Submitted:
19 December 2025
Posted:
22 December 2025
You are already at the latest version
Abstract
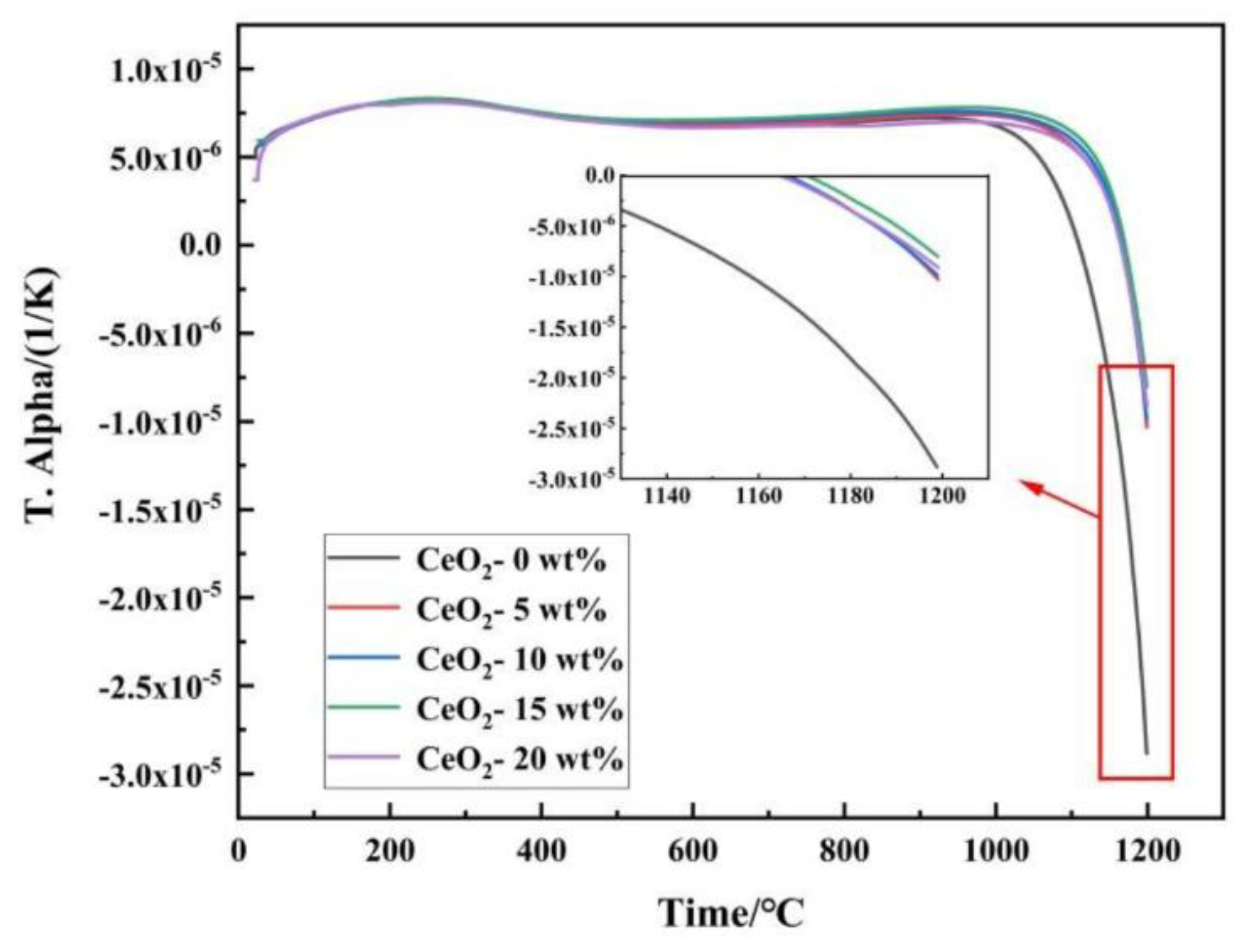
Zirconium oxide (ZrO₂) ceramics are widely used in thermal barrier coatings and high temperature structural parts due to their excellent high temperature performance and thermal insulation characteristics. However, its high temperature phase transition, thermal expansion coefficient mismatch and thermal conductivity increase limit its further application. In order to improve the comprehensive properties of ZrO₂ ceramics, the effects of different CeO₂ doping levels (0-20 wt.%) on the microstructure, mechanical properties, tribological behavior and thermophysical properties of ZrO₂ ceramics were systematically investigated. The sample was prepared by a simple and efficient method of ball milling combined with pressure-free sintering, which has simple process and low cost, and was conducive to achieving the uniformity of composition and controllable microstructure. The results showed that 15 wt.% CeO₂ was the optimal doping concentration. At this time, the density of the material was the highest, and the hardness was 310 HV₁, which was 27.64% higher than that of the undoped sample. The friction coefficient and wear rate were reduced to 0.205 and 1.81×10⁻³ mm³/N·m, respectively, showing the optimal wear resistance. At 1200 °C, the thermal expansion coefficient decreased by 72.21%, and the thermal conductivity decreased to 0.612 W/(m·K). The improved performance was mainly attributed to the solid solution enhancement of Ce⁴⁺, grain refinement and phonon scattering effect of enhanced oxygen vacancy. This study provided an important basis for optimizing the comprehensive properties of ZrO₂ ceramics by component design.
Keywords:
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
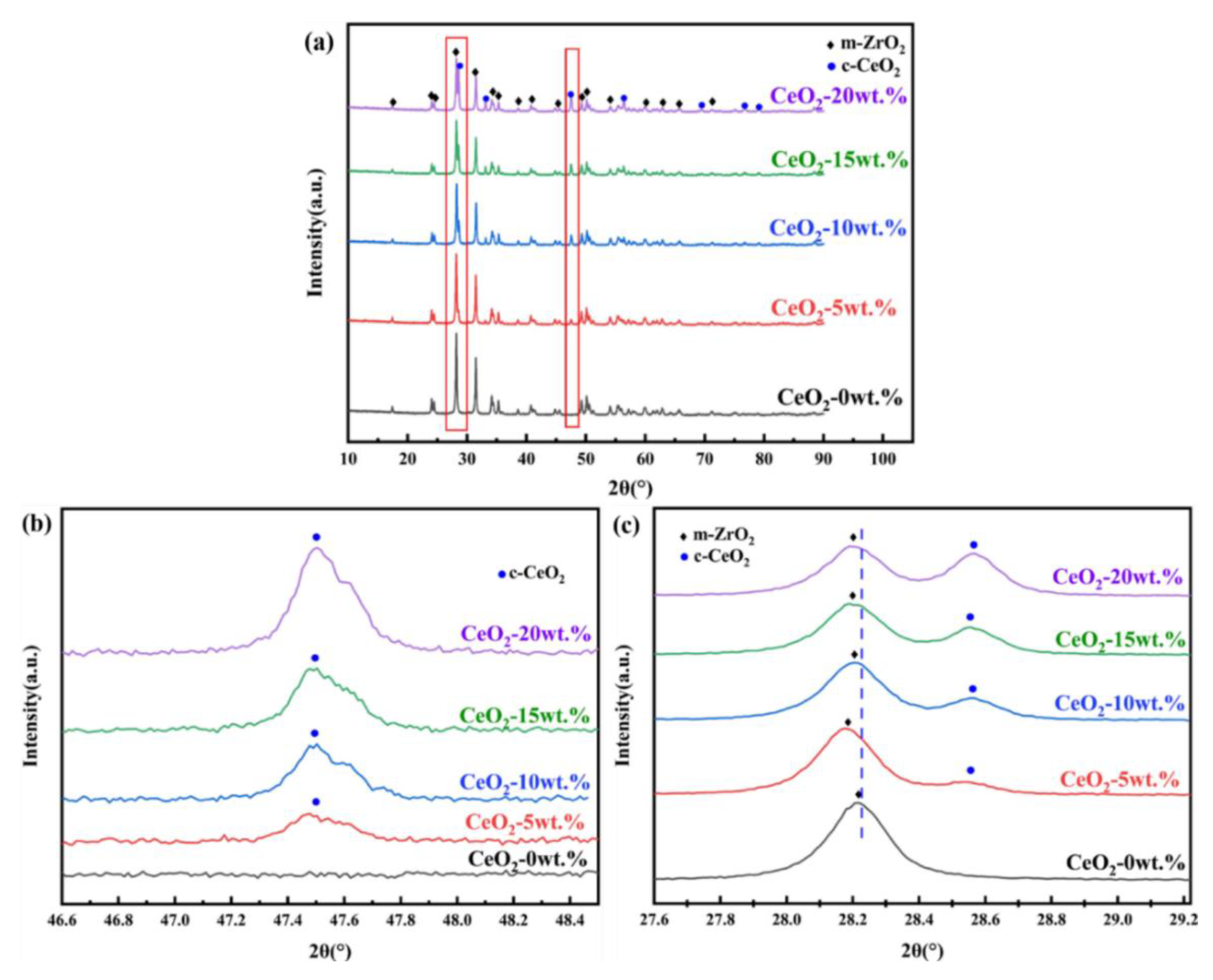
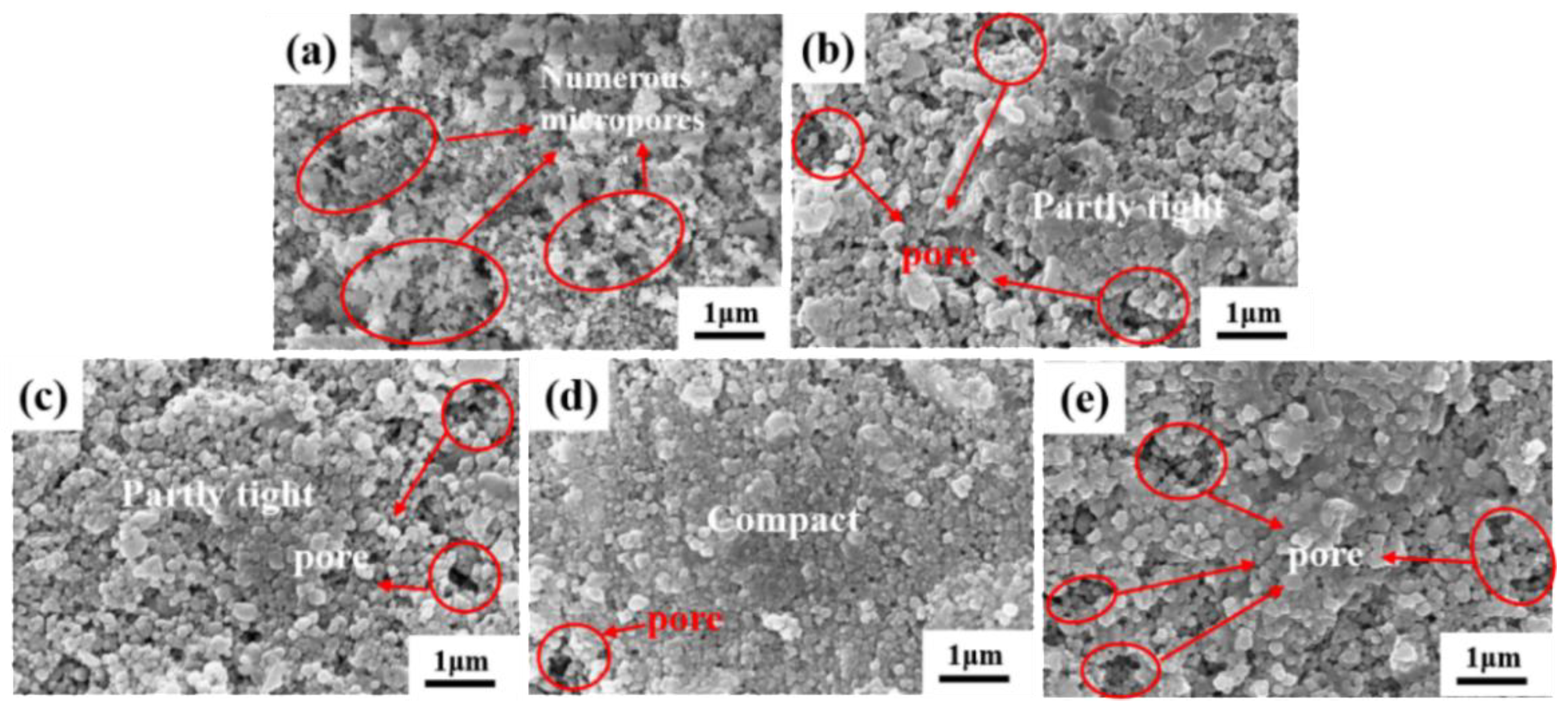
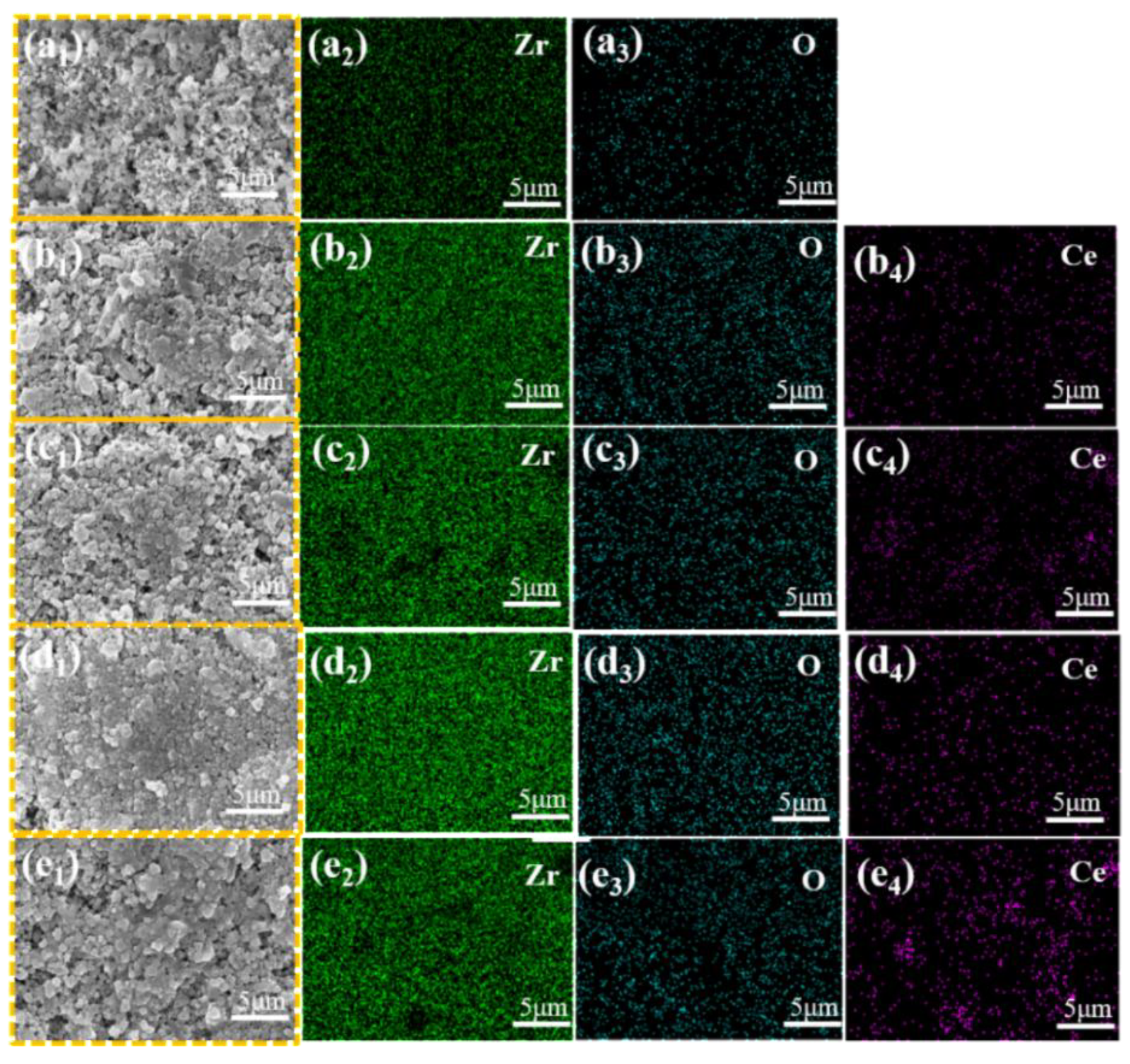
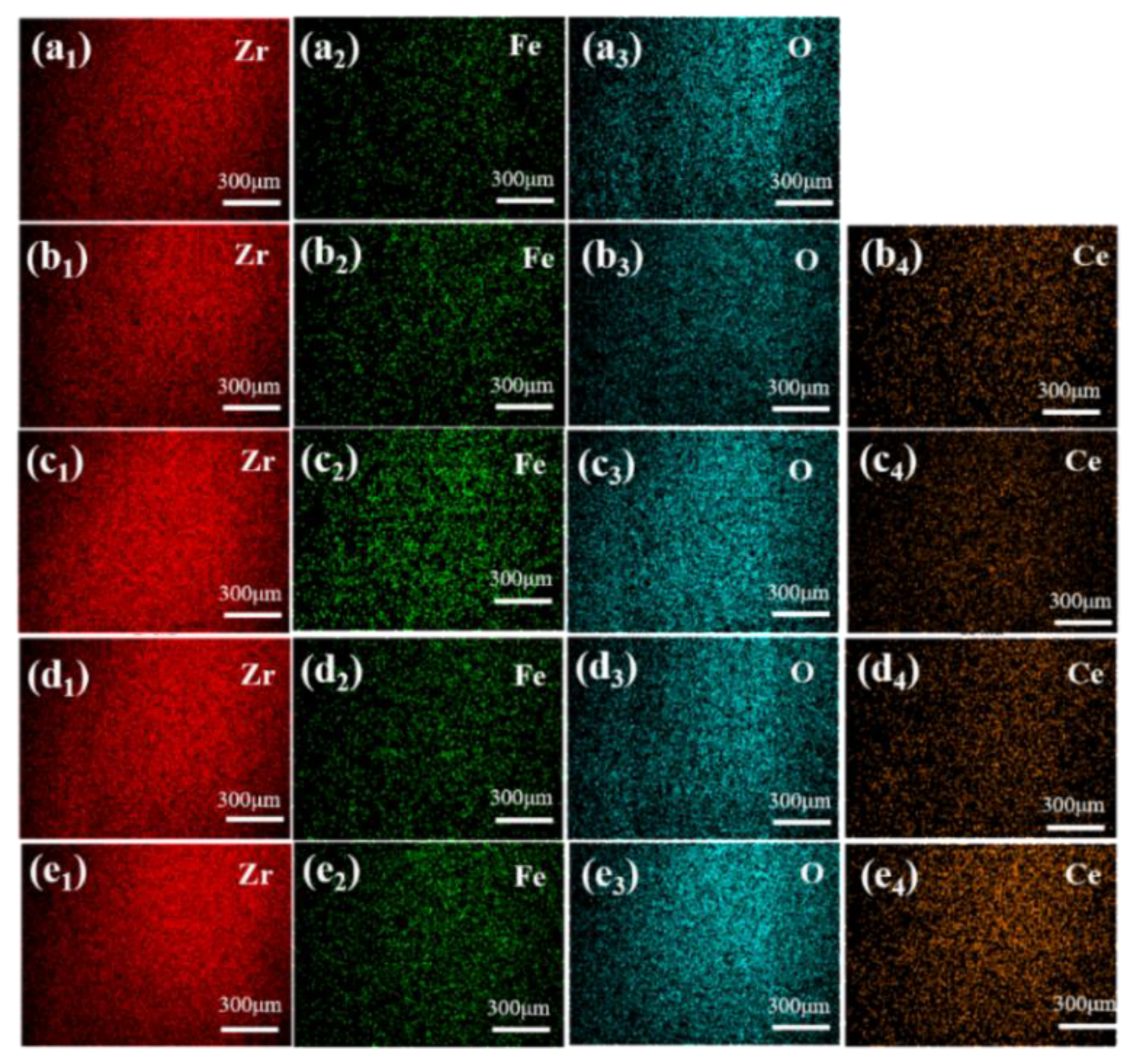
3.1. Phase Composition and Microstructure
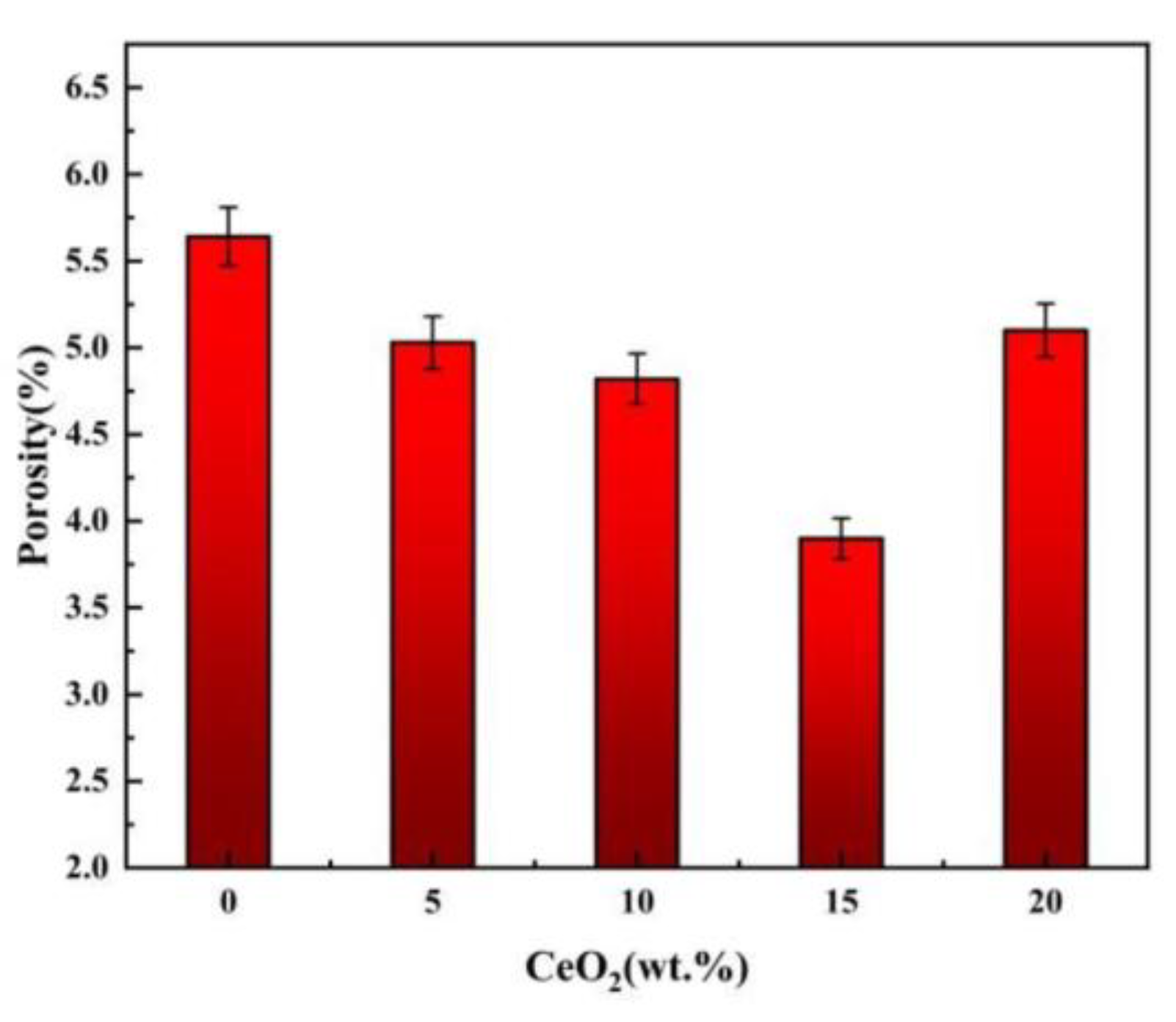
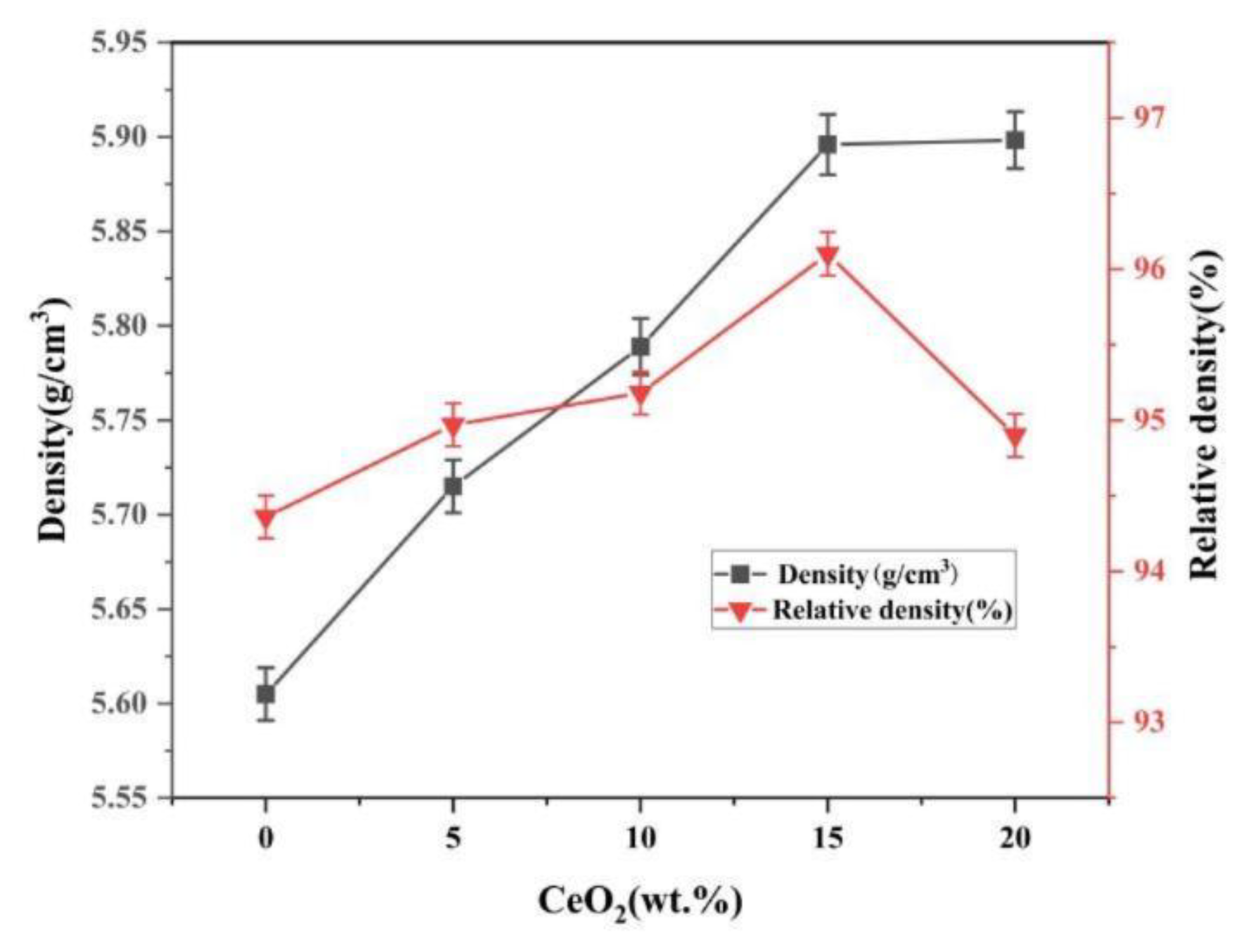
3.2. Density, Porosity and Relative Density
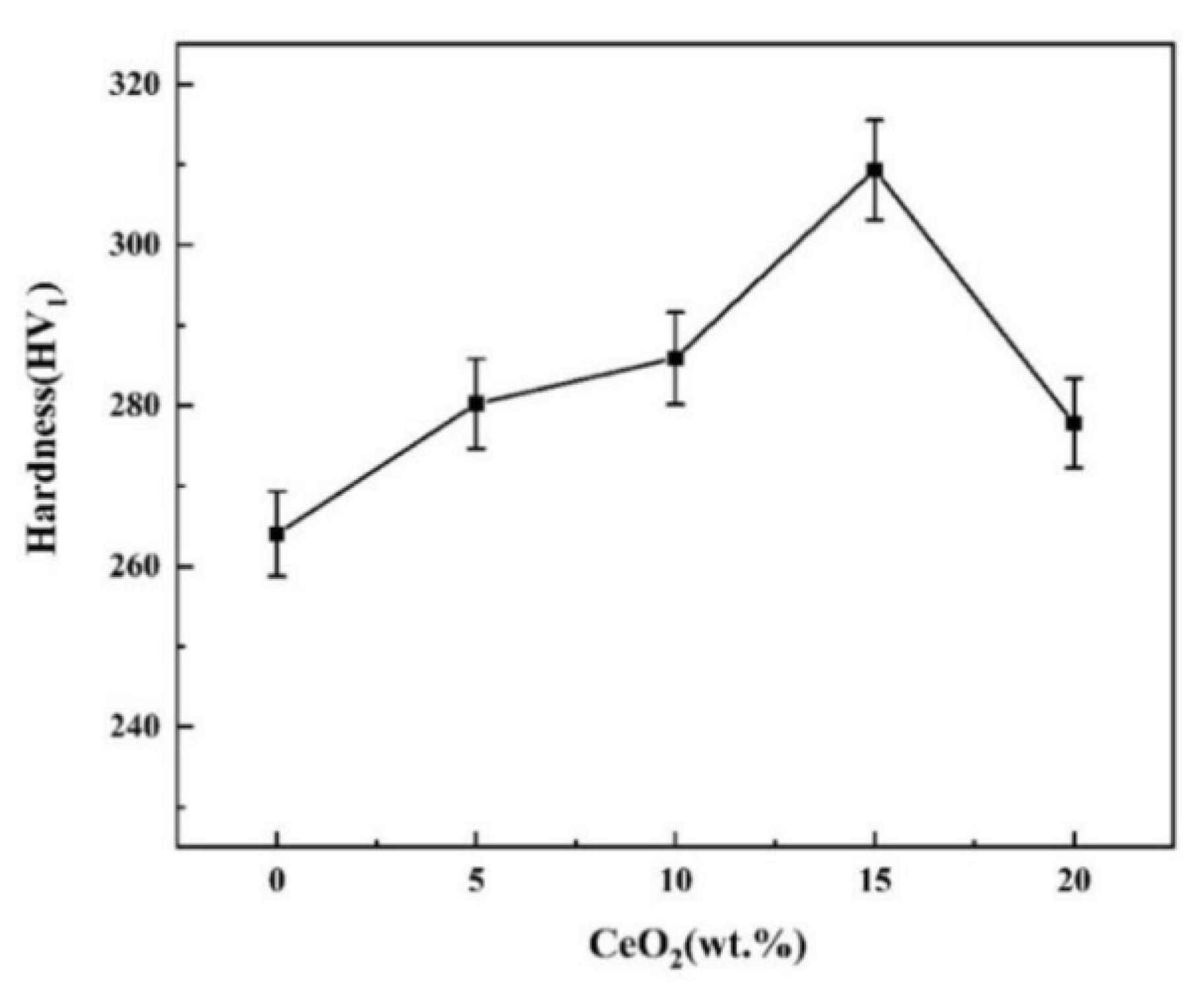
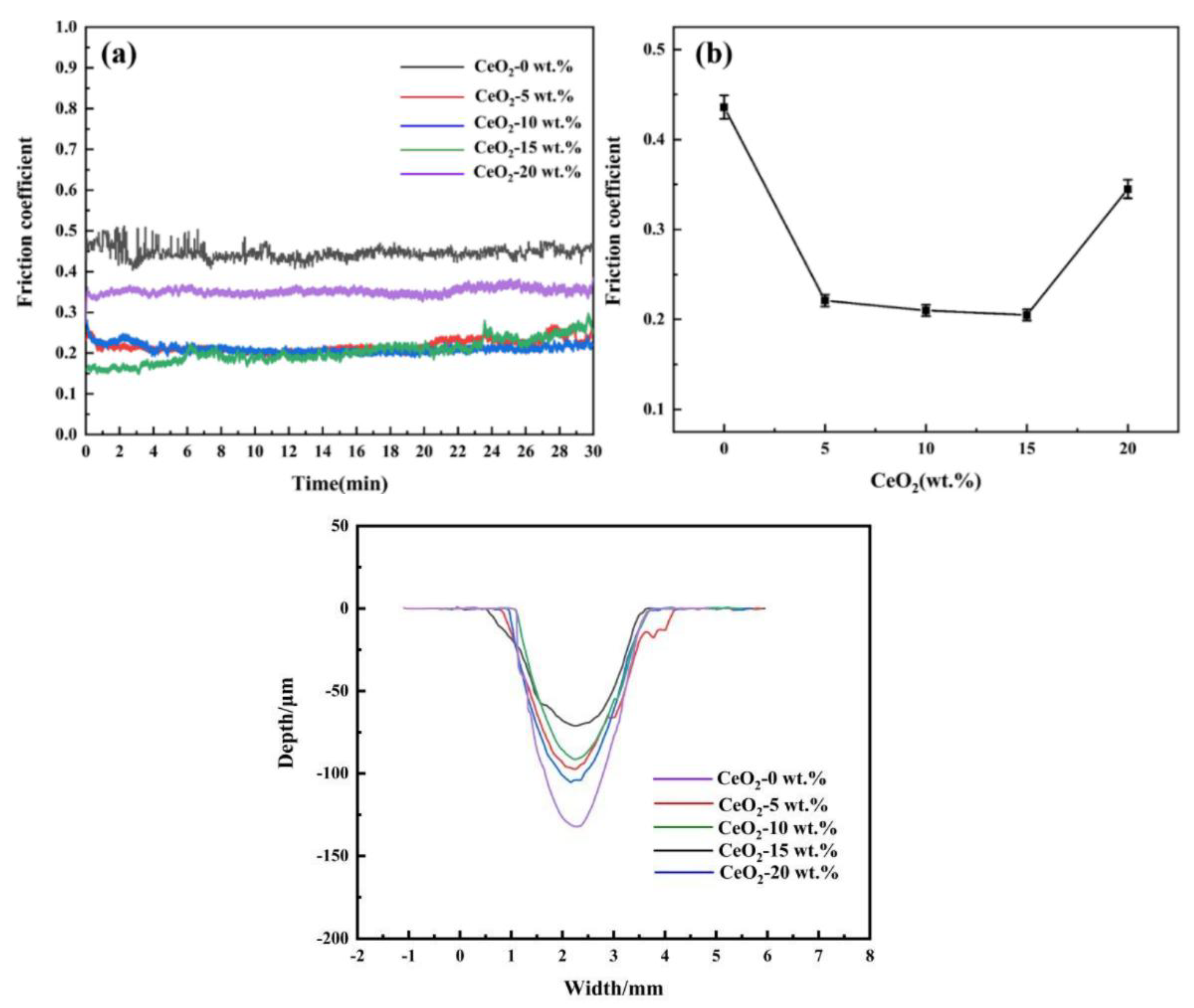
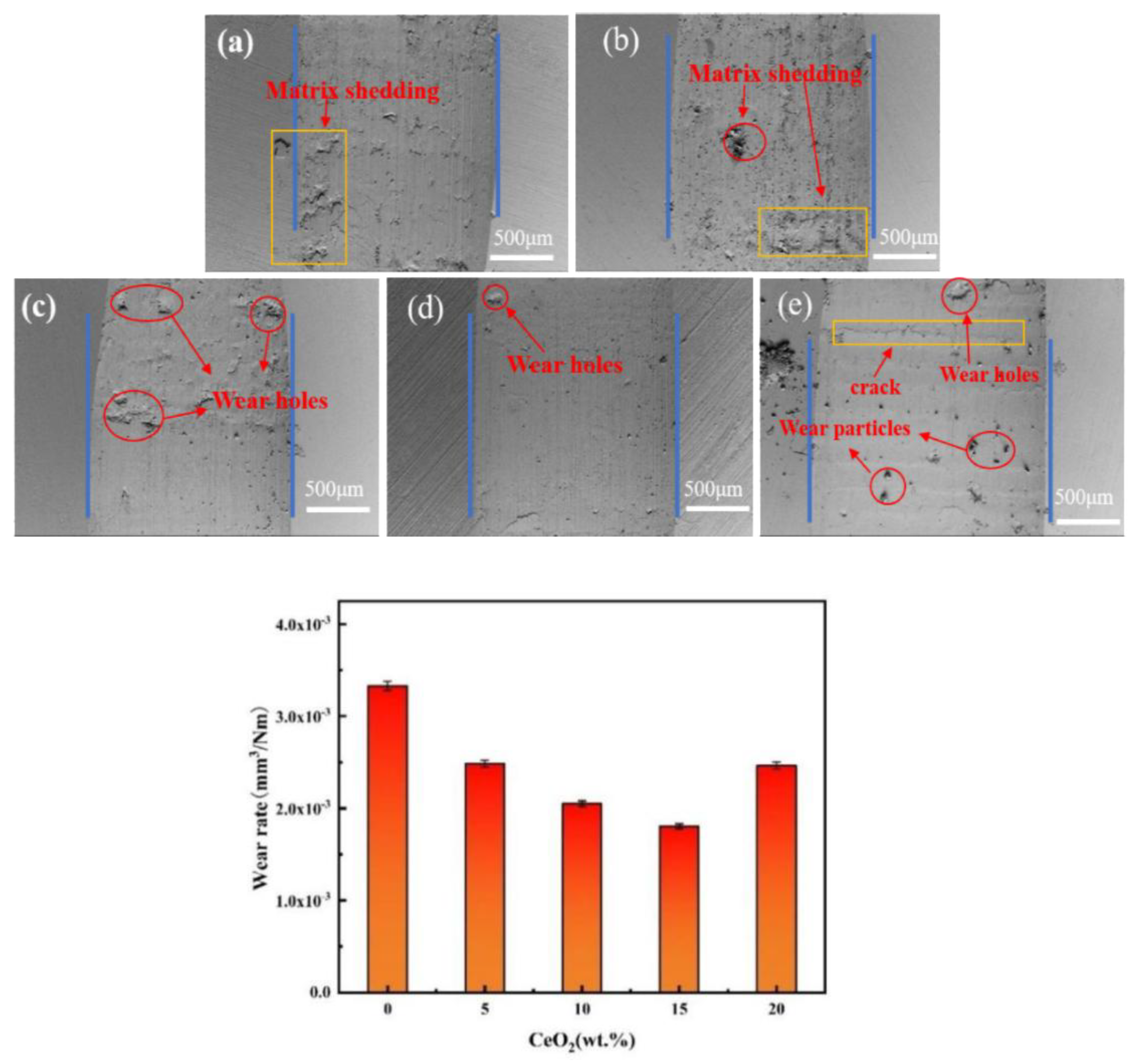
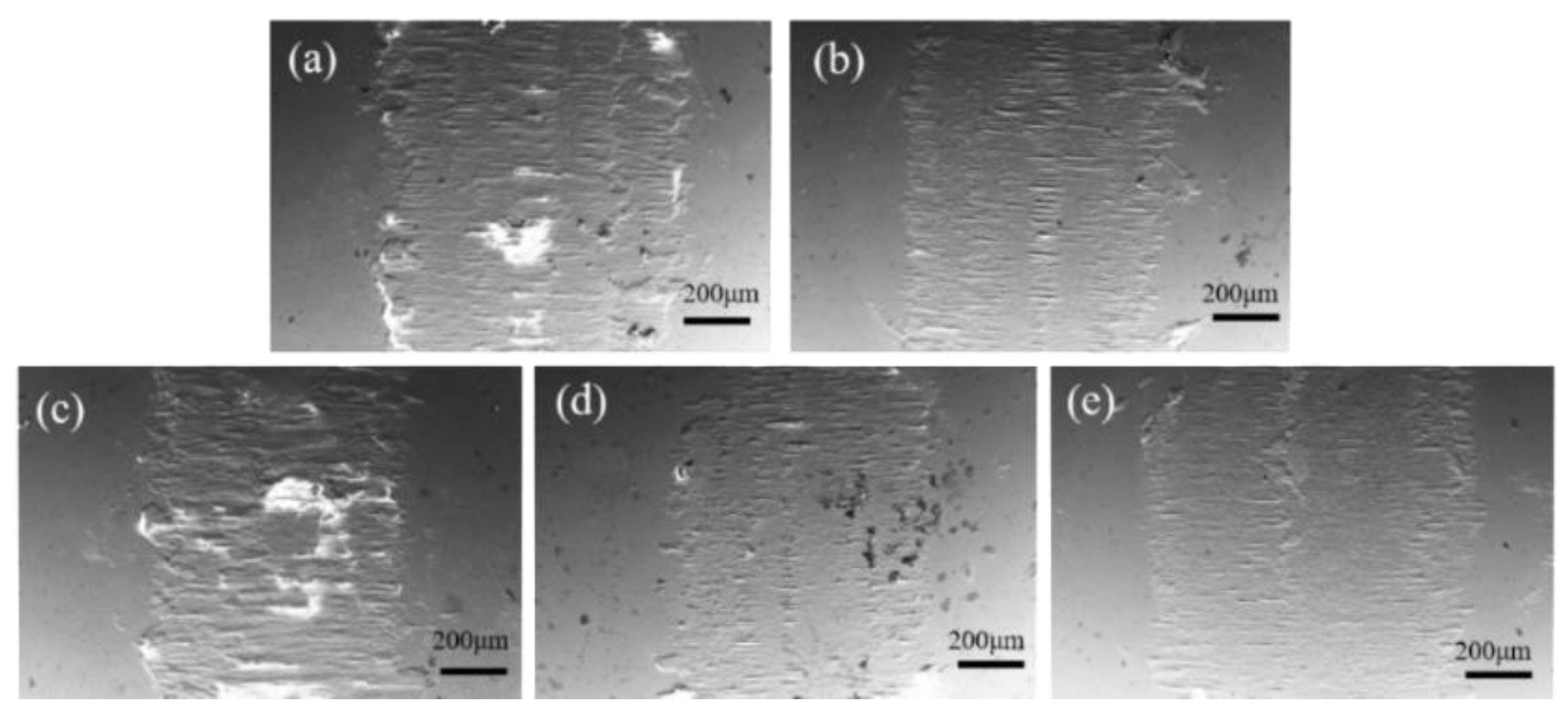
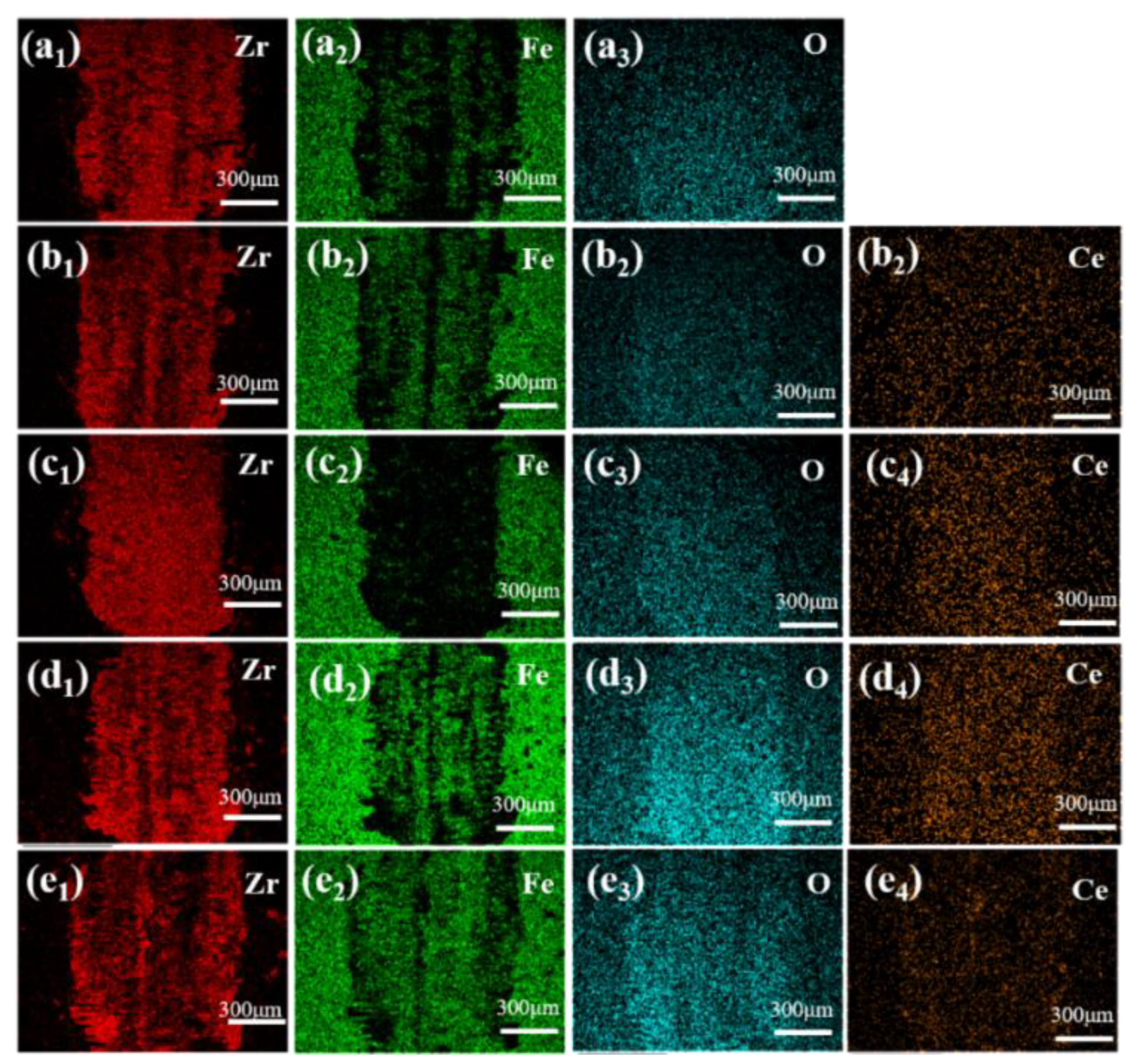
3.3. Hardness, Friction and Wear Performance
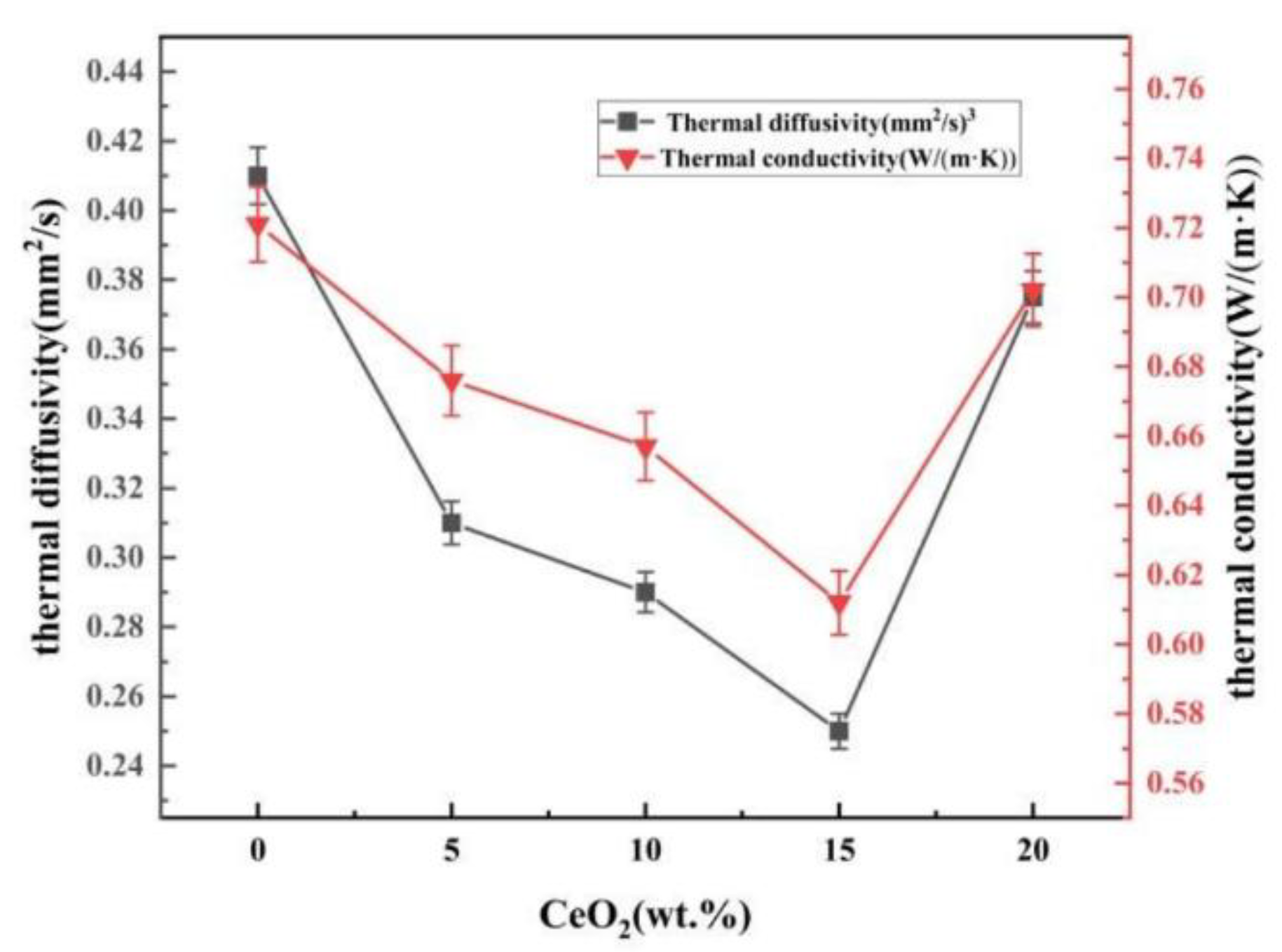
3.4. Thermal Properties
4. Conclusions
Author Contributions
Acknowledgments
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Du, L.Z. Stabilization of a novel mixed solution precursor used for preparing YSZ abradable sealing coatings. Colloid Surf A 2024, 562, 354–360. [Google Scholar] [CrossRef]
- He, Y.; Zhang, S.; He, Y.; Song, R.; Zhang, Z.; Liu, B.; Li, H.; Shangguan, J. Effects of yttrium-stabilized zirconia (different yttrium content) doping on the structure, corrosion resistance and wear resistance of Ni-P electroless coating. Colloid Surf A 2022, 654, 130059. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, K.Z.; Li, H.J.; Zhang, Y.L.; Wang, Y.J. Effects of microstructures on the ablation behaviors of ZrC deposited by CVD. Surf Coat Tech. 2024, 240, 450–455. [Google Scholar] [CrossRef]
- Reddy Channu, V.S.; Kalluru, R.R.; Schlesinger, M.; Mehring, M.; Holze, R. Synthesis and characterization of ZrO2 nanoparticles for optical and electrochemical applications. Colloid Surf A 2011, 386, 151–157. [Google Scholar] [CrossRef]
- Liu, B.X.; Lin, X.J.; Zhu, L.Y.; Wang, X.Q.; Xu, D. Fabrication of calcium zirconate fibers by the solgel method. Ceram Int. 2014, 40, 12525–12531. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.P.; Stevens, R. Hafnia and hafnia-toughened ceramics. J. Mater. Sci. 1992, 27, 5397–5430. [Google Scholar] [CrossRef]
- Lee, H-S.; Ko, H.; Heo, K.; Lee, H.S.; Lim, H.M. Dispersion control via crystal-phase modulation of yttrium-doped ZrO2 nanoparticle sol. Colloid Surf A 2023, 670, 131476. [Google Scholar] [CrossRef]
- Duh, J.G.; Dai, H.T.; Chiou, B.S. Sintering, microstructure, hardness, and fracture toughness behavior of Y2O3-CeO2-ZrO2. J Am Ceram Soc. 1988, 71, 813–819. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Cheng, C.; Chen, L.Y.; Cheng, X.; Xie, X.L.; Chen, H.; Ye, Y.W. Microstructure and tribological performances of W-Cu-Co-xWC alloys with various WC amounts. Tungsten 2025, 7, 314–326. [Google Scholar] [CrossRef]
- Toprak, C.B.; Dogruer, C.U. Neuro-fuzzy modelling methods for relative density prediction of stainless steel 316L metal parts produced by additive manufacturing technique. J Mech Sci Technol. 2023, 37, 107. [Google Scholar] [CrossRef]
- Sasan, G.; Hadi, H.; Ebrahim, G.; Taghi, S. Effect of quantitative textural specifications on Vickers hardness of limestones. Bull Eng Geol Environ. 2023, 82, 1–19. [Google Scholar]
- Deng, W.; Wu, X.; Xu, Z.; Liu, J.; Li, T.; Tang, L. Friction and Wear Behaviors of Perfluoropolyether-Impregnated Plasma-Sprayed Yttria-Stabilized Zirconia Coatings. J Mater Eng Perform. 2024, 33, 1369–1379. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Bi, Q.L.; Yang, J. Tribological behavior of Ni3Al alloy at dry friction and under seawater environment. Tribol Int. 2014, 75, 24–30. [Google Scholar] [CrossRef]
- Xie, X.; Guo, H.; Gong, S.; Xu, H. Lanthanum–titanium–aluminum oxide: a novel thermal barrier coating material for applications at 1300 °C. J Eur Ceram Soc. 2011, 31, 1677–1683. [Google Scholar] [CrossRef]
- Zhou, F.; Lan, H.; Sun, X.; Zhang, H.; Sun, Y.; Du, L.; Zhang, W. Investigation of phase structure stability and thermal expansion coefficient of ytterbia stabilized hafnia. Chin. J Process Eng. 2024, 24, 580–588. [Google Scholar]
- Karem, I.K.; Hamdan, S.A. The Influence of CeO2 Concentration on Some Physical Properties of Y2O3 Thin. Iraqi J Sci. 2022, 63, 2482–2491. [Google Scholar] [CrossRef]
- Wen, G.J.; Zhang, K.B.; Yin, D.; Zhang, H.B. Solid-state reaction synthesis and aqueous durability of Ce-doped zirconolite-rich ceramics(Review). J Nucl. Mater. 2015, 466, 113–119. [Google Scholar] [CrossRef]
- Grieshammer, S. Defect Interactions in the CeO2-ZrO2-Y2O3 Solid Solution. J Phys Chem C. 2017, 121, 15078–15084. [Google Scholar] [CrossRef]
- Li, L.; Sang, S.; Zhu, T.; Li, Y.; Wang, H. Enhancing Hardness and Wear Resistance of MgAl2O4/Fe-Based Laser Cladding Coatings by the Addition of CeO2. Coatings 2024, 14, 55. [Google Scholar] [CrossRef]
- Du, Y.; Song, C.; Wei, Y.; Ma, D.; Pan, B.; Sun, M.; Shi, G.; Wang, Z.; Li, Q. Effect of CeO2-Y2O3 sintering aids on the microstructure and properties of corundum-based composite ceramics. J Asian Ceram Soc. 2024, 11, 517–525. [Google Scholar] [CrossRef]
- Xia, F.; Yan, P.; Ma, C.; Wang, B.; Liu, Y. Effect of different heat-treated temperatures upon structural and abrasive performance of Ni-TiN composite nanocoatings. J Mater Res Technol. 2023, 27, 2874–2881. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Zdorovets, M.V.; Shlimas, D.I. Study of the Morphological and Structural Features of Inert Matrices Based on ZrO2–CeO2 Doped with Y2O3 and the Effect of Grain Sizes on the Strength Properties of Ceramics. Metals 2022, 12, 1687. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, R.; Zhang, T.; Li, M. Effect of CeO2 content on microstructure and properties of laser cladded self-lubricant coatings. Laser Optoelectron Prog. 2018, 55, 111401. [Google Scholar]
- Gan, X.Z.; Yu, Z.C.; Yuan, K.K.; Xu, C.H.; Zhang, G.H.; Wang, X.Q.; Zhu, L.Y.; Xu, D. Effects of cerium addition on the microstructure, mechanical properties and thermal conductivity of YSZ fibers. Ceram Int. 2018, 44, 7077–7083. [Google Scholar] [CrossRef]
- Ma, C.; Wang, C.; Xia, F.; Wang, Q.; Yan, P.; Zhang, Y. Microstructure, wear and corrosion resistances of Ni–ZrO2–CeO2 nano coatings. Ceram Int. 2024, 50, 20949–20957. [Google Scholar] [CrossRef]
- Lee, G.; Park, T.; Choi, S.; Kim, J.; An, G.; Lee, I.; Oh, Y. Core-shell Structured YSZ/CeO2 Composite Thermal Barrier Coating Fabrication and Properties. Korean J Met Mater. 2024, 62, 495–502. [Google Scholar] [CrossRef]
- Ye, F.; Shao, W.; Ye, X.; Liu, M.; Xie, Y.; Bian, P.; Wang, X.; Liu, L.; Wu, H. Microstructure and Corrosion Behavior of Laser-Cladding CeO2-Doped Ni-Based Composite Coatings on TC4. J Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Schoell, R.; Reyes, A.; Suman, G.; Lam, M.N.; Hamil, J.; Rosenberg, S.G.; Treadwell, L.R.; Hattar, K.; Lang, E. Hot Isostatic Pressing Control of Tungsten-Based Composites. Inorganics 2023, 11, 82. [Google Scholar] [CrossRef]
- Lucas, T.J.; Lawson, N.C.; Janowski, G.M.; Burgess, J.O. Effect of grain size on the monoclinic transformation, hardness, roughness, and modulus of aged partially stabilized zirconia. Dent Mater. 2015, 31, 1487–1492. [Google Scholar] [CrossRef]
- Moshtaghioun, B.M.; Gomez-Garcia, D.; Dominguez-Rodriguez, A.; Todd, R.I. Grain size dependence of hardness and fracture toughness in pure near fully-dense boron carbide ceramics. J Eur Ceram Soc. 2016, 36, 1829–1834. [Google Scholar] [CrossRef]
- Bijalwan, V.; Tofel, P.; Holcman, V. Grain size dependence of the microstructures and functional properties of (Ba0.85 Ce0.1-x Cex ) (Zr0.1 Ti0.9 ) O3 lead-free piezoelectric ceramics(Article). J Asian Ceram Soc. 2018, 6, 384–393. [Google Scholar] [CrossRef]
- Venkataraman, R.; Das, G.; Singh, S.R.; Pathak, L.C.; Ghosh, R.N.; Venkataraman, B.; Krishnamurthy, R. Study on influence of porosity, pore size, spatial and topological distribution of pores on microhardness of as plasma sprayed ceramic coatings. MSE 2007, 445, 269–274. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, M.; Miao, X.; Cui, C. Effect of CeO2 on crack sensitivity and tribological properties of Ni60A coatings prepared by laser cladding. AIME 2021, 13, 1–12. [Google Scholar]
- Shu, D.; Cui, X.; Li, Z.; Sun, J.; Wang, J.; Chen, X.; Dai, S.; Si, W. Effect of the rare earth oxide CeO2 on the microstructure and properties of the Nano-WC-reinforced Ni-based composite coating(Article). Metals 2020, 10, 383. [Google Scholar] [CrossRef]
- Shanmugasamy, S.; Balakrishnan, K.; Subasri, A.; Ramalingam, S.; Subramania, A. Development of CeO2 nanorods reinforced electrodeposited nickel nanocomposite coating and its tribological and corrosion resistance properties. J Rare Earths 2018, 36, 1319–1325. [Google Scholar] [CrossRef]
- Sui, X.; Weng, Y.; Zhang, L.; Lu, J.; Huang, X.; Long, F.; Zhang, W. Uncovering the Effect of CeO2 on the Microstructure and Properties of TiAl/WC Coatings on Titanium Alloy. Coatings 2024, 14, 543. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Wang, X.; Zhao, L.; Chu, Y.; Chen, F.; Ge, C.; Fang, S. Preparation and Thermophysical Properties of New Multi-Component Entropy-Stabilized Oxide Ceramics for Thermal Barrier Coatings. Coatings 2023, 13, 937. [Google Scholar] [CrossRef]
- Hou, Z.; Yang, W.; Zhan, Y.; Zhang, X.; Zhang, J. Effect of Calcination Temperature on the Microstructure, Composition and Properties of Agglomerated Nanometer CeO2-Y2O3-ZrO2 Powders for Plasma Spray-Physical Vapor Deposition (PS-PVD) and Coatings Thereof. Nanomaterials-Basel 2024, 14, 995. [Google Scholar] [CrossRef]
- Zhang, H.; Su, J.B.; Duo, S.W.; Zhou, X.; Yuan, J.Y.; Dong, S.J.; Yang, X.; Zeng, J.Y.; Jiang, J.N.; Deng, L.H. Thermal and mechanical properties of Ta2O5 doped La2Ce2O7 thermal barrier coatings prepared by atmospheric plasma spraying. J Eur Ceram Soc. 2019, 39, 2379–2388. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Li, F.; Du, S.; Chen, Z.; Zhao, S.; Zhao, D.; Fan, B.; Wang, B.; Chen, K.; Liu, G. Yb0.5Ca0.75Si7.5Al4.5O1.5N14.5 α-SiAlON ceramics: A hard material with low thermal conductivity. J Eur Ceram Soc. 2024, 44, 5957–5964. [Google Scholar] [CrossRef]
- Che, J.; Huang, W.; Ren, G.; Linghu, J.; Wang, X. Dual-channel phonon transport leads to low thermal conductivity in pyrochlore La2Hf2O7. Ceram Int. 2024, 50, 22865–22873. [Google Scholar] [CrossRef]
- Wang, A.; Li, S.H.; Bao, H. Thermal transport mechanism of electrons and phonons in pristine and defective HfB2. Rare Met. 2023, 42, 3651–3661. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, P.; Yang, F. Role and determining factor of substitutional defects on thermal conductivity: A study of La2(Zr1-xBx)2O7 (B = Hf, Ce, 0 ≤ x ≤ 0.5) pyrochlore solid solutions. Acta Mater. 2014, 68, 106. [Google Scholar] [CrossRef]
- Knoblauch, N.; Mechnich, P. A Novel Method for the Preparation of Fibrous CeO2-ZrO2-Y2O3 Compacts for Thermochemical Cycles. Crystals 2021, 11, 885. [Google Scholar] [CrossRef]
- Li, Q.S.; Zhang, Y.J.; Gong, H.Y.; Sun, H.B.; Li, T.; Guo, X.; Ai, S.H. Effects of graphene on the thermal conductivity of pressureless-sintered SiC ceramics. Ceram Int. 2015, 41, 13547–13552. [Google Scholar] [CrossRef]
| No. | Sample Name | ZrO2 (wt.%) | CeO2 (wt.%) |
|---|---|---|---|
| 1 | ZrO2 | 100 | 0 |
| 2 | 5Ce-ZrO2 | 95 | 5 |
| 3 | 10Ce-ZrO2 | 90 | 10 |
| 4 | 15Ce-ZrO2 | 85 | 15 |
| 5 | 20Ce-ZrO2 | 80 | 20 |
| CeO2 doping amount (wt.%) | Width (mm) | Depth (μm) |
|---|---|---|
| 0 | 2.21 | 132.15 |
| 5 | 2.86 | 97.26 |
| 10 | 2.65 | 91.36 |
| 15 | 2.44 | 71.17 |
| 20 | 2.72 | 105.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





