1. Introduction
Tricuspid regurgitation (TR) is a common acquired valvular heart disease, primarily affecting the elderly and often leading to disabling symptoms.[
1] In recent years, tricuspid transcatheter edge-to-edge repair (T-TEER) has become a widely available treatment option for patients with TR. This approach, characterized by a favorable safety profile, has been proven to reduce the severity of clinical symptoms more effectively than pharmacological therapy.[
2]
As a result, there has been a significant increase in the number of patients with TR being referred to tertiary care centers for evaluation and consideration for interventional treatment. However, not all referred patients ultimately derive clinical benefit from the T-TEER procedure. During the initial assessment, a substantial proportion of patients are deemed ineligible for T-TEER, mainly due to advanced stage of heart failure or anatomical constraints. Additionally, in some patients undergoing transcatheter valve repair, the procedure fails to achieve the expected reduction in regurgitation severity. Patients who are ineligible for T-TEER due to anatomical limitations or in whom the procedure does not result in sufficient regurgitation reduction may be candidates for alternative transcatheter tricuspid valve interventions (TTVI) such as orthotopic and heterotopic tricuspid valve implantation. [
3,
4]
Up to now, most studies focused on patients undergoing T-TEER and comprehensive data on the overall characteristics of all patients referred to tertiary centers for evaluation and qualification for interventional treatment of TR remains limited. Furthermore, the population of patients who may benefit from TTVI other than T-TEER has not been clearly defined. Consequently, both the demand for these alternative procedures and the clinical profile of potential TTVI candidates require further investigation.
Therefore, in this single-center prospective observational study, we aim to:
1) provide a characterization of patients with severe or greater TR referred to a tertiary care center for transcatheter treatment
2) assess the immediate outcomes of treatment in patients selected for T-TEER
3) characterize the group of potential candidates for TTVI other than T-TEER
2. Material and Methods
2.1. Study Design
This single-center prospective observational study (ChAracterization of Patients and Treatment oUtcomes in severe tricuspid Regurgitation – CAPTURE; NCT 06838611) enrolls consecutive patients that have been referred to the 1st Chair and Department of Cardiology, Medical University of Warsaw for evaluation and consideration for interventional TR treatment. Eligible patients have at least severe TR confirmed in a transthoracic echocardiography (TTE), are over 18 years old and have signed an informed consent form. Patients who at baseline presented with acute coronary syndrome, cardiogenic shock and a known disseminated malignancy are excluded from the study. Prior tricuspid surgery or previous T-TEER/TTVI were not considered exclusion criteria; however, no patients with these characteristics were present in the preliminary cohort. The enrollment started in November 2023 and is still ongoing. Presented data is a preliminary analysis of the patients enrolled until the end of December 2024.
The study design has been previously reported.[
5] In brief, all of the patients followed the same decision pathway, described below. After admission, the patients underwent a thorough clinical evaluation and optimization of medical treatment. The severity of TR was confirmed on TTE and categorized according to the five-grade scale. Since only patients with at least severe TR were eligible, all study participants fell into one of the following categories: severe, massive, or torrential. Subsequently, each patient underwent transesophageal echocardiography for the assessment of the tricuspid valve anatomy and T-TEER feasibility.[
6] Based on this data, during a Heart Team evaluation, patients were either: (1) qualified for T-TEER, (2) disqualified from T-TEER or (3) qualified for another, non-tricuspid-valve-dedicated procedure, that was deemed necessary prior to the treatment of TR. The patients were disqualified from T-TEER based on: (1) lack of heart failure symptoms (asymptomatic TR); (2) clinical futility of the procedure (e.g., poor mobility, advanced frailty, end stage heart failure or other conditions limiting the expected survival); (3) tricuspid valve anatomy not suitable for T-TEER (e.g., large coaptation gap, short or tethered leaflets, severe leaflet degeneration, clear interaction with right ventricular lead); (4) TEE visualization not sufficient for the T-TEER attempt. Patients who were initially qualified for another cardiovascular intervention prior to TR treatment (e.g.,mitral intervention) underwent a secondary clinical and echocardiographic assessment 1–3 months after completion of the prerequisite procedure, in order to reassess TR severity and eligibility for subsequent transcatheter TR therapy. For the purpose of this analysis, patients were grouped into those who were disqualified from T-TEER due to anatomical ineligibility and those who underwent T-TEER but did not achieve adequate early TR reduction. For this preliminary analysis, the unsuccessful T-TEER was defined as TR severity greater than moderate in a TTE performed on the day following the procedure. This grouping was performed solely for descriptive and comparative purposes and did not involve any additional intervention or modification of clinical management.
Protocol of the study was accepted by the Ethics Committee of Medical University of Warsaw. All enrolled patients will sign an informed consent form. The study is conducted according to good clinical practice, the Declaration of Helsinki and in compliance with local legal requirements.
2.2. Statistical Analysis
Statistical analysis was carried out with IBM SPSS Statistics package (version 29.0; IBM, New York, New York, United States). The Shapiro–Wilk test was performed to assess distribution of the continuous variables. The normally distributed variables are presented as mean and standard deviation (SD) and compared with the t test. The non-normally distributed variables are presented as median with interquartile range (IQR) and compared with the Mann–Whitney test. Categorical variables are presented as a number and percentage and compared using the χ2 test or the Fisher exact test. All the analyses are exploratory and hypothesis-generating since no adjustment for multiple testing was applied. The survival analysis was performed using the Kaplan–Meier survival analysis. Statistical significance was established at 2-sided P value below 0.05.
3. Results
3.1. Overall Study Group
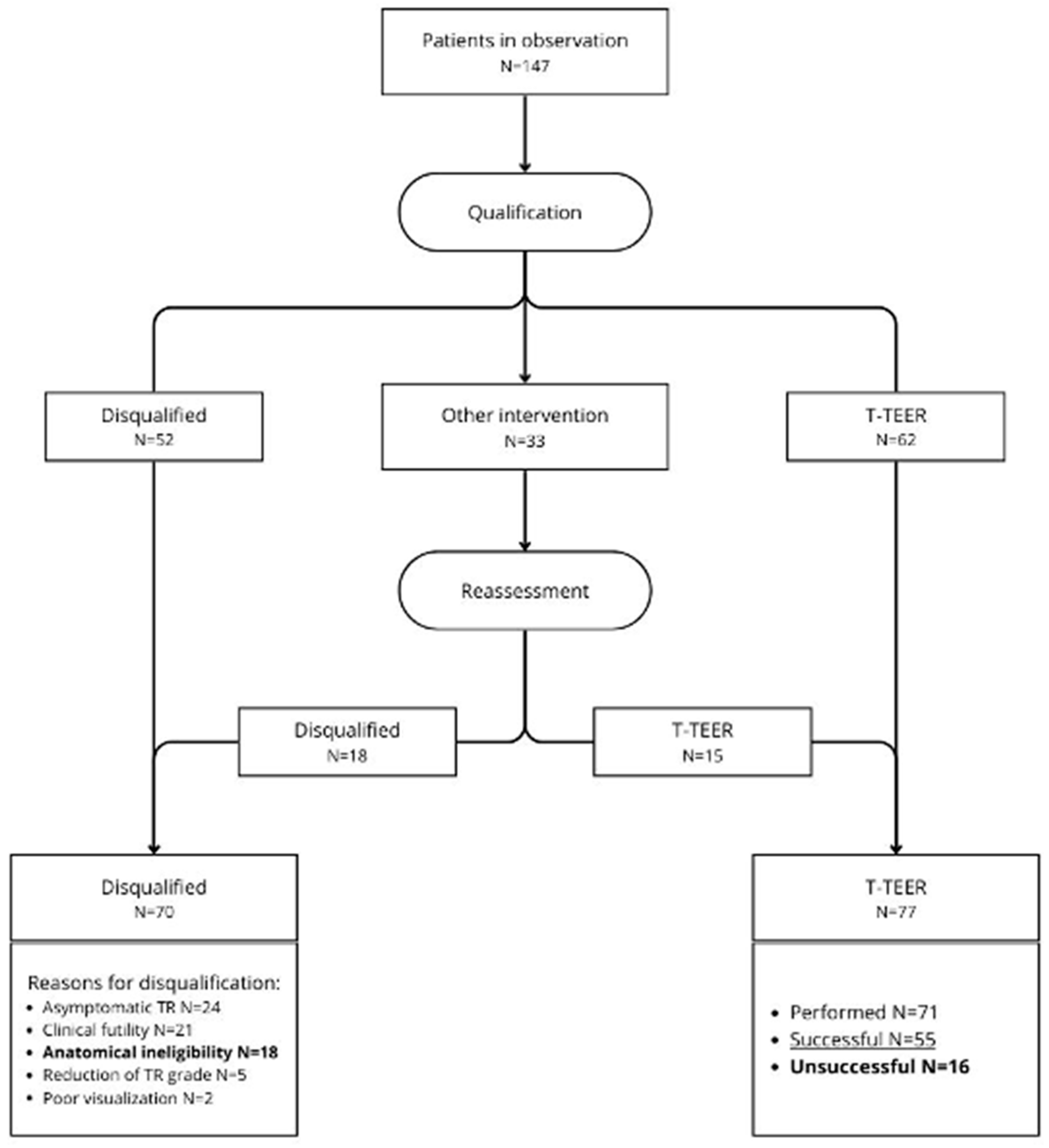
From November 2023 to the end of December 2024, a total of 147 patients with severe TR were enrolled in the study. Baseline characteristics of studied group is summarized in
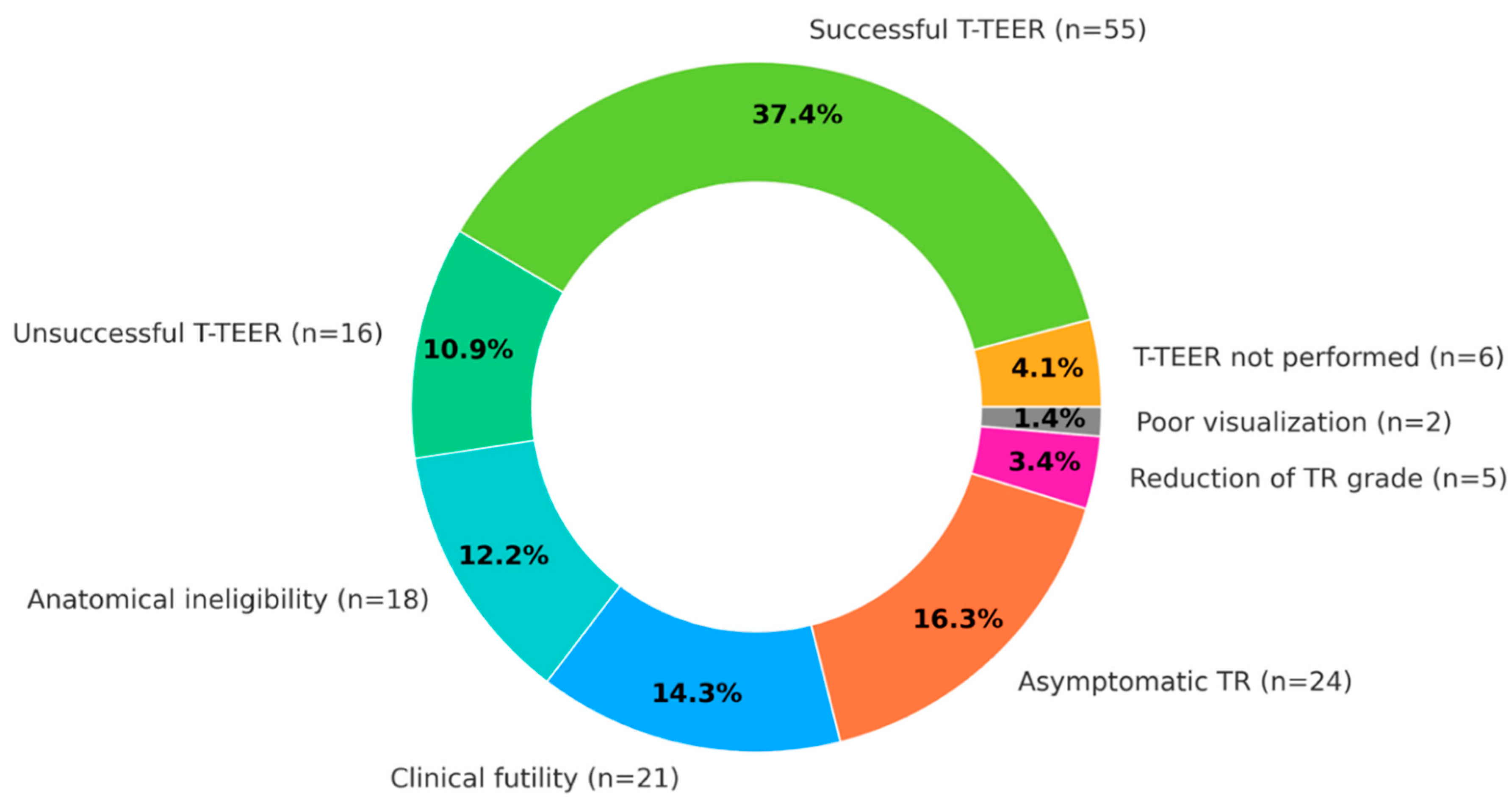
Table 1. Overall, 70 (47.6%) patients were disqualified from T-TEER. The most common causes for disqualification were: asymptomatic TR in 24 (16.3%) patients, clinical futility in 21 (14.3%) patients, anatomical ineligibility in 18 (12.2%) patients, TR grade reduction after initial treatment in 5 (3.4%) patients and lack of adequate TEE visualization for T-TEER attempt in 2 (1.4%) patients.
Out of the 77 (52.4%) patients qualified for T-TEER, 71 (48.3%) underwent the procedure during the observation period, 6 initially qualified patients did not undergo T-TEER. Successful TR reduction was achieved in 55 (37.4% of the total population and 77.5% of treated with T-TEER) patients. The detailed decision pathway and decision summary are presented in
Figure 1 and
Figure 2.
3.2. Potential Candidates for TTVI other than T-TEER
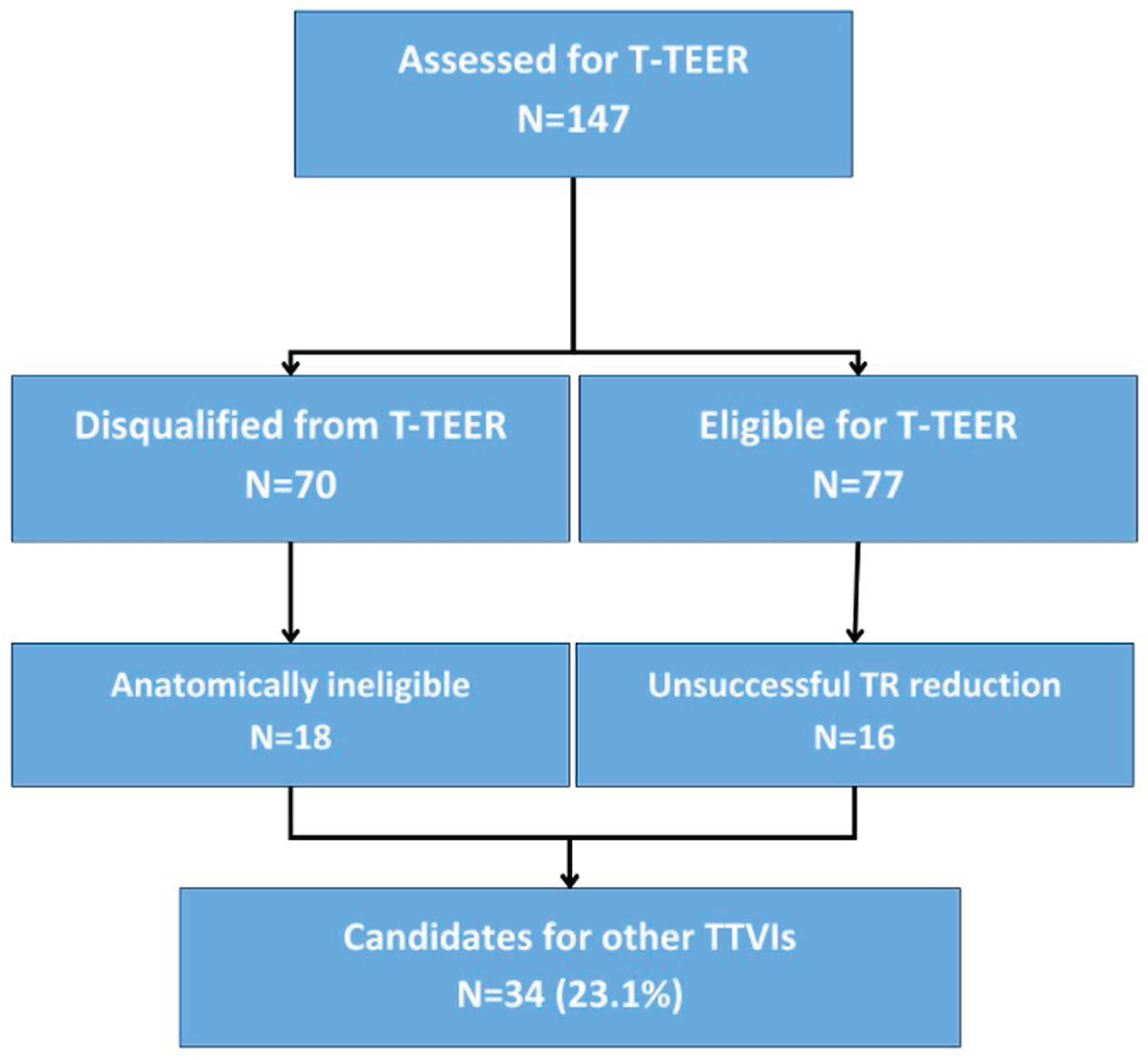
We identified a group of potential candidates for other than T-TEER TTVI comprising 18 patients anatomically ineligible for T-TEER and 16 patients with unsuccessful T-TEER attempt, 34 patients in total. The process in presented in
Figure 3. Most common anatomical reason for T-TEER ineligibility was a large coaptation gap, that was present in 13 patients. Other reasons included a possible interaction with right-ventricular lead of a cardiac implantable device (4 patients) and leaflet anatomy unsuitable for TEER device placement (1 patient). A large central coaptation deficit was also one of the main causes for T-TEER failure, identified in 4 out of 16 unsuccessful cases. Moreover in 4 cases in spite of satisfactory periprocedural TR reduction in TEE, next day TTE revealed greater than moderate TR grade.
3.3. Clinical Characteristics
We compared the candidates for other TTVI (n=34) with a group that has undergone a successful T-TEER (n=55). Potential candidates for other TTVI were on average younger, with a mean age of 76.5 (7.0) years.
Similarly to the overall study population, most of these patients suffered from severe HF symptoms, with 26 (76.5%) in NYHA functional class III or IV. In comparison, in the successful T-TEER group, there were 37 (67.3%) patients presenting with NYHA III/IV but the difference was not statistically significant. However, in potential candidates for other TTVI, significantly more patients presented with ascites – 15 (44.1%), compared to 7 (12.7%) in the successful T-TEER group (P<0.001).
The most common comorbidities were atrial fibrillation (AF), chronic kidney disease (CKD) and hypertension. There were no differences in terms of coexisting disease.
We found some notable differences in baseline laboratory tests. The potential other TTVI candidates had a significantly lower hemoglobin concentration (11.8 [1.7] g/dL vs 12.3 [1.7] g/dL; P=0.017), lower platelet count (161.0 [51.0] vs 183.0 [79.0]; P=0.015), higher international normalized ratio (INR; 1.53 [0.88] vs 1.23 [0.61]; P=0.012) and higher bilirubin concentration (1.09 [1.2] mg/dl vs 0.61 [0.42] mg/dl; P<0.003). The baseline characteristics of these groups in presented in detail in
Table 2.
3.4. Echocardiographic Characteristics
At baseline TTE, the candidates for other TTVI had a greater severity of TR than those with successful T-TEER attempt. Torrential TR was present in 26 (76.5%) of them, compared to 10 (18.2%) in the successful T-TEER group (P<0.001). Moreover, the dimensions of the right heart chambers, were significantly higher as well. Right ventricle diastolic diameter was 4.1 (0.8) cm compared to 3.6 (0.6) cm (P<0.001), right-ventricular inflow tract in apical-four chamber-view was 5.4 (0.9) cm compared to 4.6 (0.6) cm (P=0.005) and right-atrial area was 37.5 (10.1) cm2 compared to 34.0 (9.1) cm2 (P=0.003). Detailed echocardiographic characterization is presented in
Table 2.
4. Discussion
This single-center prospective observational study summarizes our initial experience in the identification of TR patients for interventional treatment in real-world clinical practice. It does not aim to define indications or contraindications for specific transcatheter techniques, but to describe screening outcomes in patients referred for transcatheter TR therapies. Importantly, all classifications presented in this study reflect analytical categorization of patients based on real-world clinical decisions and outcomes, rather than predefined treatment algorithms. The main findings of this study are as follows:
First, referral and qualification pattern – patients with severe or greater TR referred to a tertiary care center for transcatheter treatment represent a heterogeneous cohort of elderly individuals with a high burden of heart failure symptoms and multiple comorbidities. Ultimately, about half of these patients is qualified for T-TEER.
Second, immediate outcomes of T-TEER – among patients who underwent T-TEER in our study population, approximately three-quarters experienced an immediate benefit of a significant TR reduction to moderate or less, as assessed by transthoracic echocardiography (TTE) performed on the day following the procedure. Although mid-term and long-term outcomes are essential to assess clinical efficacy, immediate post-procedural TR reduction represents a critical decision point in daily practice, determining whether additional procedures are required.
Third, profile of candidates for alternative TTVI – patients that cannot benefit from T-TEER constitute almost one quarter of patients referred to tertiary care center for evaluation and consideration for interventional treatment. Since, T-TEER is usually considered as a first-line percutaneous treatment, these patients are candidates for other TTVI. Compared to patients who derive immediate benefit from T-TEER, this group is clinically characterized by a higher prevalence of right heart failure symptoms and a higher frequency of bleeding risk factors (lower hemoglobin levels, lower platelet count, and higher INR), which may be associated with impaired liver function (higher bilirubin levels). In echocardiography, this population exhibits a greater TR severity which is accompanied by greater right heart chamber dilation. Importantly, these patients emerged naturally from the real-world screening and treatment process. Furthermore, these findings should not be interpreted as contraindications to transcatheter intervention, but rather as markers of advanced disease that may influence the choice and timing of TTVI strategies.
In recent years, we have witnessed the rapid advancement of transcatheter techniques for the treatment of TR. Due to its favorable safety profile, simplicity, and prior experience with the mitral valve, transcatheter edge-to-edge repair has become the most widely utilized interventional modality in the TR population. Despite anatomical and technical obstacles, there has been also an extensive research on the development of transcatheter tricuspid valve prostheses. The EVOQUE system (Edwards Lifesciences, Irvine, CA, USA), designed for orthotopic tricuspid valve implantation, is the first device of its kind to be introduced for commercial use in Europe and the United States. In a recent randomized study, it was shown to be effective in symptoms reduction and the quality-of-life improvement when compared to medical therapy alone.[
7] Different technique, orthotopic caval valve implantation may also play a role in the treatment of patients with TR. The TricValve system (P&F Products & Features GmbH, Germany), designed for this procedure, has been evaluated in the recent TricBicaval registry, demonstrating improvements in patients’ functional status.[
8] Systems for orthotopic and heterotopic valve implantation may be used both in patients who are not eligible for T-TEER and in those who have undergone an unsuccessful transcatheter tricuspid valve repair.
To date, there are no definitive guidelines for patient selection among different transcatheter techniques. Therefore, the qualification process primarily relies on an individualized assessment of each patient’s anatomy and risk-to-benefit ratio, with T-TEER being the preferred interventional modality due to its favorable safety profile. Furthermore, qualification for interventional treatment may vary significantly between centers due to differences in operator experience and the availability of specific techniques.
Moreover, until now, only a few published studies focused on the selection of patients for invasive TR treatment. In a retrospective analysis involving 547 patients from three centers, 196 (35.8%) patients were qualified for T-TEER, while a total of 136 (24.9%) patients were referred to other transcatheter therapeutic modalities mainly direct annuloplasty and in minority of cases transcatheter valve implantation.[
9] In the latter group, larger right heart dimensions with concomitant larger TR severity were observed, indicating more advanced stage of disease in candidates for interventions other than T-TEER.
In another retrospective study involving patients evaluated for tricuspid interventions, anatomical feasibility for T-TEER and transcatheter tricuspid valve implantation were analyzed.[
11] Among 491 patients assessed for T-TEER, 157 (32.0%) were found to have unfavorable anatomy for percutaneous valve repair attempt. Also, in this case, unfavorable T-TEER candidates were characterized by more severe TR and more pronounced dilatation of the right atrium and right ventricle. It should also be emphasized that in the group of patients ineligible for T-TEER who underwent evaluation by computed tomography, 69% were also not eligible for valve implantation.
A recent analysis of a real-life population showed that out of 178 patients with TR referred to the tertiary center only 19 (10.7%) were eligible for the enrolment to the clinical trials on transcatheter TR treatment (T-TEER or other TTVI) and additional 36 (20.2%) underwent out of trial T-TEER.[
11]
Retrospective studies on patient selection for transcatheter TR treatment published so far, have differed in methodology, focused on the eligibility for different interventional techniques (annuloplasty, tricuspid valve implantation) or have analyzed study populations based on an intention-to-treat basis. Unlike prior retrospective analyses focused on anatomical feasibility, the present study prospectively captures the full clinical pathway, including patients with unsuccessful T-TEER, reflecting real-world decision making. In daily clinical practice, TTVI other than T-TEER is considered not only in patients disqualified from T-TEER due to anatomical reasons but also in those without a significant TR reduction after T-TEER. Our findings in this group confirm previous observations showing larger right heart diameters and more severe TR grade when compared to the successful T-TEER patients.
Higher frequency of bleeding risk factors in the group of potential candidates for other TTVI is a new finding from the presented analysis. In our opinion, this is a clinically relevant observation. It is also consistent with the excessive bleeding rates reported in EVOQUE and TricValve series, and highlights that comprehensive pre-procedural bleeding risk stratification should represent an important element of Heart Team discussions when considering TTVI strategies.
5. Conclusions
In real-world practice, approximately half of patients with severe tricuspid regurgitation referred for transcatheter treatment are eligible for T-TEER, whereas more than 20% may require alternative TTVI approaches. This subset of patients presents with more advanced disease and a higher prevalence of bleeding risk factors, which should be carefully weighed during the decision-making process.
Study Limitations
Several limitations of this study should be emphasized. First, although prospective in design, this registry is a single-center analysis based on patient referrals from primary care centers and outpatient clinics, making it susceptible to selection bias and potentially not fully representative of the broader TR patient population. Second, the number of T-TEER procedures performed during the first year of observation was limited, and not all qualified patients underwent treatment. This may have influenced the proportion of patients benefiting from the procedure in terms of TR reduction, thereby altering the number of potential candidates for other TTVIs. Third, the significant TR reduction observed in our T-TEER group (76.6%) is consistent with findings from large-scale registries.[
12] However, we used a different time point to assess postprocedural TR, reporting immediate results based on TTE performed the day after the procedure, rather than at 30 days. Finally, in this preliminary report we focused on clinical and basic echocardiographic data, without providing a detailed description of tricuspid valve anatomical differences between study groups, which will be addressed in subsequent analyses. Therefore, the present analysis should be interpreted as hypothesis-generating and descriptive, providing a framework for future studies.
Abbreviations and Acronyms
6MWD – 6-minute walk distance; ACE-I – angiotensin converting enzyme inhibitor; AF – atrial fibrillation; ALT – alanine transaminase; AST – aspartate transaminase; ARB – angiotensin receptor blocker; ASA – acetylsalicylic acid; AVR – aortic valve replacement; CABG – coronary artery bypass grafting; CAD – coronary artery disease; CCB – calcium channel blocker; CKD – chronic kidney disease; COPD – chronic obstructive pulmonary disease; CRP – C-reactive protein; DM – diabetes mellitus; eGFR – estimated glomerular filtration rate; EROA – effective regurgitant orifice area; HHF – hospitalization for heart failure; INR – international normalized ratio; KCCQ OS – Kansas City Cardiomyopathy Questionnaire Overall Summary Score; LAA – left atrial area; LVEF – left-ventricular ejection fraction; MI – myocardial infarction; MR – mitral regurgitation; MVR – mitral valve repair/replacement; NOAC – non-vitamin K antagonist oral anticoagulant; NT-proBNP – N-terminal pro-B-type natriuretic peptide; NYHA – New York Heart Association; PAD – peripheral artery disease; PCI – percutaneous coronary intervention; RAA – right-atrial area; RVIT A4C – right-ventricular inflow tract in apical 4-chamber view; RVOT – right-ventricular outflow tract; SLDA – single leaflet device attachment; SPAP – systolic pulmonary artery pressure; TAPSE – tricuspid annular plane systolic excursion; TAVI – transcatheter aortic valve implantation; TIA – transient ischemic attack; TEE – transesophageal echocardiography; TR – tricuspid regurgitation; TTE – transthoracic echocardiography; T-TEER – tricuspid transcatheter edge-to-edge repair; TTVI – transcatheter tricuspid valve intervention; TVG – tricuspid valve gradient; TVR – tricuspid valve repair/replacement; VKA – vitamin K antagonist;
Author Contributions
Conceptualization, AR, EP, AKC, MT, PS and FM; methodology, EP; formal analysis, AP; investigation, AR, AP, EP, EO, PP, EB, AKC, MTR and PS; data curation, AP, EO, PP, and EB; writing—original draft preparation, AR, AP, MT; writing—review and editing, EP, AKC, MT, PS and FM; visualization AKC, PS; supervision, JK and FM; project administration, AR, AP. All authors have read and agreed to the published version of the manuscript.
Financial support: none declared.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Medical University of Warsaw (approval No. AKBE/179/2023; date of approval 12 July 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Please refer to suggested Data Availability Statements in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics. If the study did not report any data, you might add “Not applicable” here.
Conflicts of Interest
All authors declare no conflict of interest regarding the contents of this article.
References
- Cahill, TJ; Prothero, A; Wilson, J; et al. Community prevalence, mechanisms and outcome of mitral or tricuspid regurgitation. Heart 2021, 107, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P; Whisenant, B; Hamid, N; et al. Transcatheter Repair for Patients with Tricuspid Regurgitation. N Engl J Med. 2023, 388, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Goldschmied, A; Droppa, M; Gawaz, M; Geisler, T. Transcatheter Tricuspid Valve Replacement Following Tricuspid Edge-To-Edge-Repair. Catheter Cardiovasc Interv. 2025, 105, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Costa, G; De Carlo, M; Spontoni, P; et al. Heterotopic Transcatheter Tricuspid Valve Replacement in Severe Tricuspid Regurgitation and Refractory Right Heart Failure. JACC Case Rep. 2022, 4, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Rdzanek, A; Piasecki, A; Tomaniak, M; et al. ChAracterization of Patients and Treatment oUtcomes in severe tricuspid Regurgitation (CAPTURE) - study design. Cardiol J 2025. [Google Scholar] [CrossRef] [PubMed]
- Praz, F; Muraru, D; Kreidel, F; et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention 2021, 17, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Hahn, RT; Makkar, R; Thourani, VH; et al. Transcatheter Valve Replacement in Severe Tricuspid Regurgitation. N Engl J Med. 2025, 392, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Recalde, A; Domínguez-Rodríguez, LM; Rosseel, L; et al. Bicaval TricValve Implantation in Patients With Severe Tricuspid Regurgitation: 1-Year Outcomes From the TricBicaval Registry. JACC Cardiovasc Interv. 2025, 18, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Gercek, M; Goncharov, A; Narang, A; et al. Characterization of Screen Failures Among Patients Evaluated for Transcatheter Tricuspid Valve Repair (TriSelect-Study). JACC Cardiovasc Interv. 2023, 16, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T; Vogelhuber, J; Ozturk, C; et al. Eligibility for Transcatheter Tricuspid Valve Interventions in Patients With Tricuspid Regurgitation. JACC Cardiovasc Interv. 2024, 17, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Abushouk, A; Layoun, H; Harb, SC; et al. Real-World Patient Eligibility and Feasibility of Transcatheter Edge-to-Edge Repair or Replacement Interventions for Tricuspid Regurgitation. J Card Fail 2024, 30, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P; Besler, C; Schmitz, T; et al. Short-Term Outcomes of Tricuspid Edge-to-Edge Repair in Clinical Practice. J Am Coll Cardiol. 2023, 82, 281–291. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).