1. Introduction
The world’s aquatic ecosystems are increasingly threatened by human activities (Dudgeon et al., 2006; Reid et al., 2019), which alter their physical and chemical conditions and exacerbate pollution processes (Søndergaard & Jeppesen, 2007). Aquatic organisms inhabiting these ecosystems are consequently exposed to environmental changes that may adversely affect their physiology (Kaloyianni et al., 2019) and survival. Among these organisms, fish are particularly responsive to environmental conditions and are widely regarded as reliable indicators of aquatic ecosystem degradation (Pinna et al., 2023). Accordingly, fish play a crucial role in ecotoxicological studies and biomonitoring surveys (Chovanec et al., 2003; Naigaga et al., 2011; Kaloyianni et al., 2019) and are commonly used as sentinel organisms across multiple levels of biological organization, ranging from community-level assessments to cellular-level approaches (Chovanec et al., 2003).
At the cellular level, pollutants can disrupt homeostasis in fish (Livingstone, 2001; López-López et al., 2011; Kaloyianni et al., 2019). A primary mechanism underlying this disruption is the induction of oxidative stress in fish tissues, resulting from the excessive production of reactive oxygen species (ROS) (López-López et al., 2011). Elevated ROS levels can trigger lipid peroxidation and lead to significant DNA damage (Juan et al., 2021). In this context, the assessment of oxidative stress–related biomarker responses in bioindicator species have been widely recommended. Biomarkers are measurable indicators of biological responses to environmental changes and provide valuable insights into the effects of pollutants on living organisms (Kaloyianni et al., 2019; Gemusse et al., 2021).
Although biomarkers can serve as crucial tools in environmental assessment, the established approach adopted within the European Union is the implementation of the European Water Framework Directive 2000/60/EC (WFD). WFD has promoted the biomonitoring of surface waters (inland, transitional, coastal) across Europe and requires all EU Member States to achieve at least good ecological status or potential for their surface water bodies by the year 2027. The assessment of ecological status is based on the condition of biological quality elements (BQEs), such as phytoplankton, aquatic macrophytes, phytobenthos, benthic macroinvertebrates and fish. Specifically, in accordance with WFD requirements, the ecological status of surface water bodies should be evaluated using biological indices that rely on well-defined pressure–response relationships. Moreover, these indices assess ecological quality/status as a deviation from reference conditions, defined as the absence of significant human disturbance, and express results on a five-class color-coded scale ranging from 0 (bad quality/status) to 1 (high quality/status).
Following this concept, the use of biomarkers could support the implementation of the WFD, as they may function as early warning systems for environmental degradation (Lomartire et al. 2021). Carassius gibelio, a widely distributed freshwater fish (Froese & Pauly, 2025), has been successfully used in ecotoxicological studies and has been proposed as a reliable bioindicator organism (Simonyan et al., 2016; Kaloyianni et al., 2019; Bobori et al., 2020; Mijošek et al., 2021). Consequently, the assessment of biomarker responses in such a widespread species could provide valuable insights into ecosystem health, particularly when focusing on specific tissues that play crucial role in species population persistence. Gonads, the reproductive organs of fish, are lipid-rich tissues that are highly responsive to pollution (Corsi et al., 2003); however, they remain relatively underutilized as target tissues in ecotoxicological research. To date, only a limited number of studies have investigated oxidative stress biomarkers in fish gonads (e.g., Kaptaner, 2015; Tyor & Pahwa, 2018; El-Shenawy et al., 2021; Pahwa et al., 2022).
The present study aimed to investigate the responses of sensitive biochemical and genotoxic biomarkers related to oxidative stress - Malondialdehyde (MDA) levels and DNA damage - in the gonads of the freshwater fish, the Prussian carp Carassius gibelio (Bloch, 1782), a successful invasive species in Greek lakes (Perdikaris et al., 2012). In detail, we focused on assessing and comparing the ecotoxicological responses of fish gonads in relation to the physicochemical characteristics and ecological quality status of four studied lakes, testing the hypothesis that differences in lake ecological quality would be reflected in variations in biomarker responses. To our knowledge, this is the first study to examine oxidative stress biomarkers in the gonads of C. gibelio in Greek lakes, and also the first attempt to correlate biochemical and genotoxic biomarkers with environmental variables and further to lakes ecological quality.
2. Materials and Methods
2.1. Study Area
The study included four natural lakes located in Northern Greece, namely Doirani, Vegoritida, Volvi, Petron (
Figure 1). Among them, Lake Doirani is transboundary and shared with the Republic of North Macedonia. The studied lakes vary in their limnological and physicochemical characteristics (
Table 1), as well as their ecological quality classification based on different national ecological quality indices (
Table 2).
2.2. Fish Species Selection and Sample Procedure
A total of 32 adult C. gibelio individuals (n=8 from each lake) were provided by local professional fishermen in January of 2023. The species was selected based on its abundance and frequent occurrence in commercial catches of the selected lakes, as well as its previous use in ecotoxicological studies (Kaloyianni et al. 2019). C. gibelio is common in shallow eutrophic lakes and is sold in local markets for human consumption, with some quantities exported to EU countries (Leonardos 2015). Specimens were immediately placed on dry-ice and transferred to the laboratory where they were measured for total length (to the nearest 1 mm) and weighed (to the nearest 0.1 g). Then dissected for gonad tissue collection. Samples were stored at -80oC until further analyses. Fish treatment was conducted in accordance with local guidelines for the care of animals, complying with the Official Journal of the Greek Government No. 106/30 April 2013 on the protection of animals used for scientific purposes.
2.3. Biochemical and Genotoxic Indicators
2.3.1. Lipid Peroxidation Quantification, MDA Levels Measurement
Gonad samples were analyzed following the method described by Niehaus and Samuelsson (1968). This experimental procedure is based on the formation of lipid peroxyl radicals and hydroperoxides as the result of the reaction of free radicals or non-radical species with polyunsaturated fatty acids (PUFAs). Results are expressed as nmol MDA per mg protein since one of the terminal products of lipid peroxidation is MDA (malondialdehyde), a reactive aldehyde which forms an adduct with two thiobarbituric acid molecules producing a pink color compound (TBARS). The concentration of TBARS was detected spectrophotometrically at 535 nm as the absorbance is proportional to MDA concentration (ε 1.5 * 105 L/ mol cm) (Wills, 1969). The quantification of the total protein concentration was conducted using bovine serum albumin as a standard solution according to the Bradford method (Bradford,1976). The results are expressed as nmol MDA/mg protein. The procedure followed was described in detail in Dimitriadi et al. (2021).
2.3.2. Alkaline Single-Cell Gel Electrophoresis (Comet Assay)
Gonad samples were analyzed following the comet assay protocol as modified by Dailianis et al. (2005). The procedure followed was described in detail in Dimitriadi et al. (2021). In brief, gonad cells were treated with collagenase, embedded in agarose, underwent cell lysis and electrophoresis under alkaline conditions. Finally, after ethidium bromide staining, the presence of comets was examined under an inverted fluorescence microscope. Four slides per sample were measured as technical replicates. One hundred cells were randomly selected and scored from each slide using the software Tritek CometScoreTM 2.0 (Tritek Corporation, Wilmington, DE, USA). The findings are expressed as a percentage of DNA in the tail (%DNA in tail) (
Figure 2). %DNA in tail and Olive moment in positive control data (1 μM H
2O
2) were 28.3 ± 5.2 and 40 ± 6.3, respectively.
2.4. Data Acquire and Statistical Analysis
Morphometric data for the studied lakes were obtained from the Greek River Basins Management Plans (2nd Revision 2024) of the Special Secretariat for Waters of the Greek Ministry of Environment (
https://wfdver.ypeka.gr/el/home-gr/), assessed on August 2025, and Zervas et al. (2021). The lakes’ physicochemical characteristics were derived from the publicly available data of the Greek Biotope/Wetland Centre (EKBY,
https://wfd.ekby.gr/apotelesmata/biologika-physikochimika-dedomena/) and assessed on August 2025. The latter correspond to the years 2020-2022, a period reflecting the presence of the sampled fish individuals in the lakes.
All data were first explored for normality using the Shapiro-Wilk test and for homogeneity of variances using Levene’s test. As the majority of the biomarker, total length, and weight data deviated from normality, non-parametric statistical tests were applied throughout the study. Thus, the independent Kruskal-Wallis tests were performed to test whether fish total length, weight and biomarker values differed significantly across lakes (p < 0.05). When significant differences were detected, pairwise comparisons were contacted using Mann-Whitney U tests with Bonferroni correction to identify specific differences between lakes for each biomarker (p < 0.05). Relationships of biomarker responses with environmental variables were assessed using the non-parametric Spearman’s rank correlation. Moreover, to examine the association between biomarker responses and lake ecological quality, fish biomarker values were regressed against the corresponding lake EQR values, as estimated using various ecological indices. All statistical analyses were conducted using IBM SPSS ver. 29 (SPSS, Inc. Chicago, USA) with significance level set at p < 0.05.
3. Results
3.1. Fish Morphometrics
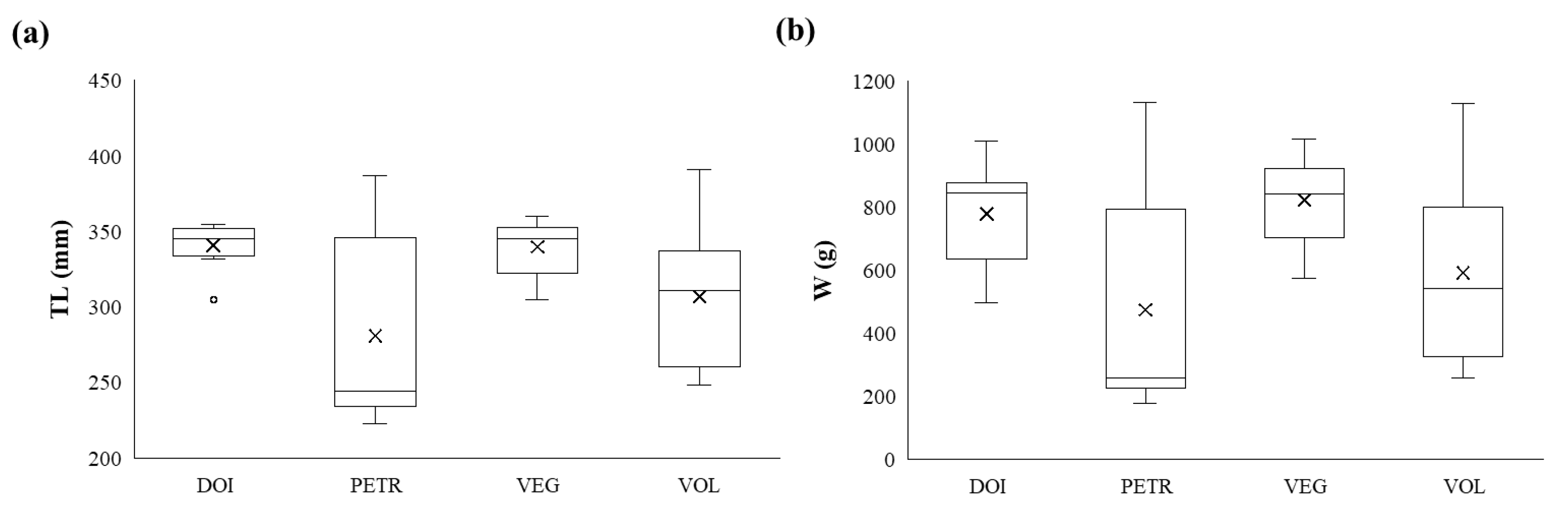
Fish mean total length (TL ± standard error) was 341
± 5.7 mm for specimens from Lake Doirani, 281
± 22.6 mm for specimens from Lake Petron, 340
± 6.8 mm for specimens from Lake Vegoritida, 307
± 17 mm for specimens from lake Volvi. Accordingly, fish weight averaged 765
± 66.9 g for specimens from Lake Doirani, 399
± 132.8 g for Lake Petron, 858
± 41.3 g for Lake Vegoritida and 562
± 118.9 g for Lake Volvi. The distribution of fish total lengths and weights (
Figure 2) did not differ significantly among lakes (Kruskal-Wallis, p > 0.05). These measurements indicate that the sampled individuals were predominantly 2–3 years old (Zhelev et al., 2015). This age class corresponds to the period 2020–2022, reflecting the time during which the sampled fish were present in the lakes and, therefore, their period of exposure to environmental stressors.
3.2. Oxidative Stress Biomarkers
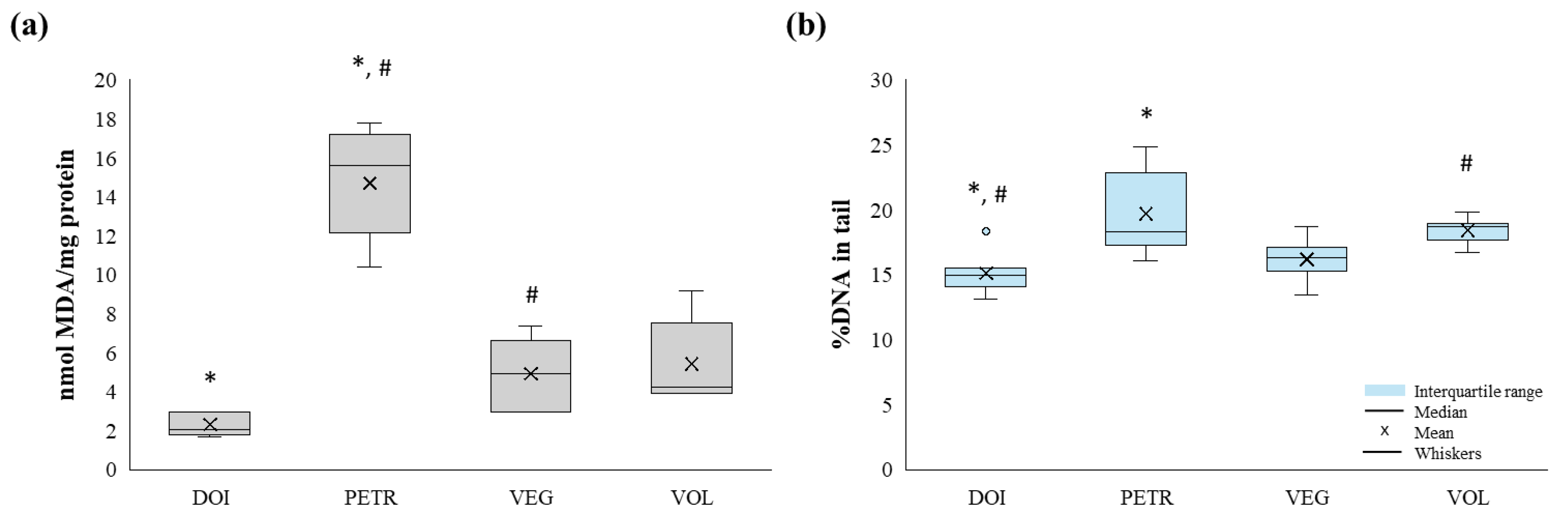
MDA values differed significantly across lakes (Kruskal-Wallis test, p < 0.01;
Table 3,
Figure 3). Mean MDA levels were higher in the gonads of fish collected from Lake Petron compared to those from the other studied lakes (
Table 3). More specifically, mean MDA levels were significantly higher (approximately sixfold) in fish from Lake Petron than in specimens from Lake Doirani (Mann-Whitney, p < 0.01) where the lowest mean MDA value was observed, as well as those from Lake Vegoritida (Mann-Whitney test, p < 0.01).
Mean DNA damage values also differed significantly among lakes (Kruskal–Wallis test, p < 0.01), following the same pattern observed for MDA. Gonads of fish collected from Lake Petron exhibited a higher degree of DNA fragmentation compared to those from the other studied lakes (
Table 3,
Figure 3). Specifically, mean DNA damage values were 1.3-fold higher in fish from Lake Petron than in those from Lake Doirani (Mann–Whitney test, p < 0.01). In addition, mean DNA damage values in samples from Lake Volvi were significantly higher than those from Lake Doirani (Mann–Whitney test, p < 0.05), while specimens from Lake Vegoritida and Lake Volvi exhibited mean DNA damage values that were 1.1- and 1.2-fold higher, respectively, compared to samples from Lake Doirani.
Overall, both biomarkers followed a consistent trend, with gonadal samples from Lake Petron exhibiting the highest biomarker values, whereas those from Lake Doirani showed the lowest.
3.3. Biomarker- Environmental Parameter Relationships
MDA levels and DNA damage showed no significant correlation (p > 0.05) with the examined morphological and environmental lake parameters (
Table 4). However, a significant interrelationship (p < 0.01) was observed between MDA levels and DNA damage (
Table 4).
3.4. Biomarker-ecological quality relationships
Biomarker values were regressed against lake water ecological quality, as assessed by various biological indices (
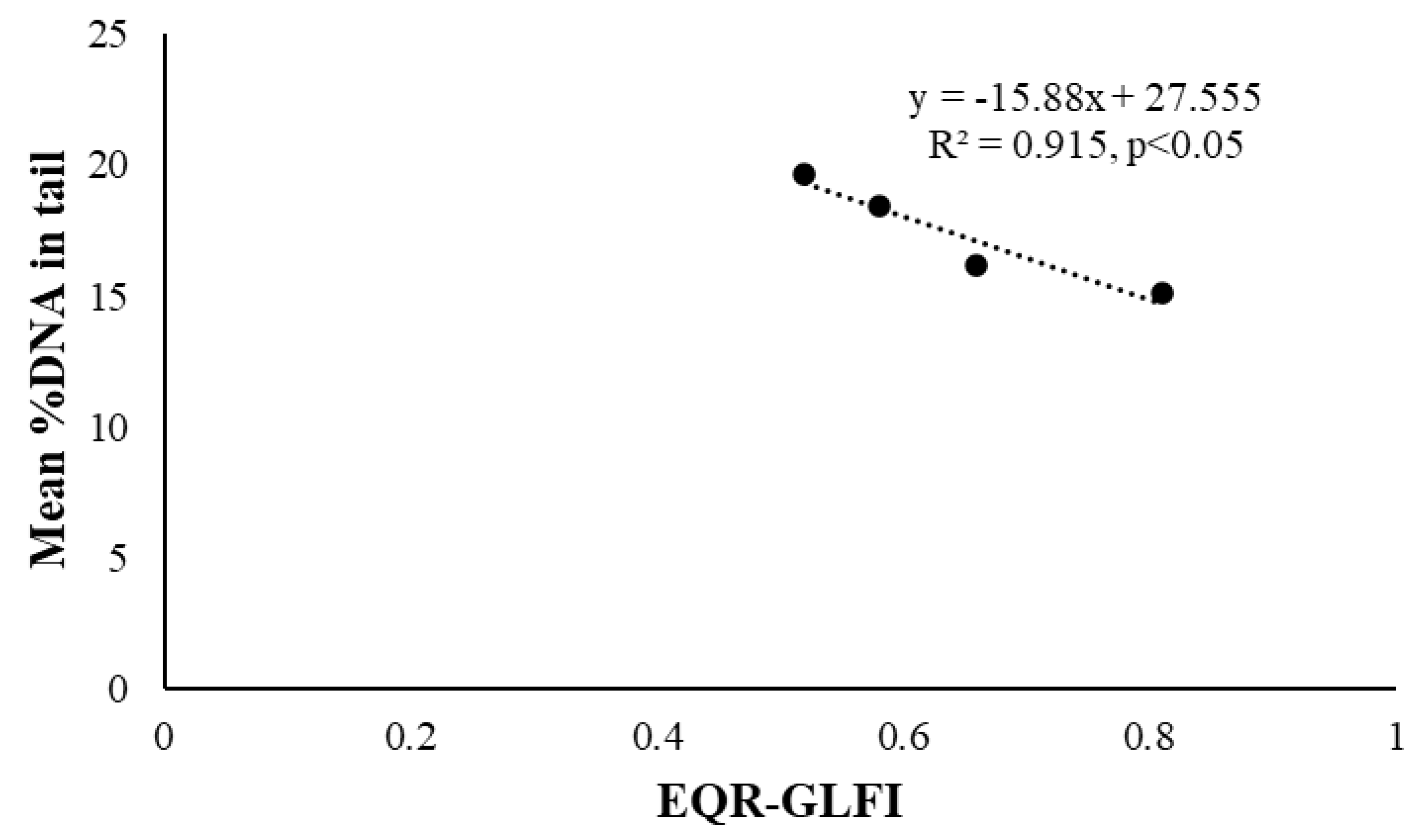
Table 2). Lipid peroxidation levels (MDA) did not show significant relationships with any of the tested ecological quality indices. In contrast, mean %DNA in tail exhibited a significant negative association only with the fish-based ecological quality index (GLFI; R
2 = 0.915, p < 0.05). Specifically, DNA damage increased as GLFI values decreased (
Figure 4). Overall, genotoxic biomarkers appeared to be more sensitive to variations in lake ecological quality than lipid peroxidation. This negative correlation is more obvious in
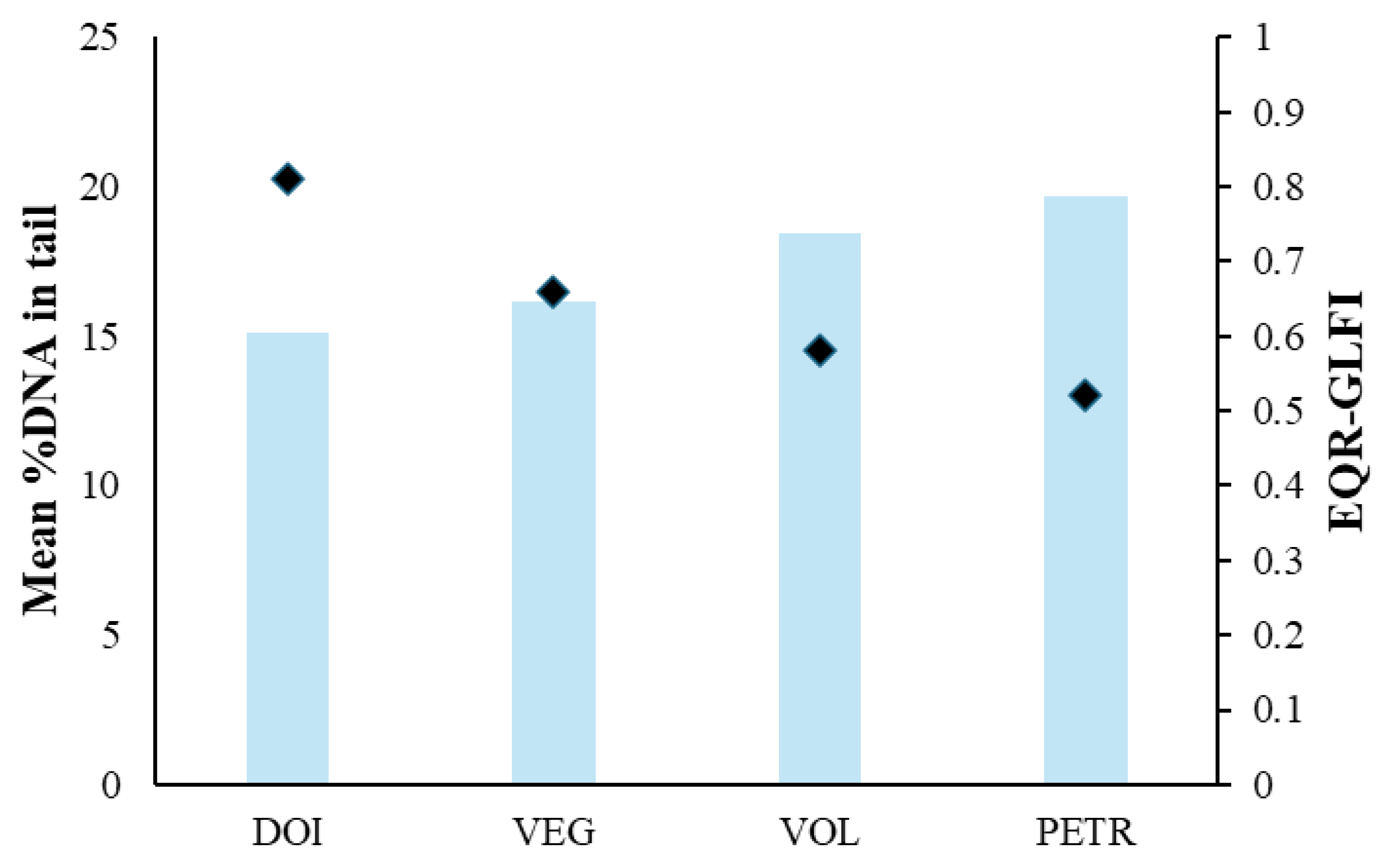
Figure 5 where the mean values of the two biomarkers are presented in relation to the EQR values of the lakes.
4. Discussion
There is a growing body of research employing biochemical and molecular biomarkers in fish as effective tools for environmental biomonitoring of aquatic ecosystems (Santana et al., 2018; Kaloyianni et al., 2019; Bobori et al., 2020; Lombardero et al., 2024). Among these, malondialdehyde (MDA), a marker of lipid peroxidation, and DNA damage, a genotoxicity indicator, have been widely proposed as sensitive biomarkers responding to a variety of environmental stressors and pollutants in fish (Kaloyianni et al., 2019; 2020; 2021).
In the present study, these two biomarkers were assessed in fish gonads collected from four lakes, with the aim of exploring potential associations between biomarker responses and the ecological quality of lake waters. Most ecotoxicological studies investigating oxidative stress and genotoxicity in fish have focused on tissues such as gills, liver, spleen, heart, and muscle (Farombi et al., 2007; Oliva et al., 2012; Orso et al., 2023), whereas fish gonads have been used comparatively rarely (Kroon et al., 2017). Gonadal tissue was selected in this study due to its high content of polyunsaturated fatty acids (PUFAs) (Ezenwosu et al., 2021), which renders it particularly susceptible to oxidative damage and pollutant-induced stress (Santos et al., 2013; Kaptaner et al., 2016; Tyor & Pahwa, 2018; El-Shenawy et al., 2021).
Elevated lipid peroxidation and DNA damage in fish gonads have been consistently reported in contaminated environments (Gagnaire et al., 2017; Barros et al., 2017; Tyor & Pahwa, 2017; Bhat et al., 2023). MDA, in particular, has been proposed as a reliable biomarker against heavy metal contamination in the gonads of Oreochromis niloticus (El-Shenawy et al., 2021). Kaptaner (2015) reported an increase in lipid peroxidation and histological lesions in the gonads of Alburnus tarichi from impacted lake systems, followed by reduction in gonadosomatic index. Moreover, Kaptaner et al. (2016) have linked histological abnormalities to increased oxidative stress responses, suggesting that water pollution in lake waters may affect the reproductive health of fish. In addition, Tyor & Pahwa (2018), observed oxidative stress-related increases of MDA in testes of Clarias gariepinus from contaminated areas of a riverine system. Also, Pahwa et al. (2022) reported a significant decline in antioxidant enzyme activity in the ovaries of Clarias gariepinus from the polluted areas. Finally, in vitro exposure experiments have demonstrated that pollutants can induce increased MDA levels in fish gonads (Zhang et al., 2016; Sumi & Chitra, 2019; Sha et al., 2021; Kutluyer et al., 2022; Sutha et al., 2022)
With respect to genotoxicity, both field and laboratory studies have demonstrated that environmental contaminants can induce DNA damage in fish gonads (Faßbender & Braunbek, 2013; Liu et al., 2014; Beaton et al., 2019). Santos et al. (2013) demonstrated a link between sperm DNA integrity and reproductive impairment in Gasterosteus aculeatus. In general, genotoxicity is suggested to disrupt reproductive processes through mechanisms such as gamete loss via cell death, increased embryonic mortality, and harmful heritable mutations, all of which can affect recruitment rates and population dynamics (Anderson & Wild, 1994; Santos et al., 2013). In parallel, high oxidative stress in gonads may be indicative of impaired reproductive capacity (Samarin et al., 2019). According to Devaux et al. (2011), DNA damage in the sperm cells of Salmo trutta and Salvelinus alpinus is associated with decreased reproductive rates. Additionally, environmental contaminants have been shown to reduce the motility and fertilization ability of fish spermatozoa that result to a high level of infertility (Ezenwosu et al., 2021; Hatef et al., 2013). In line with this notion, it's plausible to speculate that the lower biomarker levels observed in fish gonads from Lake Doirani may indicate reduced oxidative stress and, consequently, a higher potential for reproductive health compared to fish from the other studied lakes. In contrast, the elevated MDA and DNA damage levels observed in the gonads of fish from lake Petron – a shallower and more eutrophic lake - may suggest impaired reproductive performance, as pollutant-induced alterations and lesions in gonadal tissue can lead to reduced fertility (Ezenwosu et al., 2021).
Our results revealed clear differences in biomarker levels among the studied lakes. Specifically, fish from Lake Doirani exhibited the lowest mean levels of both MDA and DNA damage, suggesting comparatively lower oxidative and genotoxic stress in this system. In contrast, fish collected from Lake Petron showed markedly elevated levels of both biomarkers, indicating increased oxidative stress in gonadal tissue, as reflected by enhanced lipid peroxidation and DNA fragmentation. These findings are consistent with the observed environmental status of the lakes: Lake Petron is characterized by higher concentrations of total phosphorus and total suspended solids, as well as lower Secchi disc depth, indicative of greater eutrophication pressure. Furthermore, Lake Petron is classified as having moderate ecological quality based on the Greek Lake Fish Index (GLFI), exhibiting the lowest ecological quality ratio (EQR) among the studied lakes. The GLFI is an ecological quality index designed to reflect the response of lake fish communities to eutrophication, particularly total phosphorus levels, by incorporating metrics such as the relative contribution of omnivorous fish species to total catch (Petriki et al., 2017). Although no direct relationships were detected between the studied biomarkers and individual environmental parameters, higher GLFI values were generally associated with lower MDA levels and reduced DNA damage. This pattern supports a negative relationship between oxidative/genotoxic stress in fish gonads and overall ecological quality. Conversely, the low biomarker levels observed in Lake Doirani are in agreement with its classification as having high ecological quality according to GLFI, suggesting that the biomarker responses measured here reflect differences in lake ecological condition.
In general, environmental parameters linked to eutrophication and anthropogenic pressure appear to play an important role in shaping oxidative stress responses in fish tissues. Previous studies have shown that eutrophication can elevate oxidative stress while suppressing antioxidant defenses (Stoliar & Luschack, 2012). Furthermore, the presence of high chlorophyll levels in lake systems correlates with increased MDA values, as observed in the gills of Liza aurata (Pereira et al., 2010). MDA levels were also increased in the liver of Cyprinus carpio and Hypophthalmichthys nobilis from eutrophic lakes affected by cyanobacterial blooms (Moutou et al., 2012; Sun et al., 2013). In addition to eutrophication, elevated levels of BOD5 and TSS have been associated to increased MDA levels in fish tissues (Pandey et al., 2003; Morais et al., 2016). Moreover, low dissolved oxygen levels and highwater turbidity contribute to elevated MDA and DNA damage in fish from rivers under anthropogenic stress (Barros et al., 2017; Rubio-Vargas et al., 2024). According to Castro et al. (2020), increased lipid peroxidation and DNA damage in the sperm cells of Colossoma macropomum, due to elevated water temperature, high pH, and hypoxia, may adversely affect reproduction and reduce sperm quality. These observations support the interpretation that the biomarker patterns observed in the present study may be related to differences in water quality and trophic status among the lakes.
Overall, our research enhances the comprehension of the correlation between biomarkers associated with oxidative stress and lake water ecological quality, as assessed by fish-based indices, in accordance with the WFD. While community-level biological metrics remain central to ecological status assessment under the WFD, the present findings suggest that MDA levels and DNA damage in fish gonads may provide complementary, early-warning signals of ecosystem disturbance. Following this concept, community metrics and ecotoxicological approaches seem to be complementary (Martinez-Haro et al., 2022; Novais et al., 2023), reinforcing the need to use combined approaches of different disciplines to achieve the best and early evaluation of the ecosystem health and ecological water quality. Moreover, the integration of ecotoxicological biomarkers with biological indices may enhance the sensitivity and timeliness of lake water quality assessment. Further research is required to validate these biomarkers across broader spatial and temporal scales and to support their potential incorporation into harmonized ecological assessment and classification schemes.
5. Conclusions
The current study suggests that measuring MDA levels and DNA damage in fish gonads may reveal variations in environmental conditions and differences in ecological quality across different lake water bodies. Notably, significant correlations were observed between DNA damage and the Greek Lake Fish Index (GLFI). Higher levels of oxidative stress biomarkers were associated with lower ecological quality, indicating that these biomarkers could be useful as early indicators of water quality. However, additional research is needed to confirm these results and to assess the broader applicability of biomarkers in evaluating ecological quality and biomonitoring in freshwater ecosystems.
Author Contributions
Conceptualization, D.B. and M.K.; methodology, D.P.; investigation, D.P.; data curation, D.P. and O.P.; writing—original draft preparation, D.P. and O.P.; writing—review and editing, D.B. and M.K.; visualization, D.P. and O.P.; supervision, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Fish treatment was conducted in accordance with local guidelines for the care of animals, complying with the Official Journal of the Greek Government No. 106/30 April 2013 on the protection of animals used for scientific purposes.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anderson, S. L.; Wild, G. C. Linking genotoxic responses and reproductive success in ecotoxicology. Environmental Health Perspectives 1994, 102, 9–12. [Google Scholar] [CrossRef]
- Barros, I. T.; Ceccon, J. P.; Glinski, A.; Liebel, S.; Grötzner, S. R.; Randi, M. A. F.; Benedito, E.; Orolani-Machado, C. F.; Neto, F. F.; de Oliveira Ribeiro, C. A. Environmental risk assessment in five rivers of Parana River basin, Southern Brazil, through biomarkers in Astyanax spp. Environmental Science and Pollution Research 2017, 24(19), 16228–16240. [Google Scholar] [CrossRef]
- Beaton, E. D.; Gosselin, I.; Festarini, A.; Gagnaire, B.; Farrow, F.; Cavalié, I.; Shultz, C.; Kim, S. B.; Walsh, S.; Chen, H. Q.; Adam-Guillermin, C.; Stuart, M. Correlated responses for DNA damage, phagocytosis activity and lysosomal function revealed in a comparison between field and laboratory studies: fathead minnow exposed to tritium. Science of the Total Environment 2019, 662, 990–1002. [Google Scholar] [CrossRef]
- Bhat, R. A.; Bakhshalizadeh, S.; Guerrera, M. C.; Kesbiç, O. S.; Fazio, F. Toxic effect of heavy metals on ovarian deformities, apoptotic changes, oxidative stress, and steroid hormones in rainbow trout. Journal of Trace Elements in Medicine and Biology 2023, 75, 127106. [Google Scholar] [CrossRef]
- Bobori, D.; Dimitriadi, A.; Karasiali, S.; Tsoumaki-Tsouroufli, P.; Mastora, M.; Kastrinaki, G.; Feidantsis, K.; Printzi, A.; Koumoundrouros, G.; Kaloyianni, M. Common mechanisms activated in the tissues of aquatic and terrestrial animal models after TiO2 nanoparticles exposure. Environment International 2020, 138, 105611. [Google Scholar] [CrossRef]
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry 1976, 72(1–2), 248–254. [Google Scholar] [CrossRef]
- Castro, J. S.; Braz-Mota, S.; Campos, D. F.; Souza, S. S.; Val, A. L. High temperature, pH, and hypoxia cause oxidative stress and impair the spermatic performance of the Amazon fish Colossoma macropomum. Frontiers in Physiology 2020, 11, 772. [Google Scholar] [CrossRef]
- Chovanec, A.; Hofer, R.; Schiemer, F. Fish as bioindicators. In Trace metals and other contaminants in the environment; Elsevier, 2003; Vol. 6, pp. 639–676. [Google Scholar]
- Corsi, I.; Mariottini, M.; Sensini, C.; Lancini, L.; Focardi, S. Cytochrome P450, acetylcholinesterase and gonadal histology for evaluating contaminant exposure levels in fishes from a highly eutrophic brackish ecosystem: the Orbetello Lagoon, Italy. Marine Pollution Bulletin 2003, 46(2), 203–212. [Google Scholar] [CrossRef]
- Dailianis, S.; Piperakis, S. M.; Kaloyianni, M. Cadmium effects on ROS production and DNA damage via adrenergic receptors stimulation: role of Na+/H+ exchanger and PKC. Free Radical Research 2005, 39(10), 1059–1070. [Google Scholar] [CrossRef]
- Devaux, A.; Fiat, L.; Gillet, C.; Bony, S. Reproduction impairment following paternal genotoxin exposure in brown trout (Salmo trutta) and Arctic charr (Salvelinus alpinus). Aquatic Toxicology 2011, 101(2), 405–411. [Google Scholar] [CrossRef]
- Dimitriadi, A.; Papaefthimiou, C.; Genizegkini, E.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Bobori, D. C.; Kastrinaki, G.; Koumoundouros, G.; Lambropoulou, D. A.; Kyzas, G. Z.; Bikiaris, D. N. Adverse effects polystyrene microplastics exert on zebrafish heart–Molecular to individual level. Journal of Hazardous Materials 2021, 416, 125969. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; Sullivan, C.A. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological reviews 2006, 81(2), 163–182. [Google Scholar] [CrossRef]
- El-Shenawy, N. S.; EL-Hak, H. N. G.; Ghobashy, M. A.; Mansour, F. A.; Soliman, M. F. Using antioxidant changes in liver and gonads of Oreochromis niloticus as biomarkers for the assessment of heavy metals pollution at Sharkia province, Egypt. Regional Studies in Marine Science 2021, 46, 101863. [Google Scholar] [CrossRef]
- Ezenwosu, S. U.; Nnamonu, E. I.; Odo, G. E.; Ikele, B. C.; Ani, O. C. Evaluation of lambda-cyhalothrin oxidative stress and gonad histoarchitecture toxicity potency in Clarias gariepinus. The journal of basic and applied zoology 2021, 82(1), 3. [Google Scholar] [CrossRef]
- Farombi, E. O.; Adelowo, O. A.; Ajimoko, Y. R. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African catfish (Clarias gariepinus) from Nigeria Ogun River. International journal of environmental research and public health 2007, 4(2), 158–165. [Google Scholar] [CrossRef]
- Faßbender, C.; Braunbeck, T. Reproductive and genotoxic effects in zebrafish after chronic exposure to methyl methanesulfonate in a multigeneration study. Ecotoxicology 2013, 22(5), 825–837. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase; World Wide Web electronic publication. www.fishbase.org, 2025. [Google Scholar]
- Gagnaire, B.; Adam-Guillermin, C.; Festarini, A.; Cavalié, I.; Della-Vedova, C.; Shultz, C.; Kim, S. B.; Ikert, H.; Dubois, C.; Walsh, S.; Farrow, F.; Beaton, D.; Tan, E.; Wen, K.; Stuart, M. Effects of in situ exposure to tritiated natural environments: A multi-biomarker approach using the fathead minnow, Pimephales promelas. Science of the Total Environment 2017, 599, 597–611. [Google Scholar] [CrossRef]
- Gemusse, S. L.; Folle, N. M. T.; da Costa Souza, A. T.; Azevedo-Linhares, M.; Neto, F. F.; Ortolani-Machado, C. F.; de Oliveira Ribeiro, C. A. Micropollutants impair the survival of Oreochromis niloticus and threat local species from Iguaçu River, Southern of Brazil. Environmental Toxicology and Pharmacology 2021, 83, 103596. [Google Scholar] [CrossRef]
- Greek Biotope/Wetland Centre (EKBY). 8 2025. Available online: https://wfd.ekby.gr/apotelesmata/biologika-physikochimika-dedomena/.
- Hatef, A.; Alavi, S. M. H.; Golshan, M.; Linhart, O. Toxicity of environmental contaminants to fish spermatozoa function in vitro—A review. Aquatic toxicology 2013, 140, 134–144. [Google Scholar] [CrossRef]
- Juan, C. A.; Pérez de la Lastra, J. M.; Plou, F. J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. International journal of molecular sciences 2021, 22(9), 4642. [Google Scholar] [CrossRef]
- Kaloyianni, M.; Feidantsis, K.; Nteli, I.; Stergiou, P.; Tsoulia, T.; Dimitriadi, A.; Antonopoulou, E.; Bobori, D. Biochemical and molecular responses of cyprinids in two Mediterranean lacustrine ecosystems: Opportunities for ecological assessment and biomonitoring. Aquatic toxicology 2019, 211, 105–115. [Google Scholar] [CrossRef]
- Kaloyianni, M.; Dimitriadi, A.; Ovezik, M.; Stamkopoulou, D.; Feidantsis, K.; Kastrinaki, G.; Gallios, G.; Tsiaoussis, I.; Koumoundouros, G.; Bobori, D. Magnetite nanoparticles effects on adverse responses of aquatic and terrestrial animal models. Journal of hazardous materials 2020, 383, 121204. [Google Scholar] [CrossRef]
- Kaloyianni, M.; Bobori, D. C.; Xanthopoulou, D.; Malioufa, G.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Kastrinaki, G.; Dimitriadi., A.; Koumoundouros, G.; Lambropoulou, D. A.; Kyzas, G. Z.; Bikiaris, D. N. Toxicity and functional tissue responses of two freshwater fish after exposure to polystyrene microplastics. Toxics 2021, 9(11), 289. [Google Scholar] [CrossRef]
- Kaptaner, B. Relation between increased oxidative stress and histological abnormalities in the ovaries of Alburnus tarichi in Lake Van, Turkey. Environmental Monitoring and Assessment 2015, 187(11), 702. [Google Scholar] [CrossRef]
- Kaptaner, B.; Kankaya, E.; Dogan, A.; Durmuş, A. Alterations in histology and antioxidant defense system in the testes of the lake Van fish (Alburnus tarichi Güldenstädt, 1814). Environmental monitoring and assessment 2016, 188(8), 474. [Google Scholar] [CrossRef]
- Kroon, F.; Streten, C.; Harries, S. A protocol for identifying suitable biomarkers to assess fish health: A systematic review. PloS one 2017, 12(4), e0174762. [Google Scholar] [CrossRef]
- Kutluyer, F.; Çakir Sahilli, Y.; Kocabaş, M.; Aksu, Ö. Sperm quality and oxidative stress in chub Squalius orientalis and Padanian barbel Barbus plebejus (Teleostei: Cyprinidae) after in vitro exposure to low doses of bisphenol A. Drug and Chemical Toxicology 2022, 45(1), 8–13. [Google Scholar] [CrossRef]
- Leonardos, I.D. Craig, J.F., Ed.; Freshwater fisheries ecology. In Fisheries ecology of Greece; Chichester, UK; John Wiley & Sons, Ltd, 2015; pp. 292–303. [Google Scholar]
- Liu, J.; Lu, G.; Wu, D.; Yan, Z. A multi-biomarker assessment of single and combined effects of norfloxacin and sulfamethoxazole on male goldfish (Carassius auratus). Ecotoxicology and environmental safety 2014, 102, 12–17. [Google Scholar] [CrossRef]
- Livingstone, D. R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine pollution bulletin 2001, 42(8), 656–666. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J. C.; Gonçalves, A. M. Biomarkers based tools to assess environmental and chemical stressors in aquatic systems. Ecological Indicators 2021, 122, 107207. [Google Scholar] [CrossRef]
- Lombardero, L. R.; Pérez, D. J.; Medici, S. K.; Mendieta, J. R.; Iturburu, F. G.; Menone, M. L. Usefulness of oxidative stress biomarkers in native species for the biomonitoring of pesticide pollution in a shallow lake of the Austral Pampas, Argentina. Chemosphere 2024, 353, 141578. [Google Scholar] [CrossRef]
- López-López, E.; Sedeño-Díaz, J. E.; Soto, C.; Favari, L. Responses of antioxidant enzymes, lipid peroxidation, and Na+/K+-ATPase in liver of the fish Goodea atripinnis exposed to Lake Yuriria water. Fish physiology and biochemistry 2011, 37(3), 511–522. [Google Scholar] [CrossRef]
- Martinez-Haro, M.; Acevedo, P.; Pais-Costa, A. J.; Neto, J. M.; Vieira, L. R.; Ospina-Alvarez, N.; Taggart, M. A.; Guilhermino, L.; Ribeiro, R.; Marques, J. C. Ecotoxicological tools in support of the aims of the European Water Framework Directive: A step towards a more holistic ecosystem-based approach. Ecological Indicators 2022, 145, 109645. [Google Scholar] [CrossRef]
- Mavromati, E.; Kemitzoglou, D.; Tsiaoussi, V.; Lazaridou, M. A new WFD—compliant littoral macroinvertebrate index for monitoring and assessment of Mediterranean lakes (HeLLBI). Environmental monitoring and assessment 2021, 193(11), 745. [Google Scholar] [CrossRef]
- Mijošek, T.; Marijić, V. F.; Dragun, Z.; Ivanković, D.; Krasnići, N.; Redžović, Z.; Erk, M. Intestine of invasive fish Prussian carp as a target organ in metal exposure assessment of the wastewater impacted freshwater ecosystem. Ecological indicators 2021, 122, 107247. [Google Scholar] [CrossRef]
- Morais, C. R.; Carvalho, S. M.; Araujo, G. R.; Souto, H. N.; Bonetti, A. M.; Morelli, S.; Júnior, E. O. C. Assessment of water quality and genotoxic impact by toxic metals in Geophagus brasiliensis. Chemosphere 2016, 152, 328–334. [Google Scholar] [CrossRef]
- Moutou, K. A.; Tsikogias, S.; Papadimitriou, T.; Kagalou, I. Oxidative stress in Cyprinus carpio to analyze microcystin impact in eutrophic shallow lakes: a preliminary study. Journal of Environmental Monitoring 2012, 14(8), 2195–2203. [Google Scholar] [CrossRef]
- Naigaga, I.; Kaiser, H.; Muller, W. J.; Ojok, L.; Mbabazi, D.; Magezi, G.; Muhumuza, E. Fish as bioindicators in aquatic environmental pollution assessment: A case study in Lake Victoria wetlands, Uganda. Physics and Chemistry of the Earth, parts A/B/C 2011, 36(14–15), 918–928. [Google Scholar] [CrossRef]
- Niehaus, W. G.; Samuelson, B. Formation of malondialdehyde from and glucose 6-phosphate dehydrogenase from fermenting yeast and phospholipids arachidonate during microsomal lipid peroxidation. European Journal of Biochemistry 1968, 6(1), 126–30. [Google Scholar] [CrossRef]
- Novais, M. H.; Penha, A. M.; Catarino, A.; Martins, I.; Fialho, S.; Lima, A.; Morais, M.; Palma, P. The usefulness of ecotoxicological tools to improve the assessment of water bodies in a climate change reality. Science of the total environment 2023, 901, 166392. [Google Scholar] [CrossRef]
- Ntislidou, C.; Lazaridou, M.; Tsiaoussi, V.; Bobori, D. C. A new multimetric macroinvertebrate index for the ecological assessment of Mediterranean lakes. Ecological Indicators 2018, 93, 1020–1033. [Google Scholar] [CrossRef]
- Oliva, M.; Vicente, J. J.; Gravato, C.; Guilhermino, L.; Galindo-Riaño, M. D. Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a Huelva estuary (SW Spain): seasonal and spatial variation. Ecotoxicology and environmental safety 2012, 75, 151–162. [Google Scholar] [CrossRef]
- Orso, G.; Imperatore, R.; Coccia, E.; Rinaldi, G.; Cicchella, D.; Paolucci, M. A Deep Survey of Fish Health for the Recognition of Useful Biomarkers to Monitor Water Pollution. Environments 2023, 10(12), 219. [Google Scholar] [CrossRef]
- Pahwa, K.; Sharma, R. K.; Tyor, A. K. Biochemical and Ultrastructural Analysis of Ovaries of African Sharptooth Catfish, Clarias gariepinus (Burchell) Exposed to Pollutants from River Yamuna in Delhi Region, India. Biology Bulletin 2022, 49(5), 491–497. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Science of the total environment 2003, 309(1–3), 105–115. [Google Scholar]
- Perdikaris, C.; Ergolavou, A.; Gouva, E.; Nathanailides, C.; Chantzaropoulos, A.; Paschos, I. Carassius gibelio in Greece: the dominant naturalised invader of freshwaters. Reviews in Fish Biology and Fisheries 2012, 22(1), 17–27. [Google Scholar] [CrossRef]
- Pereira, P.; de Pablo, H.; Vale, C.; Pacheco, M. Combined use of environmental data and biomarkers in fish (Liza aurata) inhabiting a eutrophic and metal-contaminated coastal system–Gills reflect environmental contamination. Marine Environmental Research 2010, 69(2), 53–62. [Google Scholar] [CrossRef]
- Petriki, O.; Lazaridou, M.; Bobori, D. C. A fish-based index for the assessment of the ecological quality of temperate lakes. Ecological Indicators 2017, 78, 556–565. [Google Scholar] [CrossRef]
- Pinna, M.; Zangaro, F.; Saccomanno, B.; Scalone, C.; Bozzeda, F.; Fanini, L.; Specchia, V. An Overview of Ecological Indicators of Fish to Evaluate the Anthropogenic Pressures in Aquatic Ecosystems: From Traditional to Innovative DNA-Based Approaches. Water 2023, 15(5), 949. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; Smol, J.P.; Taylor, W.W.; Tockner, K.; Vermaire, J.C.; Dudgeon, D.; Cooke, S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 2019, 94(3), 849–873. [Google Scholar] [CrossRef]
-
River basin management plans, Special Secretariat for Waters, Greek Ministry of Environment. 8 2025. Available online: https://wfdver.ypeka.gr/el/home-gr/.
- Rubio-Vargas, D. A.; de Morais, T. P.; Randi, M. A. F.; Neto, F. F.; de Castro Martins, C.; Oliveira, A. P.; Nazario, M. C.; da Silva Ferreira, F. C. A.; Opuskevitch, I.; Penner, D.; Esquivel-Muelbert, J.; Prodocimo, M. M.; Choueri, R. B.; de Oliveira Ribeiro, C. A. Pollutant bioaccumulation in sentinel fish chronically exposed in Iguaçu River reservoirs (Southern Brazil) and human health risk of fish consumption. Chemosphere 2024, 349, 140812. [Google Scholar] [CrossRef] [PubMed]
- Samarin, A. M.; Samarin, A. M.; Policar, T. Cellular and molecular changes associated with fish oocyte ageing. Reviews in Aquaculture 2019, 11(3), 619–630. [Google Scholar] [CrossRef]
- Santana, M. S.; Yamamoto, F. Y.; Sandrini-Neto, L.; Neto, F. F.; Ortolani-Machado, C. F.; Ribeiro, C. A. O.; Prodocimo, M. M. Diffuse sources of contamination in freshwater fish: Detecting effects through active biomonitoring and multi-biomarker approaches. Ecotoxicology and environmental safety 2018, 149, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Palos-Ladeiro, M.; Besnard, A.; Porcher, J. M.; Bony, S.; Sanchez, W.; Devaux, A. Relationship between DNA damage in sperm after ex vivo exposure and abnormal embryo development in the progeny of the three-spined stickleback. Reproductive Toxicology 2013, 36, 6–11. [Google Scholar] [CrossRef]
- Sha, W.; Cai, F.; Li, Y.; Wang, Y.; Liu, C.; Wang, R.; Gao, P. Biomarker responses and histological damage in the gill, liver, and gonad of Cyprinus carpio with benzo (a) pyrene exposure. Environmental Science and Pollution Research 2021, 28(43), 61290–61301. [Google Scholar] [CrossRef]
- Simonyan, A.; Gabrielyan, B.; Minasyan, S.; Hovhannisyan, G.; Aroutiounian, R. Genotoxicity of water contaminants from the basin of lake Sevan, Armenia evaluated by the Comet Assay in Gibel carp (Carassius auratus gibelio) and Tradescantia Bioassays. Bulletin of environmental contamination and toxicology 2016, 96(3), 309–313. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E. Anthropogenic impacts on lake and stream ecosystems, and approaches to restoration. Journal of Applied Ecology 2007, 44(6), 1089–1094. [Google Scholar] [CrossRef]
- Stoliar, O. B.; Lushchak, V. I. Lushchak, V.I., Ed.; Environmental pollution and oxidative stress in fish. In Oxidative stress-environmental induction and dietary antioxidants; IntechOpen; London, UK, 2012; pp. 131–166. [Google Scholar]
- Sumi, N.; Chitra, K. C. Fullerene C60 nanomaterial induced oxidative imbalance in gonads of the freshwater fish, Anabas testudineus (Bloch, 1792). Aquatic Toxicology 2019, 210, 196–206. [Google Scholar] [CrossRef]
- Sun, H.; Wang, W.; Geng, L.; Chen, Y.; Yang, Z. In situ studies on growth, oxidative stress responses, and gene expression of juvenile bighead carp (Hypophthalmichthys nobilis) to eutrophic lake water dominated by cyanobacterial blooms. Chemosphere 2013, 93(2), 421–427. [Google Scholar] [CrossRef]
- Sutha, J.; Anila, P. A.; Gayathri, M.; Ramesh, M. Long term exposure to tris (2-chloroethyl) phosphate (TCEP) causes alterations in reproductive hormones, vitellogenin, antioxidant enzymes, and histology of gonads in zebrafish (Danio rerio): In vivo and computational analysis. Comparative biochemistry and physiology part C: toxicology & pharmacology 2022, 254, 109263. [Google Scholar]
- Tsiaoussi, V.; Zervas, D.; Tsiripidis, I. Report on the Development of the National Method for the Assessment of the Ecological Status of Natural Lakes in Greece, Using the Biological Quality Element “Phytoplankton”, 1st ed.; Greek Biotope/Wetland Centre and Special Secretariat for Waters, Ministry of Environment; Thermi, Greece, 2017. [Google Scholar]
- Tyor, A. K.; Pahwa, K. Pollutants induced oxidative stress, DNA damage and cellular deformities in Clarias gariepinus (burchell), from river Yamuna in Delhi region, India. Bulletin of environmental contamination and toxicology 2017, 99(1), 33–38. [Google Scholar] [CrossRef]
- Tyor, A. K.; Pahwa, K. Testicular oxidative stress and cellular deformities in Clarias gariepinus (Burchell) from river Yamuna in Delhi region, India. Bulletin of environmental contamination and toxicology 2018, 100(5), 659–664. [Google Scholar] [CrossRef]
- Water Framework Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. In Official Journal of the European Communities; Luxembourg; Volume L327/1.
- Wills, E. D. Lipid peroxide formation in microsomes. General considerations. Biochemical journal 1969, 113(2), 315–324. [Google Scholar] [CrossRef]
- Zervas, D.; Tsiaoussi, V.; Tsiripidis, I. HeLM: A macrophyte-based method for monitoring and assessment of Greek lakes. Environmental Monitoring and Assessment 2018, 190(6), 326. [Google Scholar] [CrossRef]
- Zervas, D.; Tsiaoussi, V.; Kallimanis, A. S.; Dimopoulos, P.; Bergmeier, E.; Tsiripidis, I. Multiple-Facet Diversity Patterns of Aquatic Vegetation in Lakes along a Trophic Gradient. Water 2021, 13(16), 2281. [Google Scholar] [CrossRef]
- Zhelev, Z.; Boyadzhiev, P.; Angelov, M. Analysis of size-age, sexual structure and condition of populations of Carassius gibelio (Pisces: Cyprinidae) from two water basins in Galabovo region (southern Bulgaria). Trakia Journal of Sciences 2015, 2, 185–195. [Google Scholar] [CrossRef]
- Zhang, Q. F.; Li, Y. W.; Liu, Z. H.; Chen, Q. L. Reproductive toxicity of inorganic mercury exposure in adult zebrafish: Histological damage, oxidative stress, and alterations of sex hormone and gene expression in the hypothalamic-pituitary-gonadal axis. Aquatic Toxicology 2016, 177, 417–424. [Google Scholar] [CrossRef]
Figure 1.
Map of the studied lakes.
Figure 1.
Map of the studied lakes.
Figure 2.
Box plots of (a) total length (TL, mm) and (b) weights (W, g) of Carassius gibelio from lakes Doirani, Petron, Vegoritida and Volvi. The lower and upper boundaries of the boxes represent the 25th and 75th percentiles, respectively. The lines (whiskers) indicate the maximum and minimum values of the measurements. The interior line of the box represents the median, while the dot indicates the mean and the circles the outlier values.
Figure 2.
Box plots of (a) total length (TL, mm) and (b) weights (W, g) of Carassius gibelio from lakes Doirani, Petron, Vegoritida and Volvi. The lower and upper boundaries of the boxes represent the 25th and 75th percentiles, respectively. The lines (whiskers) indicate the maximum and minimum values of the measurements. The interior line of the box represents the median, while the dot indicates the mean and the circles the outlier values.
Figure 3.
Boxplots of (a) MDA levels and (b) %DNA in tail estimated in the gonads of Carassius gibelio in lakes Doirani (DOI), Petron (PETR), Vegoritida (VEG) and Volvi (VOL). The lower and upper boundaries of the boxes represent the 25th and 75th percentiles, respectively. The lines (whiskers) indicate the maximum and minimum values of the measurements. The interior line of the box represents the median, while the dot indicates the mean and the circles the outlier values. * and # indicate statistically significant differences (Mann-Whitney pairwise comparisons, p-value<0.05) between biomarker values across lakes.
Figure 3.
Boxplots of (a) MDA levels and (b) %DNA in tail estimated in the gonads of Carassius gibelio in lakes Doirani (DOI), Petron (PETR), Vegoritida (VEG) and Volvi (VOL). The lower and upper boundaries of the boxes represent the 25th and 75th percentiles, respectively. The lines (whiskers) indicate the maximum and minimum values of the measurements. The interior line of the box represents the median, while the dot indicates the mean and the circles the outlier values. * and # indicate statistically significant differences (Mann-Whitney pairwise comparisons, p-value<0.05) between biomarker values across lakes.
Figure 4.
Linear regression of mean %DNA in tail values against the ecological quality ratio (EQR) of each of the studied lake as defined by the Greek Lake Fish Index (GLFI).
Figure 4.
Linear regression of mean %DNA in tail values against the ecological quality ratio (EQR) of each of the studied lake as defined by the Greek Lake Fish Index (GLFI).
Figure 5.
Mean %DNA in tail values (columns) and EQR values (dots) as assessed by the Greek Lake Fish Index (GLFI). DOI, VEG, VOL, PETR for Lakes Doirani, Vegoritida, Volvi and Petron, respectively.
Figure 5.
Mean %DNA in tail values (columns) and EQR values (dots) as assessed by the Greek Lake Fish Index (GLFI). DOI, VEG, VOL, PETR for Lakes Doirani, Vegoritida, Volvi and Petron, respectively.
Table 1.
Morphometric and physicochemical characteristics (mean ± standard error, minimum-maximum values) of the studied lakes. Data acquired from: aRiver Basins Management Plans of the Special Secretariat for Waters, Ministry of Environment, Greece, bZervas et al. (2021) and con line available data for the years 2020-2022 of the Greek Biotope/Wetland Centre (EKBY). DO: Dissolved Oxygen, EC: Electrical Conductivity, TP: Total Phosphorus, NO3: Nitrates, TSS: Total Suspended Solids, BOD5: Biochemical Oxygen Demand.
Table 1.
Morphometric and physicochemical characteristics (mean ± standard error, minimum-maximum values) of the studied lakes. Data acquired from: aRiver Basins Management Plans of the Special Secretariat for Waters, Ministry of Environment, Greece, bZervas et al. (2021) and con line available data for the years 2020-2022 of the Greek Biotope/Wetland Centre (EKBY). DO: Dissolved Oxygen, EC: Electrical Conductivity, TP: Total Phosphorus, NO3: Nitrates, TSS: Total Suspended Solids, BOD5: Biochemical Oxygen Demand.
| Lake |
Areaa (km2) |
Mean depthb
(m)
|
Secchi depthc (m) |
pHc |
DOc
(mg/L)
|
ECc (μS/cm) |
TPc (mg/L) |
NO3c
(mg/L)
|
TSSc (mg/L) |
BOD5c
(mg/L)
|
| |
Mean (± standard error) (minimum-maximum values) |
| Doirani |
38.87 |
4 |
1.48 (±0.14)
(0.8-4.0) |
8.90
(±0.04)
(8.53-9.26) |
10.16 (±0.28) (7.33-13.01) |
686.45
(±14.98)
(583-965.9) |
0.044
(±0.004)
(0.019-0.085) |
0.47 (±0.08) (0.27-2.1) |
7.93 (±0.66)
(1.76-14.89) |
4.52 (±0.49) (0.8-8.5) |
| Petron |
12.36 |
3 |
0.34 (±0.04)
(0.20-0.70) |
8.91 (±0.09)
(8.2-9.55) |
1051 (±0.69)
(7.65-18.39) |
979.82 (±32.88)
(737.7-1162) |
0.066
(±0.006)
(0.022-0.127) |
1.41 (±0.59) (0.27-7.1) |
29.51
(±5.26)
(10.4-96.7) |
5.4 (±0.86) (7.65-18.39) |
| Vegoritida |
53.96 |
25 |
3.14
(±0.28)
(1.10-5.20) |
8.84 (±0.06)
(8.11-9.16) |
9.66 (±0.29)
(7.66-13.26) |
629.27 (±14.54)
(510.2-727.3) |
0.042
(±0.005)
(0.016-0.091) |
0.75 (±0.21) (0.27-3.6) |
2.26
(±0.32)
(0.35-5.67) |
2.92 (±0.5) (0.3-7.3) |
| Volvi |
72.07 |
13 |
1.62
(±0.08)
(1.1-2.5) |
8.94 (±0.05)
(8.5-9.35) |
9.64 (±0.46)
(6.3-13.5) |
923.87 (±23.15) (773.3-1069) |
0.060
(±0.007)
(0.023-0.132) |
1.48 (±0.57) (0.27-7.0) |
3.94
(±0.34)
(0.69-6.75) |
4.7 (±0.7) (6.3-13.5) |
Table 2.
Ecological quality classification of the studied lakes assessed by different Greek indices covering the last decade. EQR: Ecological Quality Ratio, aGLFI: Greek Lake Fish Index (Petriki et al., 2017), bGLBiI: Greek Lake Benthic macroinvertebrate Index (Ntislidou et al., 2018), cHeLPhy: Hellenic Lake Phytoplankton (Tsiaoussi et al., 2017), dHeLM: Hellenic Lake Macrophytes (Zervas et al., 2018), eHelLBI: Hellenic Lake Littoral Benthic macroinvertebrate Index (Mavromati et al., 2021).
Table 2.
Ecological quality classification of the studied lakes assessed by different Greek indices covering the last decade. EQR: Ecological Quality Ratio, aGLFI: Greek Lake Fish Index (Petriki et al., 2017), bGLBiI: Greek Lake Benthic macroinvertebrate Index (Ntislidou et al., 2018), cHeLPhy: Hellenic Lake Phytoplankton (Tsiaoussi et al., 2017), dHeLM: Hellenic Lake Macrophytes (Zervas et al., 2018), eHelLBI: Hellenic Lake Littoral Benthic macroinvertebrate Index (Mavromati et al., 2021).
| Lake |
Quality class (EQR) per Index |
|
GLFIa
|
GLBiIb
|
HelPhyc
|
HeLMd
|
HelLBIe
|
|
| Doirani |
High
(0.81) |
Good
(0.69) |
Moderate
(0.57) |
Good
(0.77) |
Moderate
(0.47) |
|
| Petron |
Moderate
(0.52) |
Moderate
(0.54) |
Good (0.63) |
- |
Moderate
(0.55) |
|
| Vegoritida |
Good (0.66) |
Moderate
(0.54) |
Good
(0.64) |
Good
(0.62) |
Good
(0.69) |
|
| Volvi |
Moderate
(0.58) |
Moderate
(0.41) |
Moderate
(0.46) |
Good
(0.73) |
Moderate
(0.44) |
|
Table 3.
Mean MDA (nmol/mg protein) and %DNA in tail values. Standard error, minimum and maximum values are also provided.
Table 3.
Mean MDA (nmol/mg protein) and %DNA in tail values. Standard error, minimum and maximum values are also provided.
| |
Lake |
| Parameter |
Doirani |
Petron |
Vegoritida |
Volvi |
| Mean (± standard error), minimum - maximum values |
| MDA (nmol/mg protein) |
2.31 (±0.28)
1.67-2.97 |
14.70 (±0.81)
10.39-17.81 |
4.97 (±0.64)
2.97-7.35 |
5.42 (±1)
3.91-9.19 |
| %DNA in tail |
15.1 (±0.54)
13.14-18.35 |
19.68 (±1.15)
16.08-24.81 |
16.19 (±0.53)
13.41-18.73 |
18.44 (±0.34)
16.69-19.82 |
Table 4.
Spearman correlation analysis of environmental parameters and the estimated mean MDA (nmol/mg protein) and %DNA in tail values. ** statistically significant correlations (p < 0.01).
Table 4.
Spearman correlation analysis of environmental parameters and the estimated mean MDA (nmol/mg protein) and %DNA in tail values. ** statistically significant correlations (p < 0.01).
| |
Area |
Mean depth
|
Secchidepth
|
pH |
DO |
EC |
TP |
NO3 |
BOD |
TSS |
Chla |
MeanMDA
|
Mean %DNA in tail
|
| Area |
1 |
0.80 |
0.80 |
0.20 |
-1** |
-0.40 |
-0.40 |
0.40 |
-0.40 |
-0.80 |
-0.80 |
-0.20 |
-0.20 |
| Mean depth |
|
1 |
1** |
-0.40 |
-0.80 |
-0.80 |
-0.80 |
0.01 |
-0.80 |
-1** |
-1** |
-0.40 |
-0.40 |
| Secchi depth |
|
|
1 |
-0.40 |
-0.80 |
-0.80 |
-0.80 |
0.01 |
-0.80 |
-1** |
-1** |
-0.40 |
-0.40 |
| pH |
|
|
|
1 |
-0.20 |
0.80 |
0.80 |
0.80 |
0.80 |
0.40 |
0.40 |
0.60 |
0.60 |
| DO |
|
|
|
|
1 |
0.40 |
0.40 |
-0.40 |
0.40 |
0.80 |
0.80 |
0.20 |
0.20 |
| EC |
|
|
|
|
|
1 |
1** |
0.60 |
1** |
0.80 |
0.80 |
0.80 |
0.80 |
| TP |
|
|
|
|
|
|
1 |
0.60 |
1** |
0.80 |
0.80 |
0.80 |
0.80 |
| NO3 |
|
|
|
|
|
|
|
1 |
0.60 |
0.01 |
0.01 |
0.80 |
0.80 |
| BOD |
|
|
|
|
|
|
|
|
1 |
0.80 |
0.80 |
0.80 |
0.80 |
| TSS |
|
|
|
|
|
|
|
|
|
1 |
1** |
0.40 |
0.40 |
| Chla |
|
|
|
|
|
|
|
|
|
|
1 |
0.40 |
0.40 |
| Mean MDA |
|
|
|
|
|
|
|
|
|
|
|
1 |
1** |
| Mean %DNA in tail |
|
|
|
|
|
|
|
|
|
|
|
|
1 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).