods: We conducted a retrospective evaluation of standard-of-care OFCs to cow’s milk and hen’s egg white performed between January 2014 and July 2025 at the Pediatric Allergy Center. Eligible participants had suspected or confirmed IgE-mediated allergy to cow’s milk protein (CMP) or hen’s egg white protein (HEWP), and were classified according to the presence or absence of previous anaphylaxis to the challenged food. Clinical data, including age, gender, allergic comorbidities, family history of atopy, and levels of specific IgE (sIgE) were obtained from medical records and compared between groups. Open OFCs were conducted according to PRACTALL guidelines (2012/2024) under inpatient supervision with full emergency support. Specific IgE levels were measured using the ImmunoCAP® assay (Phadia, Uppsala, Sweden). Logistic regression models were used to assess the relationship between comorbidities, sIgE concentrations and OFC outcomes. Receiver operating characteristic (ROC) analysis evaluated diagnostic accuracy of sIgE levels in predicting OFC outcomes. Results: The analysis included 192 pediatric patients (median age 4.75 years, 66.1% male) undergoing OFCs: 106 to CMP and 86 to HEWP. Six challenges (3.1%) were inconclusive, giving 186 valid results. The overall OFC failure rate was 32.3% (30.2% to CMP and 34.9% to HEWP). Patients with a past history of anaphylaxis more frequently underwent cow’s milk challenges (p = 0.01). Atopic dermatitis was a more common comorbidity in those without prior anaphylaxis (p = 0.04), regardless of the trigger. In hen’s egg challenges, children with a history of anaphylaxis reacted to significantly lower cumulative doses (p = 0.03) than patients without. Logistic regression analysis identified atopic dermatitis as a predictor of OFC failure in children without prior anaphylaxis (p = 0.02), and asthma as a borderline predictor in those with previous systemic reactions (p = 0.05). Specific IgE levels correlated with OFC outcomes across allergens, with casein-sIgE showing the highest discriminative performance (AUC = 0.81) in children without previous anaphylaxis. Conclusions: Atopic dermatitis and asthma were identified as potential risk factors influencing OFC outcomes, depending on the patient’s history of anaphylaxis. The predictive accuracy of sIgE was different in groups stratified by presence of prior anaphylaxis. Casein-sIgE showed the highest diagnostic accuracy in children without previous severe reactions to CMP. A prior history of anaphylaxis was associated with increased allergen sensitivity but did not predict a higher risk of severe reactions during supervised OFCs. Presence of anaphylactic reactions in the past is an important consideration when selecting children for OFCs to CMP and HEWP, since it delineates distinct risk factors for challenge failure in these patient populations.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Specific IgE Testing
2.3. Oral Food Challenges
2.4. Statistical Methodology
3. Results
3.1. General Information and Patient Demographics
3.2. Comparison Between Patient Groups by History of Anaphylaxis to the Challenged Food
3.3. Factors Influencing OFC Outcome in Studied Groups
- • Coef. → The estimated coefficient of the independent variable in the ordinal logistic regression model. It represents the effect size of the predictor on the dependent variable.
- • Std.Err. → Standard error of the coefficient estimate, indicating the level of variability in the estimate. A smaller value suggests higher precision.
- • z → The z-score (test statistic), which measures how many standard deviations the coefficient is away from zero. Higher absolute values indicate stronger relationships.
- • P>|z| → The p-value, representing the probability that the coefficient is different from zero due to random chance. A value below 0.05 typically suggests statistical significance.
- • 0.025 → The lower bound of the 95% confidence interval for the coefficient estimate.
- • 0.975 → The upper bound of the 95% confidence interval for the coefficient estimate.
3.4. Specific IgE Level as a Predictor of OFC Outcome in Studied Groups
4. Discussion
4.1. General Information and Patient Demographics
4.2. Comparison Between Patient Groups by History of Anaphylaxis to the Challenged Food and Factors Influencing OFC Outcome in Studied Groups
4.3. Specific IgE Level as a Predictor of OFC Outcome in Studied Groups
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FA | food allergy |
| EAACI | European Academy of Allergy and Clinical Immunology |
| CMP | cow’s milk protein |
| HEWP | hen’s egg white protein |
| sIgE | specific IgE |
| CRD | component-resolved diagnostics |
| BAT | basophil activation test |
| MAT | mast cell activation test |
| EDN | eosinophil-derived neurotoxin |
| OFC | oral food challenge |
| OIT | oral immunotherapy |
| FPIES | food protein-induced enterocolitis syndrome |
| ROC | receiver operating characteristic |
| AUC | area under curve |
| AD | atopic dermatitis |
References
- Arens, A.; Lange, L.; Stamos, K. Epidemiology of Food Allergy. Allergo J Int 2025, 34, 121–126. [Google Scholar] [CrossRef]
- Spolidoro, G.C.I.; Amera, Y.T.; Ali, M.M.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Frequency of Food Allergy in Europe: An Updated Systematic Review and Meta-Analysis. Allergy: European Journal of Allergy and Clinical Immunology 2023, 78, 351–368. [Google Scholar] [CrossRef]
- Sampath, V.; Abrams, E.M.; Adlou, B.; Akdis, C.; Akdis, M.; Brough, H.A.; Chan, S.; Chatchatee, P.; Chinthrajah, R.S.; Cocco, R.R.; et al. Food Allergy across the Globe. Journal of Allergy and Clinical Immunology 2021, 148, 1347–1364. [Google Scholar] [CrossRef]
- Elghoudi, A.; Narchi, H. Food Allergy in Children-the Current Status and the Way Forward. World J Clin Pediatr 2022, 11, 253–269. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food Allergy: A Review and Update on Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Journal of Allergy and Clinical Immunology 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Lyons, S.A.; Clausen, M.; Knulst, A.C.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Bieli, C.; Dubakiene, R.; Fernandez-Perez, C.; Jedrzejczak-Czechowicz, M.; et al. Prevalence of Food Sensitization and Food Allergy in Children Across Europe. Journal of Allergy and Clinical Immunology: In Practice 2020, 8, 2736–2746.e9. [Google Scholar] [CrossRef]
- Wang, Y.; Allen, K.J.; Suaini, N.H.A.; McWilliam, V.; Peters, R.L.; Koplin, J.J. The Global Incidence and Prevalence of Anaphylaxis in Children in the General Population: A Systematic Review. Allergy: European Journal of Allergy and Clinical Immunology 2019, 74, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Campbell, D.E.; Motosue, M.S.; Campbell, R.L. Global Trends in Anaphylaxis Epidemiology and Clinical Implications. Journal of Allergy and Clinical Immunology: In Practice 2020, 8, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Rueter, K.; Moseley, N.; Ta, B.; Bear, N.; Borland, M.L.; Prescott, S.L. Increasing Emergency Department Visits for Anaphylaxis in Very Early Childhood: A Canary in the Coal Mine. Acta Paediatrica, International Journal of Paediatrics 2023, 112, 2182–2188. [Google Scholar] [CrossRef]
- Robinson, L.B.; Arroyo, A.C.; Faridi, M.K.; Rudders, S.A.; Camargo, C.A. Trends in US Hospitalizations for Anaphylaxis among Infants and Toddlers: 2006 to 2015. Annals of Allergy, Asthma and Immunology 2021, 126, 168–174.e3. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGalvin, A.; Garvey, L.H.; Riggioni, C.; de Silva, D.; Angier, E.; Arasi, S.; et al. EAACI Guidelines: Anaphylaxis (2021 Update). Allergy: European Journal of Allergy and Clinical Immunology 2022, 77, 357–377. [Google Scholar] [CrossRef]
- Błażowski, Ł.; Kurzawa, R.; Majak, P. Food-Induced Anaphylaxis in Children - State of Art. Pediatria i Medycyna Rodzinna 2021, 17, 8–16. [Google Scholar] [CrossRef]
- Tanno, L.K.; Demoly, P. Anaphylaxis in Children. Pediatric Allergy and Immunology 2020, 31, 8–10. [Google Scholar] [CrossRef]
- Gaspar, Â.; Santos, N.; Faria, E.; Pereira, A.M.; Gomes, E.; Câmara, R.; Rodrigues-Alves, R.; Borrego, L.M.; Carrapatoso, I.; Carneiro-Leão, L.; et al. Anaphylaxis in Children and Adolescents: The Portuguese Anaphylaxis Registry. Pediatric Allergy and Immunology 2021, 32, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Stoianova, S.; Sharma, V.; Boyle, R.J. Fatal Food Anaphylaxis in Children: A Statutory Review in England. Clinical and Experimental Allergy 2025, 55, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Şengül Emeksiz, Z.; Ertuğrul, A.; Uygun, S.D.; Özmen, S. Evaluation of Emotional, Behavioral, and Clinical Characteristics of Children Aged 1–5 with a History of Food-Related Anaphylaxis. Pediatr Neonatol 2023, 64, 154–159. [Google Scholar] [CrossRef]
- Baseggio Conrado, A.; Patel, N.; Turner, P.J. Global Patterns in Anaphylaxis Due to Specific Foods: A Systematic Review. Journal of Allergy and Clinical Immunology 2021, 148, 1515–1525.e3. [Google Scholar] [CrossRef]
- Dölle-Bierke, S.; Höfer, V.; Francuzik, W.; Näher, A.F.; Bilo, M.B.; Cichocka-Jarosz, E.; Lopes de Oliveira, L.C.; Fernandez-Rivas, M.; García, B.E.; Hartmann, K.; et al. Food-Induced Anaphylaxis: Data From the European Anaphylaxis Registry. Journal of Allergy and Clinical Immunology: In Practice 2023, 11, 2069–2079.e7. [Google Scholar] [CrossRef] [PubMed]
- Pouessel, G.; Jean-Bart, C.; Deschildre, A.; Van der Brempt, X.; Tanno, L.K.; Beaumont, P.; Dumond, P.; Sabouraud-Leclerc, D.; Beaudouin, E.; Ramdane, N.; et al. Food-Induced Anaphylaxis in Infancy Compared to Preschool Age: A Retrospective Analysis. Clinical and Experimental Allergy 2020, 50, 74–81. [Google Scholar] [CrossRef]
- Su, Y.; Wen, J.; Zhang, H.; Zou, Z.; Cai, Y.; Zhang, C. Clinical Characteristics of Anaphylaxis in Children Aged 0-16 Years in Xi’an, China. Int Arch Allergy Immunol 2023, 184, 220–227. [Google Scholar] [CrossRef]
- Cichocka-Jarosz, E.; Dölle-Bierke, S.; Jedynak-Wąsowicz, U.; Sabouraud-Leclerc, D.; Köhli, A.; Lange, L.; Papadopoulos, N.G.; Hourihane, J.; Nemat, K.; Scherer Hofmeier, K.; et al. Cow’s Milk and Hen’s Egg Anaphylaxis: A Comprehensive Data Analysis from the European Anaphylaxis Registry. Clin Transl Allergy 2023, 13. [Google Scholar] [CrossRef]
- Klim, L.; Michalik, M.; Figura, N.; Cichocka-Jarosz, E.; Jedynak-Wąsowicz, U. Food-Induced Anaphylaxis in Children Less than 2 Years of Age. Postepy Dermatol Alergol 2025, 42, 378–386. [Google Scholar] [CrossRef]
- Novembre, E.; Gelsomino, M.; Liotti, L.; Barni, S.; Mori, F.; Giovannini, M.; Mastrorilli, C.; Pecoraro, L.; Saretta, F.; Castagnoli, R.; et al. Fatal Food Anaphylaxis in Adults and Children. Ital J Pediatr 2024, 50. [Google Scholar] [CrossRef]
- Barni, S.; Liccioli, G.; Sarti, L.; Giovannini, M.; Novembre, E.; Mori, F. Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina (Lithuania) 2020, 56. [Google Scholar] [CrossRef] [PubMed]
- Riggioni, C.; Ricci, C.; Moya, B.; Wong, D.; van Goor, E.; Bartha, I.; Buyuktiryaki, B.; Giovannini, M.; Jayasinghe, S.; Jaumdally, H.; et al. Systematic Review and Meta-Analyses on the Accuracy of Diagnostic Tests for IgE-Mediated Food Allergy. Allergy: European Journal of Allergy and Clinical Immunology 2024, 79, 324–352. [Google Scholar] [CrossRef] [PubMed]
- Cafarotti, A.; Giovannini, M.; Begìn, P.; Brough, H.A.; Arasi, S. Management of IgE-Mediated Food Allergy in the 21st Century. Clinical and Experimental Allergy 2023, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Knyziak-Mędrzycka, I.; Majsiak, E.; Gromek, W.; Kozłowska, D.; Swadźba, J.; Beata, B.J.; Kurzawa, R.; Cukrowska, B. The Sensitization Profile for Selected Food Allergens in Polish Children Assessed with the Use of a Precision Allergy Molecular Diagnostic Technique. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Popielarz, M.; Krogulska, A. The Importance of Component-Resolved Diagnostics in IgE-Mediated Cow’s Milk Allergy. Allergol Immunopathol (Madr) 2021, 49, 30–41. [Google Scholar] [CrossRef]
- Chong, K.W.; Sultana, R.; Lee, M.P.; Tan, L.L.; Goh, A.; Goh, S.H.; Loh, W. Diagnostic Accuracy of Skin Prick Test, Food-Specific IgE and Component Testing for IgE-Mediated Peanut, Egg, Milk and Wheat Allergy. Clinical and Experimental Allergy 2025, 55, 187–189. [Google Scholar] [CrossRef]
- Bégin, P.; Waserman, S.; Protudjer, J.L.P.; Jeimy, S.; Watson, W. Immunoglobulin E (IgE)-Mediated Food Allergy. Allergy, Asthma and Clinical Immunology 2024, 20. [Google Scholar] [CrossRef]
- Karadağ, Ş.İ.K.; Öztürk, F.; Sancak, R.; Yildiran, A. The Role of Basophil Activation Test in the Diagnosis of Pediatric Egg Allergy in Turkey: A Comparison of Clinical and Laboratory Findings with Real-Life Data. Allergol Immunopathol (Madr) 2025, 53, 32–39. [Google Scholar] [CrossRef]
- Bartha, I.; Boyd, H.; Foong, R.X.; Krawiec, M.; Marques-Mejias, A.; Marshall, H.F.; Radulovic, S.; Harrison, F.; Antoneria, G.; Jama, Z.; et al. The Basophil Activation Test Is the Most Accurate Test in Predicting Allergic Reactions to Baked and Fresh Cow’s Milk During Oral Food Challenges. Allergy: European Journal of Allergy and Clinical Immunology 2025. [Google Scholar] [CrossRef]
- Licari, A.; D’Auria, E.; De Amici, M.; Castagnoli, R.; Sacchi, L.; Testa, G.; Marseglia, G.L. The Role of Basophil Activation Test and Component-Resolved Diagnostics in the Workup of Egg Allergy in Children at Low Risk for Severe Allergic Reactions: A Real-Life Study. Pediatric Allergy and Immunology 2023, 34. [Google Scholar] [CrossRef]
- Foong, R.X.; Santos, A.F. Biomarkers of Diagnosis and Resolution of Food Allergy. Pediatric Allergy and Immunology 2021, 32, 223–233. [Google Scholar] [CrossRef]
- Wasserman, R.L. A Diagnostic Approach to IgE-Mediated Food Allergy: A Practical Algorithm. Journal of Food Allergy 2024, 6, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Boyd, H.; Santos, A.F. Novel Diagnostics in Food Allergy. Journal of Allergy and Clinical Immunology 2025, 155, 275–285. [Google Scholar] [CrossRef]
- Krzych-Fałta, E.; Białek, S.; Sybilski, A.J.; Tylewicz, A.; Samoliński, B.; Wojas, O. Differential Diagnostics of Food Allergy as Based on Provocation Tests and Laboratory Diagnostic Assays. Postepy Dermatol Alergol 2023, 40, 709–715. [Google Scholar] [CrossRef]
- Bahbah, W.A.; Abo Hola, A.S.; Bedair, H.M.; Taha, E.T.; El Zefzaf, H.M.S. Serum Eosinophil-Derived Neurotoxin: A New Promising Biomarker for Cow’s Milk Allergy Diagnosis. Pediatr Res 2024, 96, 1812–1821. [Google Scholar] [CrossRef]
- Sampson, H.A.; Gerth Van Wijk, R.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.J.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing Double-Blind, Placebo-Controlled Oral Food Challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL Consensus Report. Journal of Allergy and Clinical Immunology 2012, 130, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Arasi, S.; Bahnson, H.T.; Ballmer-Weber, B.; Beyer, K.; Bindslev-Jensen, C.; Bird, J.A.; Blumchen, K.; Davis, C.; Ebisawa, M.; et al. AAAAI–EAACI PRACTALL: Standardizing Oral Food Challenges—2024 Update. Pediatric Allergy and Immunology 2024, 35. [Google Scholar] [CrossRef] [PubMed]
- Çelebioğlu, E.; Akarsu, A.; Şahiner, Ü.M. Ige-Mediated Food Allergy throughout Life. Turk J Med Sci 2021, 51, 49–60. [Google Scholar] [CrossRef]
- Calvani, M.; Bianchi, A.; Reginelli, C.; Peresso, M.; Testa, A. Oral Food Challenge. Medicina (Lithuania) 2019, 55. [Google Scholar] [CrossRef]
- Hsu, E.; Soller, L.; Abrams, E.M.; Protudjer, J.L.P.; Mill, C.; Chan, E.S. Oral Food Challenge Implementation: The First Mixed-Methods Study Exploring Barriers and Solutions. Journal of Allergy and Clinical Immunology: In Practice 2020, 8, 149–156.e1. [Google Scholar] [CrossRef]
- Ishikawa, F.; Miyaji, Y.; Yamamoto-Hanada, K.; Shimada, M.; Kiguchi, T.; Hirai, S.; Kram, Y.E.; Takada, K.; Noguchi, K.; Inuzuka, Y.; et al. Safer Oral Immunotherapy with Very Low-Dose Introduction for Pediatric Hen’s Egg Allergy. Journal of Allergy and Clinical Immunology: Global 2025, 100542. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, K.; Kim, J. Practical Issues of Oral Immunotherapy for Egg or Milk Allergy. Clin Exp Pediatr 2024, 67, 140–148. [Google Scholar] [CrossRef]
- Hund, S.K.; Sampath, V.; Zhou, X.; Thai, B.; Desai, K.; Nadeau, K.C. Scientific Developments in Understanding Food Allergy Prevention, Diagnosis, and Treatment. Front Immunol 2025, 16. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, N.; Minoura, T.; Kitaoka, S.; Ebisawa, M. A Three-Level Stepwise Oral Food Challenge for Egg, Milk, and Wheat Allergy. Journal of Allergy and Clinical Immunology: In Practice 2018, 6, 658–660.e10. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Patel, N.; Campbell, D.E.; Sampson, H.A.; Maeda, M.; Katsunuma, T.; Westerhout, J.; Blom, W.M.; Baumert, J.L.; Houben, G.F.; et al. Reproducibility of Food Challenge to Cow’s Milk: Systematic Review with Individual Participant Data Meta-Analysis. Journal of Allergy and Clinical Immunology 2022, 150, 1135–1143.e8. [Google Scholar] [CrossRef] [PubMed]
- Ohara, Y.; Matsumoto, Y.; Fujita, M.; Inuo, C. Comparative Safety of Baked Egg, Egg Yolk, and Egg White in Young Children with Egg Allergy. Int Arch Allergy Immunol 2025, 1–13. [Google Scholar] [CrossRef]
- Upton, J.E.M.; Lanser, B.J.; Bird, J.A.; Nowak-Węgrzyn, A. Baked Milk and Baked Egg Survey: A Work Group Report of the AAAAI Adverse Reactions to Foods Committee. Journal of Allergy and Clinical Immunology: In Practice 2023, 11, 2335–2344.e4. [Google Scholar] [CrossRef]
- Matthai, J.; Sathiasekharan, M.; Poddar, U.; Sibal, A.; Srivastava, A.; Waikar, Y.; Malik, R. Guidelines on Diagnosis and Management of Cow’s Milk Protein Allergy. Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition; Pediatric Gastroenterology Chapter of Indian Academy of Pediatrics. Guidelines on Diagnosis and Management of Cow's Milk Protein Allergy. Indian Pediatr 2020, 57(8), 723–729. [Google Scholar] [PubMed]
- Tosca, M.A.; Naso, M.; Trincianti, C.; Schiavetti, I.; Ciprandi, G. Reaction Severity to Oral Food Challenge to Milk Is Unpredictable: A Caveat for Clinical Practice. Acta Biomedica 2024, 95. [Google Scholar] [CrossRef]
- Laha, A.; Bhattacharya, S.; Moitra, S.; Saha, N.C.; Biswas, H.; Podder, S. Assessment of Egg and Milk Allergies among Indians by Revalidating a Food Allergy Predictive Model. World Allergy Organization Journal 2022, 15. [Google Scholar] [CrossRef]
- Ünsal, H.; Gökçe, Ö.B.G.; Ocak, M.; Akarsu, A.; Şahiner, Ü.M.; Soyer, Ö.; Şekerel, B.E. Oral Food Challenge in IgE Mediated Food Allergy in Eastern Mediterranean Children. Allergol Immunopathol (Madr) 2021, 49, 185–192. [Google Scholar] [CrossRef]
- Itazawa, T.; Adachi, Y.; Takahashi, Y.; Miura, K.; Uehara, Y.; Kameda, M.; Kitamura, T.; Kuzume, K.; Tezuka, J.; Ito, K.; et al. The Severity of Reaction after Food Challenges Depends on the Indication: A Prospective Multicenter Study. Pediatric Allergy and Immunology 2020, 31, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.A.; Leonard, S.; Groetch, M.; Assa’ad, A.; Cianferoni, A.; Clark, A.; Crain, M.; Fausnight, T.; Fleischer, D.; Green, T.; et al. Conducting an Oral Food Challenge: An Update to the 2009 Adverse Reactions to Foods Committee Work Group Report. Journal of Allergy and Clinical Immunology: In Practice 2020, 8, 75–90.e17. [Google Scholar] [CrossRef]
- Upton, J.E.M.; Bird, J.A. Oral Food Challenges: Special Considerations. Annals of Allergy, Asthma and Immunology 2020, 124, 451–458. [Google Scholar] [CrossRef]
- Ebisawa, M.; Ito, K.; Fujisawa, T.; Aihara, Y.; Ito, S.; Imai, T.; Ohshima, Y.; Ohya, Y.; Kaneko, H.; Kondo, Y.; et al. Japanese Guidelines for Food Allergy 2020. Allergology International 2020, 69, 370–386. [Google Scholar] [CrossRef]
- Nakanishi, K.; Okafuji, I.; Kihara, T.; Kawanami, Y.; Shimizu, A.; Kotani, S.; Kamoi, Y.; Okutani, T. Safety of Low-Dose Oral Food Challenges for Hen’s Eggs, Cow’s Milk, and Wheat: Report from a General Hospital without Allergy Specialists in Japan. 2023, Vol. 69. [Google Scholar]
- Assa’ad, A.H. Oral Food Challenges. Journal of Food Allergy 2020, 2, 31–34. [Google Scholar] [CrossRef]
- Coimbra, M.R.; Araújo, L.M.L.; Filho, N.A.R. Oral Food Challenge in Children with Contact Urticaria in Reaction to Cow’s Milk. Allergol Immunopathol (Madr) 2023, 51, 93–98. [Google Scholar] [CrossRef]
- Greiwe, J. Using Oral Food Challenges to Provide Clarity and Confidence When Diagnosing Food Allergies. Journal of Food Allergy 2021, 3, 3–7. [Google Scholar] [CrossRef]
- Nishino, M.; Yanagida, N.; Sato, S.; Nagakura, K. ichi; Takahashi, K.; Ogura, K.; Ebisawa, M. Risk Factors for Failing a Repeat Oral Food Challenge in Preschool Children with Hen’s Egg Allergy. Pediatric Allergy and Immunology 2022, 33. [Google Scholar] [CrossRef]
- Jacob, J.G.; Fernando, S.L.; Nickolls, C.; Li, J. Oral Food Challenge Outcomes in Children and Adolescents in a Tertiary Centre: A 5-Year Experience. J Paediatr Child Health 2023, 59, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, N.; Sato, S.; Asaumi, T.; Ogura, K.; Ebisawa, M. Risk Factors for Severe Reactions during Double-Blind Placebo-Controlled Food Challenges. Int Arch Allergy Immunol 2017, 172, 173–182. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, K.; Park, M.; Roh, Y.Y.; Jung, J.H.; Kim, S.Y.; Kim, J.D.; Kim, M.J.; Kim, Y.H.; Sohn, M.H.; et al. Predicting the Outcome of Pediatric Oral Food Challenges for Determining Tolerance Development. Allergy Asthma Immunol Res 2024, 16, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Koutlas, N.; Stallings, A.; Hall, G.; Zhou, C.; Kim-Chang, J.; Mousallem, T. Pediatric Oral Food Challenges in the Outpatient Setting: A Single-Center Experience. Journal of Allergy and Clinical Immunology: Global 2024, 3. [Google Scholar] [CrossRef] [PubMed]
- Klim, L.; Michalik, M.; Cichocka-Jarosz, E.; Jedynak-Wąsowicz, U. Asthma and Multi-Food Allergy Are Risk Factors for Oral Food Challenge Failure-A Single-Center Experience. Nutrients 2025, 17. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Assa’ad, A.H.; Bahna, S.L.; Bock, S.A.; Sicherer, S.H.; Teuber, S.S. Work Group Report: Oral Food Challenge Testing. Journal of Allergy and Clinical Immunology 2009, 123. [Google Scholar] [CrossRef]
- Leonard, S.A.; Caubet, J.C.; Kim, J.S.; Groetch, M.; Nowak-Wegrzyn, A. Baked Milk- and Egg-Containing Diet in the Management of Milk and Egg Allergy. Journal of Allergy and Clinical Immunology: In Practice 2015, 3, 13–23. [Google Scholar] [CrossRef]
- Sampson, H.A. Anaphylaxis and Emergency Treatment. Pediatrics 2003, 111, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.; Feeney, M.; Yerlett, N.; Meyer, R. Nutritional Management of Children with Food Allergies. Curr Treat Options Allergy 2022, 9, 375–393. [Google Scholar] [CrossRef]
- Di Cesare, G.; Carciofi, A.; Borgiani, F.; Cappelletti, D.; Correani, A.; Monachesi, C.; Gatti, S.; Lionetti, M.E. Are We Meeting the Needs? A Systematic Review of Nutritional Gaps and Growth Outcomes in Children with Multiple Food Allergies. Nutrients 2025, 17. [Google Scholar] [CrossRef]
- Kotchetkoff, E.C. de A.; de Oliveira, L.C.L.; Sarni, R.O.S. Elimination Diet in Food Allergy: Friend or Foe? J Pediatr (Rio J) 2024, 100, S65–S73. [Google Scholar] [CrossRef]
- Bird, J.A.; Crain, M.; Varshney, P. Food Allergen Panel Testing Often Results in Misdiagnosis of Food Allergy. Journal of Pediatrics 2015, 166, 97–100.e1. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.P. A Review of Food Allergy Panels and Their Consequences. Annals of Allergy, Asthma and Immunology 2023, 131, 421–426. [Google Scholar] [CrossRef]
- Herber, C.; Bogler, L.; Subramanian, S. V.; Vollmer, S. Association between Milk Consumption and Child Growth for Children Aged 6–59 Months. Sci Rep 2020, 10. [Google Scholar] [CrossRef]
- Larson, E.A.; Zhao, Z.; Bader-Larsen, K.S.; Magkos, F. Egg Consumption and Growth in Children: A Meta-Analysis of Interventional Trials. Front Nutr 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Mahfuz, M.; Alam, M.A.; Das, S.; Fahim, S.M.; Hossain, M.S.; Petri, W.A.; Ashorn, P.; Ashorn, U.; Ahmed, T. Daily Supplementation with Egg, Cow Milk, and Multiple Micronutrients Increases Linear Growth of Young Children with Short Stature. Journal of Nutrition 2020, 150, 394–403. [Google Scholar] [CrossRef]
- Corica, D.; Aversa, T.; Caminiti, L.; Lombardo, F.; Wasniewska, M.; Pajno, G.B. Nutrition and Avoidance Diets in Children With Food Allergy. Front Pediatr 2020, 8. [Google Scholar] [CrossRef]
- Rodrigues, V.C.D.C.; Speridião, P.D.G.L.; Sanudo, A.; Morais, M.B. Feeding Difficulties in Children Fed a Cows’ Milk Elimination Diet. British Journal of Nutrition 2022, 128, 1190–1199. [Google Scholar] [CrossRef]
- Dziechciarz, P.; Stróżyk, A.; Horvath, A.; Cudowska, B.; Jedynak-Wąsowicz, U.; Mól, N.; Jarocka-Cyrta, E.; Zawadzka-Krajewska, A.; Krauze, A. Nutritional Status and Feeding Difficulties in Children up to 2 Years of Age with Cow’s Milk Allergy. J Pediatr Gastroenterol Nutr 2024, 79, 131–139. [Google Scholar] [CrossRef]
- Miyagi, Y.; Yamamoto-Hanada, K.; Ogita, H.; Kiguchi, T.; Inuzuka, Y.; Toyokuni, K.; Nishimura, K.; Irahara, M.; Ishikawa, F.; Sato, M.; et al. Avoidance of Hen’s Egg Based on IgE Levels Should Be Avoided for Children With Hen’s Egg Allergy. Front Pediatr 2021, 8. [Google Scholar] [CrossRef]
- Gonzalez, P.M.; Cassin, A.M.; Durban, R.; Upton, J.E.M. Effects of Food Processing on Allergenicity. Curr Allergy Asthma Rep 2025, 25, 9. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Bloom, K.A.; Sicherer, S.H.; Shreffler, W.G.; Noone, S.; Wanich, N.; Sampson, H.A. Tolerance to Extensively Heated Milk in Children with Cow’s Milk Allergy. Journal of Allergy and Clinical Immunology 2008, 122. [Google Scholar] [CrossRef] [PubMed]
- Gruzelle, V.; Juchet, A.; Martin-Blondel, A.; Michelet, M.; Chabbert-Broue, A.; Didier, A. Benefits of Baked Milk Oral Immunotherapy in French Children with Cow’s Milk Allergy. Pediatric Allergy and Immunology 2020, 31, 364–370. [Google Scholar] [CrossRef]
- Kim, J.S.; Nowak-Węgrzyn, A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Sampson, H.A. Dietary Baked Milk Accelerates the Resolution of Cow’s Milk Allergy in Children. Journal of Allergy and Clinical Immunology 2011, 128, 125–131.e2. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.A.; Sampson, H.A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Godbold, J.; Nowak-Wegrzyn, A. Dietary Baked Egg Accelerates Resolution of Egg Allergy in Children. Journal of Allergy and Clinical Immunology 2012, 130. [Google Scholar] [CrossRef]
- Giannetti, A.; Toschi Vespasiani, G.; Ricci, G.; Miniaci, A.; Di Palmo, E.; Pession, A. Cow’s Milk Protein Allergy as a Model of Food Allergies. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Gantulga, P.; Lee, J.; Jeong, K.; Jeon, S.A.; Lee, S. Variation in the Allergenicity of Scrambled, Boiled, Short-Baked and Long-Baked Egg White Proteins. J Korean Med Sci 2024, 39. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Meyer, R.; Nowak-Wegrzyn, A.; Salvatore, S.; Venter, C.; Vieira, M.C. The Remaining Challenge to Diagnose and Manage Cow’s Milk Allergy: An Opinion Paper to Daily Clinical Practice. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Upton, J.E.M.; Wong, D.; Nowak-Wegrzyn, A. Baked Milk and Egg Diets Revisited. Annals of Allergy, Asthma and Immunology 2024, 132, 328–336.e5. [Google Scholar] [CrossRef]
- Buyuktiryaki, B.; Soyer, O.; Bingol, G.; Can, C.; Nacaroglu, H.T.; Bingol, A.; Arik Yilmaz, E.; Aydogan, M.; Sackesen, C. Milk Ladder: Who? When? How? Where? With the Lowest Risk of Reaction. Frontiers in Allergy 2024, 5. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Meyer, R.; Groetch, M.; Nowak-Wegrzyn, A.; Mennini, M.; Pawankar, R.; Kamenwa, R.; Assa’ad, A.; Amara, S.; Fiocchi, A.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines Update – XVI - Nutritional Management of Cow’s Milk Allergy. World Allergy Organization Journal 2024, 17. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.R.; Vázquez, D.; Machinena-Spera, A.; Jiménez, M. del M.F.; Lozano-Blasco, J.; Feijoo, R.J.; Sánchez, O.D.; Gibert, M.P.; Costa, M.D.; Alvaro-Lozano, M. Tolerance to Cooked Egg in Infants with Risk Factors for Egg Allergy after Early Introduction of Baked Egg. Allergol Immunopathol (Madr) 2025, 53, 59–66. [Google Scholar] [CrossRef] [PubMed]
- van Boven, F.E.; Arends, N.J.T.; Sprikkelman, A.B.; Emons, J.A.M.; Hendriks, A.I.; van Splunter, M.; Schreurs, M.W.J.; Terlouw, S.; Gerth van Wijk, R.; Wichers, H.J.; et al. Tolerance Induction in Cow’s Milk Allergic Children by Heated Cow’s Milk Protein: The IAGE Follow-Up Study. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Ballini, G.; Gavagni, C.; Guidotti, C.; Ciolini, G.; Liccioli, G.; Giovannini, M.; Sarti, L.; Ciofi, D.; Novembre, E.; Mori, F.; et al. Frequency of Positive Oral Food Challenges and Their Outcomes in the Allergy Unit of a Tertiary-Care Pediatric Hospital. Allergol Immunopathol (Madr) 2021, 49, 120–130. [Google Scholar] [CrossRef]
- Ogata, M.; Kido, J.; Yoshida, T.; Nishi, N.; Shimomura, S.; Hirai, N.; Mizukami, T.; Yanai, M.; Nakamura, K. The Efficacy and Safety of Stepwise Oral Food Challenge in Children with Hen’s Egg Allergy. Allergy, Asthma & Clinical Immunology 2024, 20, 67. [Google Scholar] [CrossRef]
- Emeksiz, Z.S.; Ertugrul, A.; Ozmen, S.; Cavkaytar, O.; Ercan, N.; Bostancl, I.B. Is Oral Food Challenge as Safe Enough as It Seems? J Trop Pediatr 2021, 67. [Google Scholar] [CrossRef]
- Uekert, S.J.; Akan, G.; Evans, M.D.; Li, Z.; Roberg, K.; Tisler, C.; DaSilva, D.; Anderson, E.; Gangnon, R.; Allen, D.B.; et al. Sex-Related Differences in Immune Development and the Expression of Atopy in Early Childhood. Journal of Allergy and Clinical Immunology 2006, 118, 1375–1381. [Google Scholar] [CrossRef]
- Honda, A.; Imai, T.; Okada, Y.; Maeda, M.; Kamiya, T. Severe Anaphylaxis Requiring Continuous Adrenaline Infusion during Oral Food Challenge: A Case Series. Annals of Allergy, Asthma and Immunology 2024. [Google Scholar] [CrossRef]
- Vininski, M.S.; Rajput, S.; Hobbs, N.J.; Dolence, J.J. Understanding Sex Differences in the Allergic Immune Response to Food. AIMS Allergy Immunol 2022, 6, 90–105. [Google Scholar] [CrossRef]
- Leffler, J.; Stumbles, P.A.; Strickland, D.H. Immunological Processes Driving IgE Sensitisation and Disease Development in Males and Females. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Sindher, S.; Long, A.J.; Purington, N.; Chollet, M.; Slatkin, S.; Andorf, S.; Tupa, D.; Kumar, D.; Woch, M.A.; O’Laughlin, K.L.; et al. Analysis of a Large Standardized Food Challenge Data Set to Determine Predictors of Positive Outcome across Multiple Allergens. Front Immunol 2018, 9. [Google Scholar] [CrossRef]
- Yagmur, I.T.; Celik, I.K.; Topal, O.Y.; Toyran, M.; Civelek, E.; Misirlioglu, E.D. Development of Anaphylaxis upon Oral Food Challenge and Drug Provocation Tests in Pediatric Patients. Allergy Asthma Proc 2023, 44, 326–332. [Google Scholar] [CrossRef]
- Aquilante, B.P.; Castro, A.P.B.M.; Yonamine, G.H.; de Barros Dorna, M.; Barp, M.F.; Martins, T.P. da R.; Pastorino, A.C. IgE-Mediated Cow’s Milk Allergy in Brazilian Children: Outcomes of Oral Food Challenge. World Allergy Organization Journal 2023, 16. [Google Scholar] [CrossRef]
- Hill, S.A.; Nurmatov, U.; DunnGalvin, A.; Reese, I.; Vieira, M.C.; Rommel, N.; Dupont, C.; Venter, C.; Cianferoni, A.; Walsh, J.; et al. Feeding Difficulties in Children with Food Allergies: An EAACI Task Force Report. Pediatric Allergy and Immunology 2024, 35. [Google Scholar] [CrossRef] [PubMed]
- Valluzzi, R.L.; Riccardi, C.; Arasi, S.; Piscitelli, A.L.; Calandrelli, V.; Dahdah, L.; Fierro, V.; Mennini, M.; Fiocchi, A. Cow’s Milk and Egg Protein Threshold Dose Distributions in Children Tolerant to Beef, Baked Milk, and Baked Egg. Allergy: European Journal of Allergy and Clinical Immunology 2022, 77, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Yonkof, J.R.; Mikhail, I.J.; Prince, B.T.; Stukus, D. Delayed and Severe Reactions to Baked Egg and Baked Milk Challenges. Journal of Allergy and Clinical Immunology: In Practice 2021, 9, 283–289.e2. [Google Scholar] [CrossRef]
- Upton, J.; Alvaro, M.; Nadeau, K. A Perspective on the Pediatric Death from Oral Food Challenge Reported from the Allergy Vigilance Network. Allergy: European Journal of Allergy and Clinical Immunology 2019, 74, 1035–1036. [Google Scholar] [CrossRef]
- Singh, A.M.; Anvari, S.; Hauk, P.; Lio, P.; Nanda, A.; Sidbury, R.; Schneider, L. Atopic Dermatitis and Food Allergy: Best Practices and Knowledge Gaps—A Work Group Report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. Journal of Allergy and Clinical Immunology: In Practice 2022, 10, 697–706. [Google Scholar] [CrossRef]
- Abrams, E.M.; Becker, A.B. Oral Food Challenge Outcomes in a Pediatric Tertiary Care Center. Allergy, Asthma and Clinical Immunology 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Papapostolou, N.; Xepapadaki, P.; Gregoriou, S.; Makris, M. Atopic Dermatitis and Food Allergy: A Complex Interplay What We Know and What We Would Like to Learn. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Trogen, B.; Verma, M.; Sicherer, S.H.; Cox, A. The Role of Food Allergy in Atopic Dermatitis. Dermatol Clin 2024, 42, 527–535. [Google Scholar] [CrossRef]
- Hui-Beckman, J.W.; Goleva, E.; Berdyshev, E.; Leung, D.Y.M. Endotypes of Atopic Dermatitis and Food Allergy. Journal of Allergy and Clinical Immunology 2023, 151, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Thongsukkaeo, S.; Suksawat, Y. Early-Life Risk Factors and Clinical Features of Food Allergy Among Thai Children. International Journal of Pediatrics (United Kingdom) 2024, 2024. [Google Scholar] [CrossRef]
- Saito-Abe, M.; Yamamoto-Hanada, K.; Pak, K.; Iwamoto, S.; Sato, M.; Miyaji, Y.; Mezawa, H.; Nishizato, M.; Yang, L.; Kumasaka, N.; et al. How a Family History of Allergic Diseases Influences Food Allergy in Children: The Japan Environment and Children’s Study. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Galletta, F.; Bencivenga, C.L.; Bettini, I.; Klain, A.; D’Addio, E.; Mori, F.; Licari, A.; Miraglia Del Giudice, M.; Indolfi, C. Food Allergy Risk: A Comprehensive Review of Maternal Interventions for Food Allergy Prevention. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Correa, N.; L. P. Protudjer, J.; Hsu, E.; Soller, L.; Chan, E.S.; Kim, H.; Jeimy, S. Canadian Parent Perceptions of Oral Food Challenges: A Qualitative Analysis. Pediatric Allergy and Immunology 2022, 33. [Google Scholar] [CrossRef]
- Skoczylas, D.; Gujski, M.; Bojar, I.; Raciborski, F. Importance of Food Allergy and Food Intolerance in Allergic Multimorbidity. Annals of Agricultural and Environmental Medicine 2020, 27, 413–417. [Google Scholar] [CrossRef]
- Purington, N.; Chinthrajah, R.S.; Long, A.; Sindher, S.; Andorf, S.; O’Laughlin, K.; Woch, M.A.; Scheiber, A.; Assa’Ad, A.; Pongracic, J.; et al. Eliciting Dose and Safety Outcomes from a Large Dataset of Standardized Multiple Food Challenges. Front Immunol 2018, 9. [Google Scholar] [CrossRef]
- Kennedy, K.; Alfaro, M.K.C.; Spergel, Z.C.; Dorris, S.L.; Spergel, J.M.; Capucilli, P. Differences in Oral Food Challenge Reaction Severity Based on Increasing Age in a Pediatric Population. Annals of Allergy, Asthma and Immunology 2021, 127, 562–567.e1. [Google Scholar] [CrossRef] [PubMed]
- Emons, J.A.M.; Gerth van Wijk, R. Food Allergy and Asthma: Is There a Link? Curr Treat Options Allergy 2018, 5, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Foong, R.X.; du Toit, G.; Fox, A.T. Asthma, Food Allergy, and How They Relate to Each Other. Front Pediatr 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xu, W.; Huang, H.; Hou, X.; Xiang, L. Anaphylaxis in Chinese Children: Different Clinical Profile Between Children with and without a History of Asthma/Recurrent Wheezing. J Asthma Allergy 2022, 15, 1093–1104. [Google Scholar] [CrossRef]
- Dribin, T.E.; Michelson, K.A.; Zhang, Y.; Schnadower, D.; Neuman, M.I. Are Children with a History of Asthma More Likely to Have Severe Anaphylactic Reactions? A Retrospective Cohort Study. Journal of Pediatrics 2020, 220, 159–164.e2. [Google Scholar] [CrossRef]
- Cunico, D.; Giannì, G.; Scavone, S.; Buono, E.V.; Caffarelli, C. The Relationship Between Asthma and Food Allergies in Children. Children 2024, 11. [Google Scholar] [CrossRef]
- Stukus, D.R.; Prince, B.T. Asthma and Food Allergy: A Nuanced Relationship. Journal of Food Allergy 2023, 5, 33–37. [Google Scholar] [CrossRef]
- Di Palmo, E.; Gallucci, M.; Cipriani, F.; Bertelli, L.; Giannetti, A.; Ricci, G. Asthma and Food Allergy: Which Risks? Medicina (Lithuania) 2019, 55. [Google Scholar] [CrossRef]
- De Boer, R.; Cartledge, N.; Lazenby, S.; Tobias, A.; Chan, S.; Fox, A.T.; Santos, A.F. Specific IgE as the Best Predictor of the Outcome of Challenges to Baked Milk and Baked Egg. Journal of Allergy and Clinical Immunology: In Practice 2020, 8, 1459–1461.e5. [Google Scholar] [CrossRef]
- Yanagida, N.; Sato, S.; Ebisawa, M. Relationship between Eliciting Doses and the Severity of Allergic Reactions to Food. Curr Opin Allergy Clin Immunol 2023, 23, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, N.; Sato, S.; Takahashi, K.; Nagakura, K.I.; Asaumi, T.; Ogura, K.; Ebisawa, M. Increasing Specific Immunoglobulin E Levels Correlate with the Risk of Anaphylaxis during an Oral Food Challenge. Pediatric Allergy and Immunology 2018, 29, 417–424. [Google Scholar] [CrossRef]
- Al Hawi, Y.; Nagao, M.; Furuya, K.; Sato, Y.; Ito, S.; Hori, H.; Hirayama, M.; Fujisawa, T. Agreement between Predictive, Allergen-Specific IgE Values Assessed by ImmunoCAP and IMMULITE 2000 3gAllergyTM Assay Systems for Milk and Wheat Allergies. Allergy Asthma Immunol Res 2021, 13, 141–153. [Google Scholar] [CrossRef]
- Sasaki, Y.; Matsunami, K.; Kondo, M.; Matsukuma, E.; Imamura, A.; Kaneko, H. Oral Food Challenge Test Results of Patients with Food Allergy with Specific. Biomed Rep 2024, 21. [Google Scholar] [CrossRef]
- Yoshida, T.; Kido, J.; Ogata, M.; Watanabe, S.; Nishi, N.; Shimomura, S.; Hirai, N.; Tanaka, K.; Yanai, M.; Mizukami, T.; et al. Safety of Oral Food Challenges for Individuals with Low Levels of Cow’s Milk-Specific Immunoglobulin e Antibodies. Int Arch Allergy Immunol 2025, 186, 543–550. [Google Scholar] [CrossRef]
- Greiwe, J. Oral Food Challenges in Infants and Toddlers. Immunol Allergy Clin North Am 2019, 39, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Gawryjołek, J.; Wycech, A.; Smyk, A.; Krogulska, A. Difficulties in Interpretation of Oral Food Challenge Results. Postepy Dermatol Alergol 2021, 38, 721–726. [Google Scholar] [CrossRef]
- Fashho, K.; Garber, M.; Yousef, E. Interpreting Serum-Specific IgE Panels: Key Insights for Pediatricians in Diagnosing Food Allergies in Children. Journal of Pediatric Health Care 2025. [Google Scholar] [CrossRef]
- Tosca, M.A.; Schiavetti, I.; Olcese, R.; Trincianti, C.; Ciprandi, G. Molecular Allergy Diagnostics in Children with Cow’s Milk Allergy: Prediction of Oral Food Challenge Response in Clinical Practice. J Immunol Res 2023. [Google Scholar] [CrossRef]
- Nieminen, O.; Palosuo, K.; Mäkelä, M.J. Casein SIgE as the Most Accurate Predictor for Heated Milk Tolerance in Finnish Children. Pediatric Allergy and Immunology 2025, 36. [Google Scholar] [CrossRef] [PubMed]
- Ayats-Vidal, R.; Valdesoiro-Navarrete, L.; García-González, M.; Asensio-De la Cruz, O.; Larramona-Carrera, H.; Bosque-García, M. Predictors of a Positive Oral Food Challenge to Cow’s Milk in Children Sensitized to Cow’s Milk. Allergol Immunopathol (Madr) 2020, 48, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.W.; Goh, S.H.; Saffari, S.E.; Loh, W.; Sia, I.; Seah, S.; Goh, A. IgE-Mediated Cow’s Milk Protein Allergy in Singaporean Children. Asian Pac J Allergy Immunol 2022, 40, 65–71. [Google Scholar] [CrossRef]
- Domínguez, O.; Riggioni, C.; Poyatos, E.; Jiménez-Feijoo, R.M.; Piquer, M.; Machinena, A.; Folqué, M.; Ortiz de Landazuri, I.; Torradeflot, M.; Lozano, J.; Alsina, L.; Pascal, M.; Alvaro-Lozano, M. Biomarkers of Tolerance to Baked Milk in Cow’s Milk-Allergic Children at High Risk of Anaphylaxis. J Investig Allergol Clin Immunol 2026, 36. [Google Scholar] [CrossRef]
- Kilic, M.; Çilkol, L.; Taşkın, E. Evaluation of Some Predictive Parameters for Baked-Milk Tolerance in Children with Cow’s Milk Allergy. Allergol Immunopathol (Madr) 2021, 49, 53–59. [Google Scholar] [CrossRef]
- Rodríguez-Catalán, J.; González-Arias, A.M.; Casas, A.V.; Camacho, G.D.R. Specific IgE Levels as an Outcome Predictor in Egg-Allergic Children. Allergol Immunopathol (Madr) 2021, 49, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.S.; dos Santos, G.M.; Sangalho, I.; Rosa, S.; Pinto, P.L. Role of Serum-Specific Immunoglobulin E in Egg Allergy: A Comprehensive Study of Portuguese Pediatric Patients. Allergol Immunopathol (Madr) 2024, 52, 53–59. [Google Scholar] [CrossRef]
- Perry, T.T.; Matsui, E.C.; Kay Conover-Walker, M.; Wood, R.A. The Relationship of Allergen-Specific IgE Levels and Oral Food Challenge Outcome. Journal of Allergy and Clinical Immunology 2004, 114, 144–149. [Google Scholar] [CrossRef]
- Delli Colli, L.; Yu, J.; Lanoue, D.; Mir, A.; Cohen, C.G.; Toscano-Rivero, D.; Sacksner, J.; Mazer, B.; Mccusker, C.; Ke, D.; et al. Predicting Cow’s Milk Challenge Outcomes in Children: Multivariate Analysis of Clinical Predictors. Int Arch Allergy Immunol 2025. [Google Scholar] [CrossRef]
- Maesa, J.M.; Dobrzynska, A.; Baños-Álvarez, E.; Isabel-Gómez, R.; Blasco-Amaro, J.A. ImmunoCAP ISAC in Food Allergy Diagnosis: A Systematic Review of Diagnostic Test Accuracy. Clinical and Experimental Allergy 2021, 51, 778–789. [Google Scholar] [CrossRef]
- Cigerci Gunaydin, N. Effects of Cow’s Milk Components, Goat’s Milk and Sheep’s Milk Sensitivities on Clinical Findings and Tolerance Development in Cow’s Milk Allergy. SiSli Etfal Hastanesi Tip Bulteni / The Medical Bulletin of Sisli Hospital 2020. [Google Scholar] [CrossRef]
- Cronin, C.; Muñoz Archidona, C.; Fernández Prudencio, B.; Gallagher, A.; Velasco Zuniga, R.; Trujillo Wurttele, J. Real-Life Use of Component-Specific IgE in IgE-Mediated Cow’s Milk Protein Allergy in a Spanish Paediatric Allergy Centre. Antibodies 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Dodi, G.; Di Filippo, P.; Di Pillo, S.; Chiarelli, F.; Attanasi, M. Total Serum IgE Levels as Predictor of the Acquisition of Tolerance in Children with Food Allergy: Findings from a Pilot Study. Front Pediatr 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, M.; Farias, R.; Mello, R.G.; Prando, C. Biomarkers Associated with Persistence and Severity of IgE-Mediated Food Allergies: A Systematic Review. J Pediatr (Rio J) 2023, 99, 315–321. [Google Scholar] [CrossRef] [PubMed]
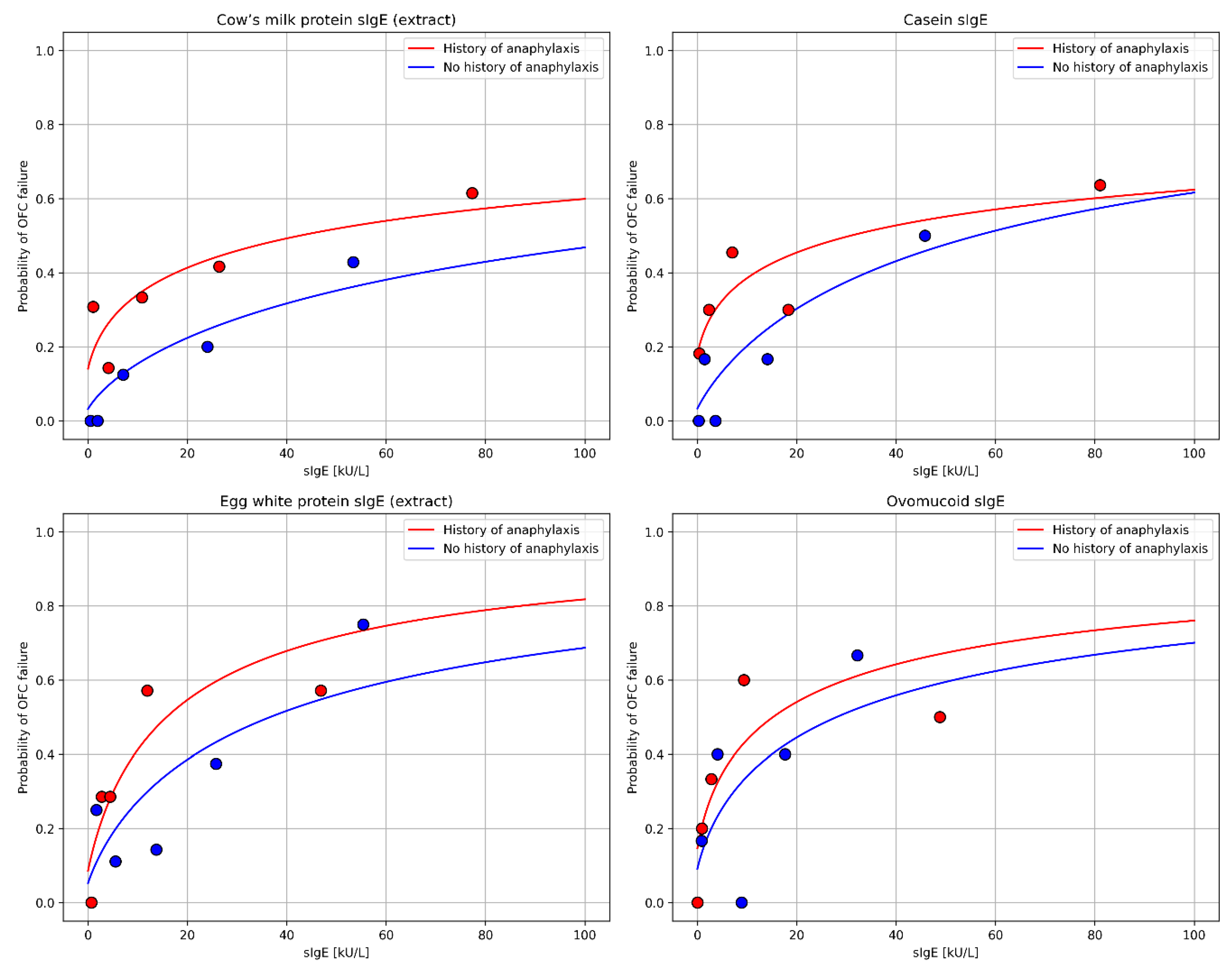
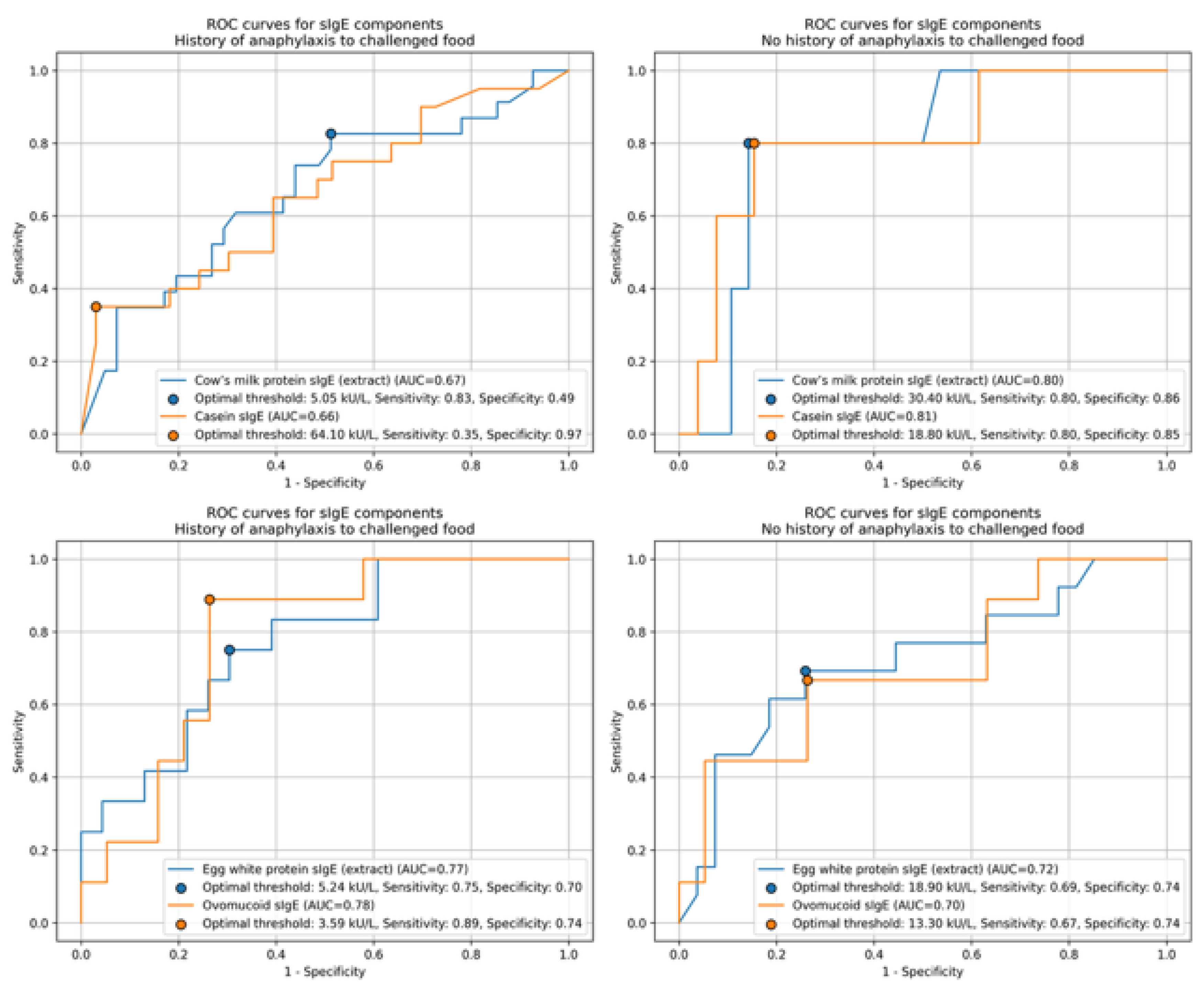
| Feature | Value |
|---|---|
| Number of patients | 192* |
| Median age [IQR], (years) | 4.75 [3.32-6.82] |
| Gender, n (%) | |
| Male | 127 (66.1%) |
| Female | 65 (33.9%) |
| Allergen challenged in the OFC, n (%) | |
| Cow’s milk protein (CMP) | 106 (55.2%) |
| Hen’s egg white protein (HEWP) | 86 (44.8%) |
| Median time from food consumption to reaction occurrence [IQR], (minutes) | 90.0 [60.0-120.0] |
| Accompanying atopic diseases | |
| Multi-food allergy | 147 (76.6%) |
| Atopic dermatitis | 120 (62.5%) |
| Asthma | 97 (50.5%) |
| Family history of atopy | 85 (44.3%) |
| History of anaphylaxis to the challenged food# | 110 (57.3%) |
| Grade 2 | 39 (35.5%) |
| Grade 3 | 34 (30.9%) |
| Grade 4 | 35 (31.8%) |
| Grade 5 | 2 (1.8%) |
| Feature | History of anaphylaxis to the challenged food* (n=110) |
No history of anaphylaxis to the challenged food* (n=82) |
p |
|---|---|---|---|
| No. of failed OFCs | 39 (35.5%) | 23 (28.1%) | 0.46 |
| Median age [IQR], (years) | 4.57 [2.96-6.87] | 5.04 [3.70-6.78] | 0.45 |
| Gender, n (%) | |||
| Male | 69 (62.7%) | 58 (70.7%) | 0.25 |
| Female | 41 (37.3%) | 24 (29.3%) | |
| Allergen challenged in the OFC, n (%) | |||
| Cow’s milk protein (CMP) | 70 (63.6%) | 36 (43.9%) | 0.01 |
| Hen’s egg white protein (HEWP) | 40 (36.4%) | 46 (50.1%) | |
| Median time from food consumption to reaction occurrence [IQR], (minutes) | 90.0 [65.0-150.0] | 90.0 [50.0-120.0] | 0.67 |
| Accompanying atopic diseases | |||
| Multi-food allergy | 81 (73.6%) | 66 (80.5%) | 0.27 |
| Atopic dermatitis | 62 (56.4%) | 58 (70.7%) | 0.04 |
| Asthma | 60 (54.6%) | 37 (45.1%) | 0.20 |
| Family history of atopy | 52 (47.3%) | 33 (40.2%) | 0.33 |
| N | CMP (g) | N | HEWP (g) | P value | |
|---|---|---|---|---|---|
| History of anaphylaxis to the challenged food | 25 | 0.41 [0.13-1.09] |
14 | 0.44 [0.29-1.02] |
0.66 |
| No history of anaphylaxis to the challenged food | 7 | 0.54 [0.14-1.12] |
16 | 1.17 [0.51-2.34] |
0.15 |
| Pvalue | 0.45 | 0.03 | |||
| Data are presented as median and [interquartile range]. N – sample size in each group. CMP – cow’s milk protein; HEWP – hen’s egg white protein; OFC – oral food challenge. | |||||
| History of anaphylaxis to food challenged in the OFC (N=106) | No history of anaphylaxis to food challenged in the OFC (N=80) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Coef. | Std. Err. | z | P>|z| | [0.025 | 0.975] | Coef. | Std. Err. | z | P>|z| | [0.025 | 0.975] | |
| Asthma | 0.83 | 0.44 | 1.90 | 0.05 | -0.03 | 1.69 | -0.50 | 0.57 | -0.88 | 0.38 | -1.61 | 0.62 | |
|
Atopic dermatitis |
-0.44 | 0.44 | -1.00 | 0.32 | -1.29 | 0.42 | 1.80 | 0.75 | 2.41 | 0.02 | 0.34 | 3.26 | |
|
Family history of atopy |
-0.14 | 0.42 | -0.33 | 0.74 | -0.97 | 0.69 | -1.11 | 0.60 | -1.86 | 0.06 | -2.28 | 0.06 | |
|
Multi-food allergy |
0.82 | 0.56 | 1.48 | 0.14 | -0.27 | 1.91 | 1.59 | 0.86 | 1.84 | 0.07 | -0.10 | 3.27 | |
| CMP-specific IgE [kU/L] | casein-specific IgE [kU/L] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Passed | N | Failed | p | N | Passed | N | Failed | p | |
| History of anaphylaxis to the challenged food | 41 | 6.19 [2.54-20.20] |
23 | 19.40 [6.71-58.10] |
0.03 | 33 | 4.99 [0.80-17.30] |
20 | 10.99 [4.37-91.00] |
0.05 |
| No history of anaphylaxis to the challenged food | 28 | 4.96 [1.25-21.55] |
5 | 30.43 [30.40-45.00] |
0.04 | 26 | 2.75 [0.53-8.90] |
5 | 30.95 [18.80-37.10] |
0.03 |
| p | 0.34 | 0.81 | 0.36 | 0.76 | ||||||
| HEWP-specific IgE [kU/L] | ovomucoid-specific IgE [kU/L] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Passed | N | Failed | p | N | Passed | N | Failed | p | |
| History of anaphylaxis to the challenged food | 23 | 3.26 [1.21-6.69] |
12 | 11.20 [4.81-33.03] |
0.01 | 19 | 1.67 [0.01-3.62] |
9 | 10.30 [3.59-16.90] |
0.02 |
| No history of anaphylaxis to the challenged food | 27 | 7.72 [3.86-20.30] |
13 | 27.00 [11.30-39.00] |
0.03 | 19 | 7.35 [1.91-14.95] |
9 | 16.60 [5.16-24.20] |
0.09 |
| p | 0.02 | 0.50 | 0.04 | 0.60 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





