1. Introduction
In recent years, increasing attention has been directed toward the human immune system and strategies to strengthen its function. A growing body of evidence indicates that physical inactivity, tobacco use, excessive alcohol consumption, diets high in ultra-processed foods, excessive intake of sugar and salt, insufficient sleep, and chronic psychological stress are associated with impaired immune function [
1]. In contrast, regular physical activity, abstinence from tobacco and excessive alcohol consumption, consumption of minimally processed, nutritionally balanced diets, adequate sleep, and effective stress management have been linked to improved immune function and greater resilience to illness [
2].
Beyond these lifestyle measures, Whole-Body cryotherapy (WBC)—the short-term therapeutic application (3 – 6 minutes) of extreme cold (approximately −90 °C)—has attracted increasing scientific and clinical interest. WBC was first developed in Japan around 40 years ago, and involves brief exposure in a cryochamber, during which participants perform light movements of the extremities and protect sensitive regions such as the ears, nose, and mouth. Initial studies in athletes reported reductions in inflammatory mediators and muscle enzymes [
3]. Since then, WBC has demonstrated promising effects across multiple domains, including alleviation of chronic pain (e.g., fibromyalgia, osteoarthritis), facilitation of post-exercise recovery, improvements in sleep quality, mood, and reductions in depressive symptoms. Additional evidence points to potential benefits in weight management, autoimmune and neurological disorders, and even cognitive function [
4,
5,
6].
More recently, mechanistic investigations have begun to clarify how WBC may influence immune and metabolic pathways. Clinical and experimental evidence suggests that WBC may modulate inflammatory cytokines, enhance adaptive immune responses, and contribute to improved metabolic flexibility and body composition [
1,
2,
3,
4,
5,
6,
8,
9,
10]. WBC has also been explored in rehabilitation contexts, including post-COVID-19 recovery, [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
22,
39] where Soluble Angiotensin-Converting Enzyme 2 (sACE2) has been identified as a biomarker of disease severity [
26,
27,
28,
29,
30,
31,
32].
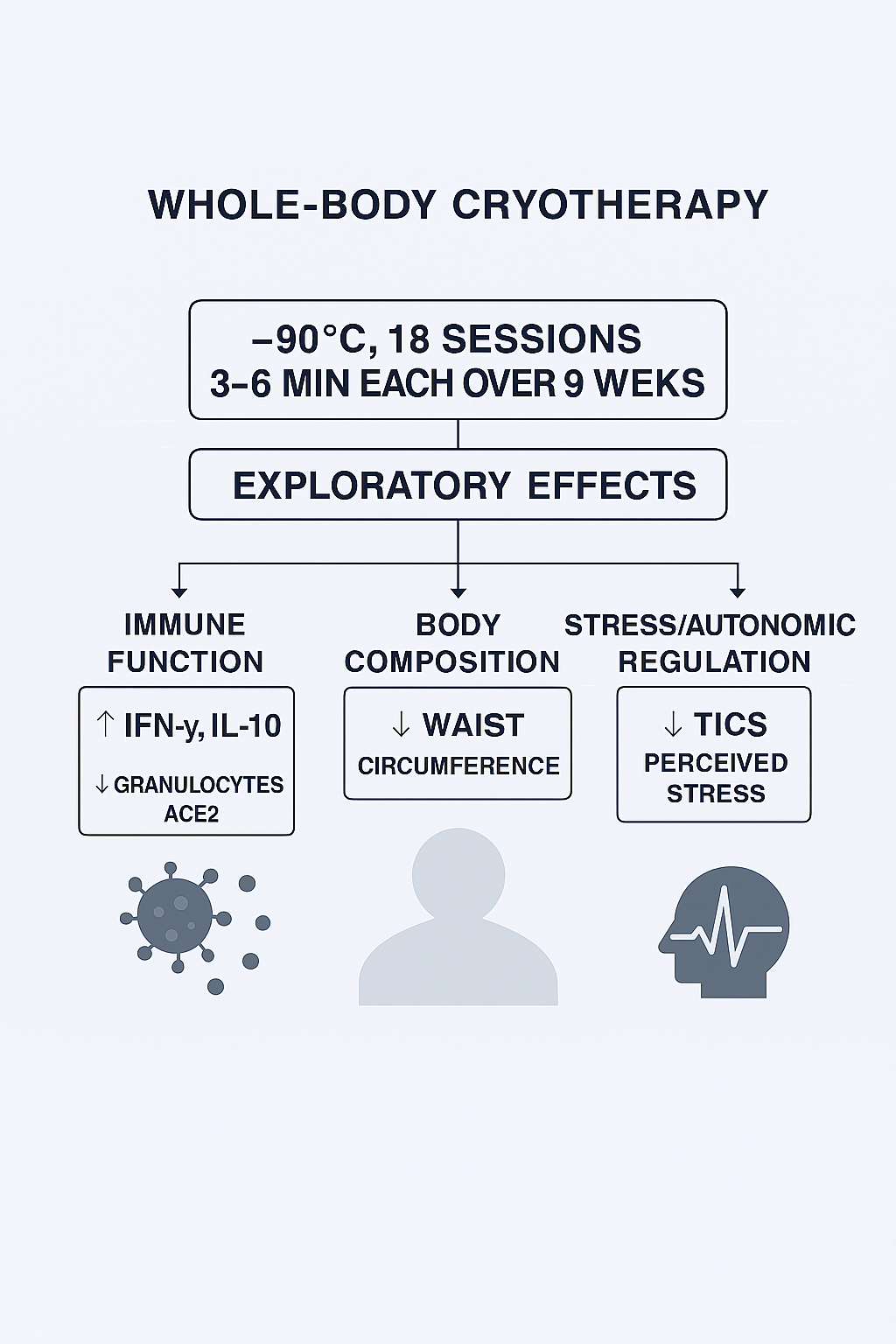
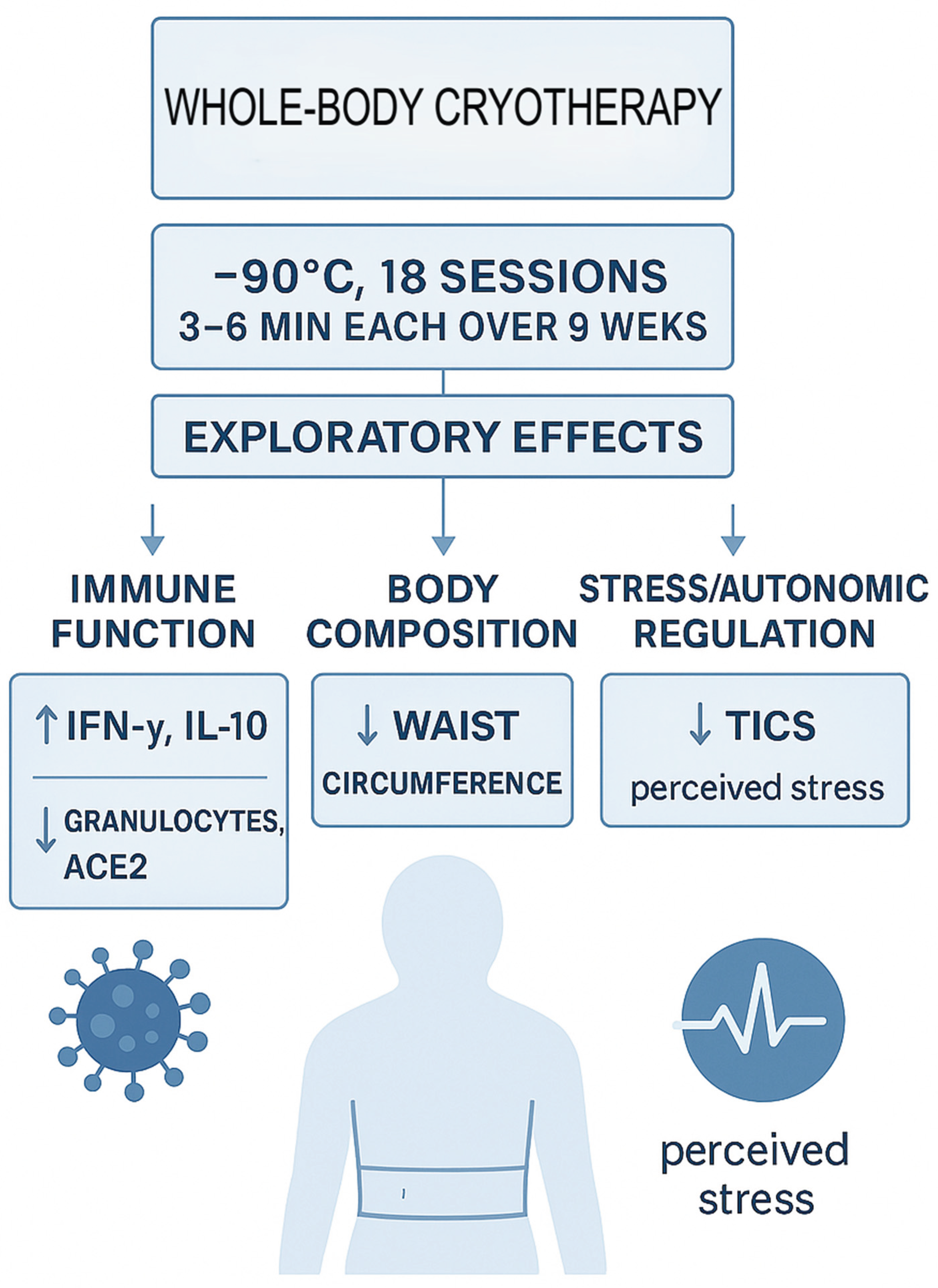
Given this background, the present exploratory study investigated the effects of a standardized 9-week WBC program in middle-aged adults. Specifically, we focused on three domains: (1) Immune function, including Interferon-gamma (IFN-γ), Interleukin 2 (IL-2), Interleukin 10 (IL-10), lymphocyte subsets, tumor necrosis factor-alpha (TNF α)and plasma soluble ACE2 (sACE2); (2) body composition, assessed by bioelectrical impedance analysis (BIA), with a focus on waist circumference and lean/fat mass distribution; and (3) Psychological stress, measured by the validated Trier Inventory for Chronic Stress (TICS) [
17,
18,
41,
42]. This pilot study aimed to generate preliminary mechanistic data on the regulatory effects of WBC in humans and provide a foundation for the design of larger controlled trials [
22,
34,
35,
37].
2. Materials and Methods
2.1. Study Design
The presented study was a one-armed prospective monocentric observational study including 20 adult participants. Patients before treatment were considered their own controls. (
Figure 1)
Participants underwent 18 sessions of cryotherapy over 9 weeks (−90 °C, 3–6 minutes each), followed by a 9-week post-intervention phase. Assessments were conducted at three time points: measurement 1 (baseline) before intervention, measurement 2 (post-intervention), and measurement 3 (follow-up). The Consort Flow-Chart is available in Supplementary File S1.
2.2. Ethical Approval and Registration
The study was registered in the German Register for Clinical Trials (trial no. DRKS00031033) after approval by the ethical committee of the Bayerische Landesärztekammer (
www.ethikkommission.blaek.de; approval number 22118). All participants provided written informed consent.
2.3. Sample Size Justification
Since the present study is an exploratory monocentric study with a pilot character, no formal sample size planning was conducted. The number of cases of n = 20 was determined on the basis of available donations for the research and the limited capacities of a therapeutic practice for reasons of feasibility. The number of cases was considered sufficient to describe the continuous target variables collected in the study in such a way that initial insights into mechanisms of action of the intervention can be provided, and sample size calculations can be carried out for future confirmatory studies.
2.4. Recruitment
Participants were recruited in the corresponding author’s practice in Murnau via a local newspaper article, a local journal, and a video on a public website. Information was also disseminated to the Murnau health department as well as general practitioners and other health professionals in the Garmisch-Partenkirchen district in Bavaria, Germany.
2.5. Eligibility Criteria
The inclusion criteria were male/female/diverse, aged 40–75 years. The exclusion criteria for WBC in this study (to be clarified by preclinical medical diagnosis by the leading medical doctor of the study) comprised: pregnancy, severe hypertension (blood pressure > 180/100), acute or recent myocardial infarction, unstable angina, arrhythmia, symptomatic cardiovascular disease, pacemaker, peripheral arterial occlusive disease, venous thrombosis, acute or recent cerebrovascular accident, uncontrolled seizures, Raynaud's syndrome, fever, tumor disease, symptomatic lung diseases, blood clotting disorders, severe anemia, infections, cold allergies, cold agglutinin disease and acute kidney and urinary tract diseases, as well as epileptic seizures. Further, increased intraocular pressure, such as glaucoma, represented an exclusion; for these patients, there is no exclusion of liability, including cases of unexplained increases in intraocular pressure. [
33] Additional exclusion criteria included consumption of more than four cups of coffee per day, more than two alcoholic beverages per day, substantial dietary changes, and extreme sports, as well as other cold applications such as ice bathing and prior cryotherapy experience.
2.6. Procedures and Measurements
The procedure of the study went as follows: Before the intervention, there were measurements of Body composition (BIA), peripheral vascular activity, stress questionnaire, fascial tissue properties, and immune parameters via blood samples. The baseline measurement was followed by a 9-week program comprising 18 sessions (3-6 min) conducted in a Cryo.One whole-body cryotherapy chamber (Mecotec GmbH, Germany). After the intervention phase, a second measuring session was conducted following the same protocol as at baseline. After a subsequent 9-week post-intervention waiting period, a third measurement session was performed. Body composition was measured using bioelectrical impedance analysis (DATA Input Systems GmbH, Pöcking, Germany). Immune parameters included whole blood count analysis, CD4/CD8 subtyping (T cell Subsets (Helper / Cytotoxic)), and cytokine profiling under stimulated conditions, as well as measurement of plasma soluble ACE2 (sACE2). Stress perception was measured using the Trier Inventory for Chronic Stress (TICS), Hogrefe Verlag, Göttingen, Germany [
41,
42]. Peripheral vascular reactivity was assessed via blood volume pulse amplitude (BVP) with Nexus 10, Mindmedia B.V., Roermond, The Netherlands. [
19,
20,
21]
2.7. Laboratory Analyses
Laboratory analysis for sACE2 was performed using the Human sACE2 Enzyme-Linked Immunosorbent Assay (ELISA) kit (by Invitrogen (Thermo Fisher Scientific, USA). The serum samples from the participants, which had been frozen at −20 °C, were thawed at room temperature and diluted 1:2 with assay diluent from the test kit. This dilution level was chosen because preliminary tests showed that the 1:8 dilution specified by the test kit yielded in signals that were too low. Except for the changed dilutions, the ELISA was performed according to the test instructions, measured on the Tecan Reader Infinite F50 Plus, and evaluated with the Tecan Magellan software. Laboratory analysis of sACE2, as well as Erythrocyte Sedimentation Rate (ESR), was conducted at the practice in Murnau.
All the other laboratory parameters were sent to and diagnosed by Lab4more Laboratory GmbH, Munich, Germany.
2.8. Protocol Deviations
During the study, it was discovered that one participant had had extensive cryotherapy exposure prior to enrollment; this participant was asked to withdraw and excluded from analysis.
2.9. Statistical Analysis
Patient cohort characteristics and clinical parameters were described using appropriate measures, depending on the scale: means and standard deviations for continuous variables medians with interquartile ranges, and total ranges for continuous variables; and absolute and relative frequencies for categorical variables. Repeated-measures analysis of variance (RM-ANOVA) was performed to analyze time courses of clinical parameters, which were illustrated using boxplots. Post hoc test of the RM-ANOVA was performed and adjusted by the Holm-Bonferroni method. Due to the exploratory character of this study, p-values were not adjusted for multiple testing (excluding post hoc tests), and no imputation of missing values was performed. Furthermore, p-values have only descriptive meaning; p-values <0.05 were determined as statistically significant, p-values <0.1 were defined as trends. All analyses were performed in R version > 4.2.0.
This study was reported in accordance with the TREND Statement for nonrandomized evaluations of behavioral and public health interventions. The completed TREND checklist is provided in Supplementary File S2.
3. Results
One participant experienced significant physical and mental strain due to family and work circumstances over the course of the study and became severely ill toward the end. This adverse event was deemed unrelated to the cryotherapy intervention. Consequently, the participant’s data was excluded from the final analysis.
3.1. Description of the Study Cohort and Received Cryotherapy
The description of the study cohort and received cryotherapy can be found in
Table 1. The cohort consisted of 19 patients with an average age of 52.9 ± 9.8, and 4 (21%) male participants. All participants received 18 sessions of cryotherapy with an average duration of 4.5 ± 0.76 min every 3.5 ± 0.4 days, indicating a consistent application of cryotherapy over all participants.
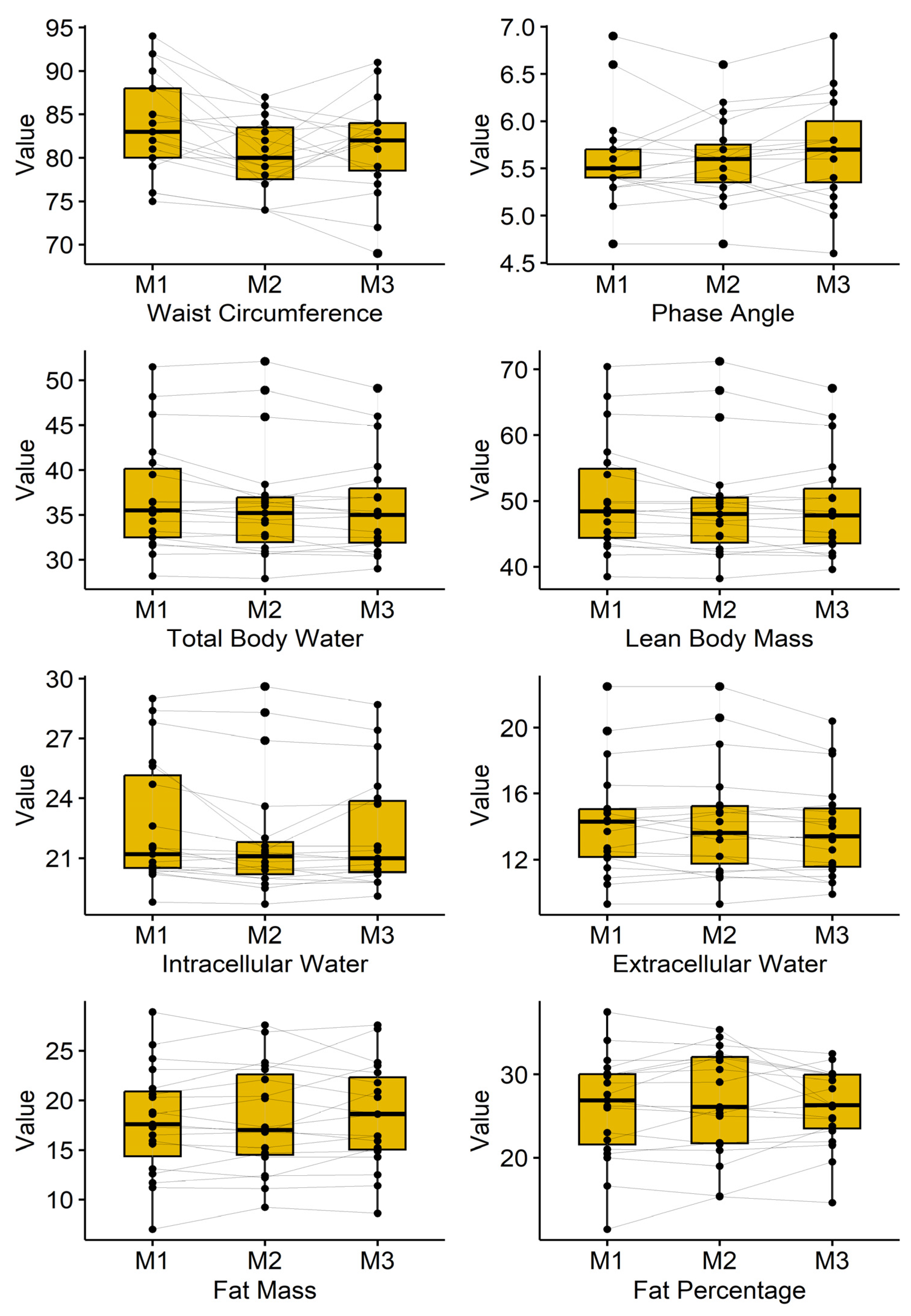
3.2. Bioimpedance Analysis
Results of the bioimpedance analysis are presented in
Table 2 and are illustrated in
Figure 2. Waist circumference dropped from 83.8 ± 5.7cm to 80.2 ± 4.2cm and ended in 81.3 ± 5.5cm (p=0.001). Furthermore, total body water, lean body mass, as well as intercellular and extracellular water, showed a significant decrease.
3.3. Immuno-Blood Parameter
Table 3 shows the time course of blood parameters related to the immune system. Effects on Lymphocytes, Monocytes, and Granulocytes can be observed, especially directly after cryotherapy (Measurement 2); however, these effects dissipate by Measurement 3. Furthermore, the effects of cryotherapy on the virus pool can be seen: IL2 and INF-γ monotonically decrease and increase, respectively; IL10 shows a substantial impact at measurement 2. In general, effects are examined particularly from measurement 1 to measurement 2, with diminishing effects afterwards.
3.4. Further Blood Parameters
The influence of cryotherapy on the further blood parameters ESR, sACE2, and BVP (mean and SD) is shown in
Table 4. No effects can be found for ESR. A significant decrease was observed for sACE2, surprisingly occurring mainly during follow-up (between measurement 2: 0.5 ± 0.7 and measurement 3: 0.3 ± 0.4; p = 0.029).
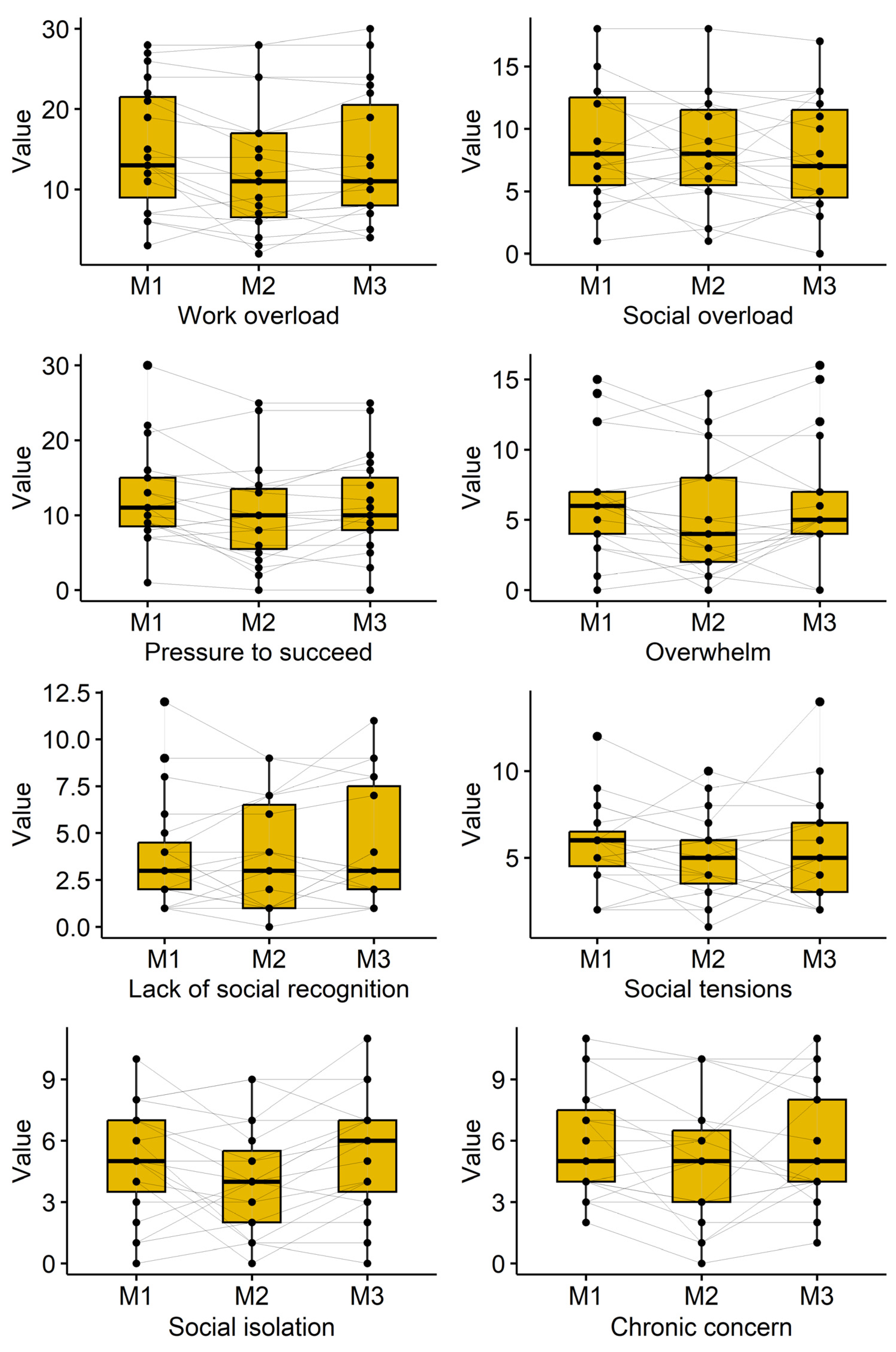
3.4. Subjective Perception of Stress
Subjective stress perception was measured using the Trier Inventory for Chronic Stress (TICS). The course of the participant’s reported subjective stress perception is shown in
Table 5 and
Figure 3. Decreases were observed for the TICS subscales “work overload” (p=0.009), “pressure to succeed” (p=0.018), and “social isolation” (p=0.049). A reduction was also observed for the scale “overwhelm” directly after cryotherapy (6.4±4.1 to 5.2±4.3; p=0.081), but values returned towards baseline at follow-up (6.2±4.4). Several other TICS subscales showed reduced scores immediately after cryotherapy, which often attenuated at follow-up.
4. Discussion
This exploratory pilot study demonstrates that a structured 9-week program of whole-body cryotherapy (WBC) at −90 °C followed by a 9-week observation period is associated with measurable systemic changes in immune function, body composition, and vascular regulation. Since this study is a single-arm pilot study in which participants served as their own pre-post controls, the findings should be interpreted with appropriate caution.
The most consistent findings were a reduction in waist circumference, an increase in lymphocytes and interferon-γ, a rise in IL-10, a decline in granulocytes, and a significant decrease in plasma soluble ACE2 (sACE2). Together, these results suggest that repeated WBC exposure may exert immunomodulatory and metabolic effects relevant to health maintenance and disease prevention.
Immune modulation: The observed increase in interferon-γ and IL-10, alongside a decline in granulocytes, indicates a shift toward a more balanced immune phenotype, consistent with literature showing cryotherapy-induced regulation of cytokine networks and leukocyte subsets [
6,
7,
8,
9,
10]. The anti-inflammatory signal of IL-10, although modest, may support a regulatory immune state, while stable or slightly reduced pro-inflammatory mediators align with earlier cold exposure findings [
11,
12,
13]. These results add to evidence that WBC may attenuate low-grade inflammation and improve immune surveillance in middle-aged adults. [
23,
24,
25,
36]
Soluble ACE2: The reduction in plasma sACE2 is of particular interest. Beyond its role as a receptor in SARS-CoV-2 infection, sACE2 is recognized as a regulator of vascular and metabolic homeostasis. Lower circulating sACE2 after WBC may reflect an adaptive endothelial or neurohumoral response, possibly mediated by catecholamine release or nitric oxide signaling. This effect has not been reported in prior WBC trials, underscoring the novelty of our protocol and its implications for vascular–immune crosstalk. Nevertheless, interpretation must remain cautious: hydration, diet, and inter-individual variability may confound sACE2 dynamics; controlled studies are needed to validate this signal.
Body composition: We observed a reduction in waist circumference without significant changes in total fat or lean mass. This may reflect regional adaptation, redistribution of body water, or subtle changes in visceral adiposity not detectable by bioimpedance.
We recognize that the lack of a control group is a significant weakness of this study; thus, the results should be read as preliminary and interpreted with care. With that in mind, the BIA data suggest a possible—though statistically non-significant—trend toward a more balanced distribution of body water between fat and lean tissue compartments. Many participants showed mild signs consistent with edema before the WBC sessions, and across the intervention, their readings moved toward lower water values and a profile the device interprets as more balanced hydration.
To date, we are not aware of published work directly linking WBC to hydration status. This means other explanations cannot be ruled out. In particular, the observed changes might reflect a general dehydration effect rather than a specific normalization of fluid distribution. We also did not track participants’ diet or fluid intake during the study, which limits what we can conclude about mechanisms. Still, using the manufacturer’s reference framework for the BIA system, the post-WBC patterns are classified as indicating improved hydration balance. We see these findings mainly as hypothesis-generating and hope they encourage future controlled studies that include careful monitoring of fluid intake and additional hydration measures. Future studies should incorporate imaging modalities such as Dual-Energy-Xray Absorptiometry (DXA) or Magnetic Resonance Imaging (MRI), as well as fluid intake questionnaires for the participants to clarify these findings.
Stress and adaptation: Although modest, stress perception measured by TICS, declined post-intervention, aligning with reports that WBC may support psychological resilience, sleep quality, and mood [
14,
15,
16,
17]. Acute changes in vascular reactivity observed during the intervention phase (e.g., transient increases in BVP amplitude) suggest stimulation of autonomic pathways, possibly linked to norepinephrine surges during cold exposure. However, these responses were not sustained, reinforcing that WBC’s primary benefits may lie in immune and metabolic modulation rather than durable autonomic changes.
Age and sex considerations: Our cohort (40–75 years, 79% female) differs from most WBC studies that focus on younger male athletes. Aging is associated with diminished mitochondrial plasticity, altered immune remodeling, and a senescence-associated secretory phenotype, all of which may blunt cytokine responses to interventions. This may explain the more modest cytokine shifts observed here compared to younger cohorts. Sex differences in cold adaptation and immune regulation are well documented, but could not be examined in this small, unbalanced sample. Larger, sex-stratified studies are warranted.
Comparison with existing literature: Our findings are consistent with reports that cryotherapy reduces IL-6, TNF-α, and CRP, improves recovery, and reduces stress perception [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
40]. Unlike protocols using −110 °C or cold-water immersion, we employed standardized −90 °C cold-air exposure without mechanical confounders, enabling clearer attribution to thermal stress. The consistent 18-session program over 9 weeks adds to the limited data on longer-term adaptations in non-athletic, middle-aged populations.
Exploratory parameters such as myofascial stiffness, joint flexibility (finger-to-floor distance), and mechanical pain sensitivity (algometry) were assessed but are not reported in detail due to limited sample sizes and non-prespecified status. These measures will be targeted for standardized collection in future confirmatory trials.
Limitations: This study is limited by its small sample size and lack of a control group, which restricts causal inference. The sex imbalance (79% female) limits generalizability. Hydration and dietary intake were not standardized, which confounded the interpretation of bioimpedance outcomes. Subgroup analyses (e.g., stiffness, algometry) were underpowered and are not reported here. While preliminary signals on sACE2 and cytokines are intriguing, they require validation in larger randomized controlled trials.
Implications: Despite limitations, the reduction in sACE2 alongside immune modulation and improved central adiposity suggests potential roles for WBC in preventive medicine and metabolic health. Given safety and feasibility, larger controlled studies are justified to define therapeutic potential and optimize protocols for specific populations, particularly older adults.
5. Conclusions
Our findings demonstrate that whole-body cryotherapy at −90 °C induces measurable and systemic physiological adaptations, most prominently a reduction in inflammatory markers, improvements in body composition parameters such as waist circumference, and modulation of immune regulation. By applying a uniform cold stimulus without mechanical confounders, this protocol reveals subtle but reproducible adaptation mechanisms, offering new insights into how the human body responds to repeated thermal stress.
These results highlight that WBC acts on multiple regulatory levels—including hydration, immune function, and vascular balance—extending beyond the traditionally reported effects of pain relief or local inflammation. The observed decrease in soluble ACE2 is of particular relevance, as it indicates a potential modulation of vascular–immune crosstalk and may represent a protective adjustment in metabolic and stress-related pathways.
Collectively, this study underscores the complex, multilevel impact of WBC and the need for system-level frameworks that can capture non-linear, time-sensitive, and individualized responses to cryotherapy. While preliminary, these findings suggest that WBC may serve as a viable non-pharmacological adjunct in metabolic regulation, stress resilience, and immune support.
This investigation did not include a proper control group. Instead, participants’ baseline health status was used as a within-subject reference for the intervention period. Although unlikely, it cannot be ruled out that comparable improvements might have occurred even without the intervention, for example, due to concurrent lifestyle changes or spontaneous remission. Future clinical trials should build on the encouraging findings of this pilot study and re-examine these effects in appropriately larger, controlled, randomized trials, exploring sex-specific responses and investigating tailored cryotherapy protocols to optimize long-term outcomes.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Punito Michael Aisenpreis: Protocol/project development, data collection and management, manuscript writing/editing. Sybille Aisenpreis: data collection, manuscript writing/editing. Manuel Feisst: data analysis, data interpretation, manuscript writing/editing. Robert Schleip: Protocol/project development, manuscript writing/editing. All authors agree to be accountable for all aspects of the work and ensure that any questions regarding the accuracy or integrity of any part of the work are properly addressed and resolved.
Funding
This study was financially supported by private contributions through the „Stiftung Immunweltmeister e.V“., a non-profit association in Murnau, Bavaria, Germany.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, was registered in the German Register for Clinical Trials (trial no. DRKS 00031033) after approval by the ethical committee of the Bayerische Landesärztekammer (
www.ethikkommission.blaek.de; approval number 22118).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to reasonable request.
Acknowledgments
We would like to express our deepest gratitude to all those who contributed to the success of this study. Special thanks go to Lena Mühmel, Elisabeth Schmid, Monika Schöps, Diana Ott, and Monika Heidrich for their meticulous support in data collection. I am sincerely grateful to Dr. Ali Grossmann (formerly Roche AG, Penzberg), Dr. Felix Quitterer (Roche AG, Penzberg), and Dr. Wolfgang Mayer (Immumed GmbH/ Lab4more GmbH, Munich) for their invaluable contributions to conceptual planning. My appreciation extends to Ursula Rammelmaier for her guidance on hygiene and planning, and to Dr. Gerti Heiss, Munich, for her leadership and expertise as the supervising medical doctor throughout this project. I also gratefully acknowledge the many contributors of the Stiftung Immunweltmeister, e.V. Murnau, whose generous financial and motivational support sustained this study over many months and played a vital role in its completion.
Conflicts of Interest
Authors Punito Michael Aisenpreis, Sybille Aisenpreis, and Manuel Feißt declare no conflicts of interest. Author RS is a board member of a non-profit fascia research charity (
www.fasciaresearchcharity.org)
Abbreviations
The following abbreviations are used in this manuscript:
| WBC: |
Whole Body Cryotherapy |
| BIA: |
Bioelectrical Impedance Analysis |
| BVP: |
Blood Volume Pulse Amplitude |
| TICS: |
Trier Inventory for Chronic Stress |
| ESR: |
Erythrocyte Sedimentation Rate (German: BKS) |
| sACE2: |
Soluble Angiotensin Converting Enzyme 2 |
| IL 2: |
Interleukin 2 |
| IL 10: |
Interleukin 10 |
| IFN γ: |
Interferon gamma |
| TNF α: |
Tumor Necrosis Factor alpha |
| CD4/CD8: |
T cell Subsets (Helper / Cytotoxic) |
| RM ANOVA: |
Repeated Measures Analysis of Variance |
References
- He, J.; Zhang, X.; Ge, Z.; et al. Whole-body cryotherapy can reduce the inflammatory response in humans: a meta-analysis based on 11 randomized controlled trials. Sci Rep 2025, 15, 7759. [Google Scholar] [CrossRef]
- Karppinen, J. E.; Suojanen, L.; Heinonen, S.; Kaye, S.; van der Kolk, B. W.; White, J. W.; Orava, J.; Lee, S. H.T.; Dillon, E.; Muniandy, M.; Rissanen, A.; le Roux, C. W.; Docherty, N.; Pajukanta, P.; Virtanen, K. A.; Pietiläinen, K. H.; et al. Effects of Whole-Body Cryotherapy Combined With Conventional Obesity Management Versus Obesity Management Alone: A Clinical Trial. Obesity 2025, 33, 2112–2127. [Google Scholar] [CrossRef] [PubMed]
- Chun, E; Joseph, R; Pojednic, R. Whole-Body Cryotherapy Reduces Systemic Inflammation in Healthy Adults: Pilot Cohort Study. Interact J Med Res 2024, 13, e60942. [Google Scholar] [CrossRef]
- Jdidi, H; Dugué, B; de Bisschop, C; Dupuy, O; Douzi, W. The effects of cold exposure (cold water immersion, whole- and partial-body cryostimulation) on cardiovascular and cardiac autonomic control responses in healthy individuals: A systematic review, meta-analysis and meta-regression. J Therm Biol 2024, 121, 103857. [Google Scholar] [CrossRef]
- Solaro, N; Giovanelli, L; Bianchi, L; Piterà, P; Verme, F; Malacarne, M; Pagani, M; Fontana, JM; Capodaglio, P; Lucini, D. Whole-Body Cold Stimulation Improves Cardiac Autonomic Control Independently of the Employed Temperature. J Clin Med 2024, 13, 7728. [Google Scholar] [CrossRef] [PubMed]
- Jurecka, A; Woźniak, A; Mila-Kierzenkowska, C; Augustyńska, B; Oleksy, Ł; Stolarczyk, A; Gądek, A. The Influence of Single Whole-Body Cryostimulation on Cytokine Status and Oxidative Stress Biomarkers during Exhaustive Physical Effort: A Crossover Study. Int J Mol Sci 2023, 24, 5559. [Google Scholar] [CrossRef] [PubMed]
- Alito, A.; Verme, F.; Mercati, G.P.; Piterà, P.; Fontana, J.M.; Capodaglio, P. Whole Body Cryostimulation: A New Adjuvant Treatment in Central Sensitization Syndromes? An Expert Opinion. Healthcare 2024, 12, 546. [Google Scholar] [CrossRef]
- Ptaszek, B; Podsiadło, S; Wójcik, A; Czerwińska-Ledwig, O; Teległów, A. The influence of whole-body cryotherapy or winter swimming on the lipid profile and selected adipokines. BMC Sports Sci Med Rehabil. 2023, 15, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, T; Linfei, D; Wang, W; Ren, J; Liu, X; Li, J. Effect of whole-body cryotherapy on recovery after high-intensity training in elite rowers. Front Physiol 2024, 15. [Google Scholar] [CrossRef]
- Cerfoglio, S; Verme, F; Fontana, JM; Alito, A; Galli, M; Capodaglio, P; Cimolin, V. Effects of whole-body cryostimulation on spinal and shoulder range of motion in individuals with obesity. Front Rehabil Sci 2025, 6. [Google Scholar] [CrossRef]
- Banfi, G; Melegati, G; Barassi, A; Dogliotti, G; Melzi d’Eril, G; Dugué, B; Corsi, MM. Effects of whole-body cryotherapy on serum mediators of inflammation and serum muscle enzymes in athletes. J Therm Biol 2009, 34, 55–59. [Google Scholar] [CrossRef]
- Lubkowska, A; Szygula, Z; Klimek, AJ; et al. Do sessions of cryostimulation have influence on white blood cell count, level of IL6 and total oxidative and antioxidative status in healthy men? Eur J Appl Physiol 2010, 109, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Leppäluoto, J.; Westerlund, T.; Huttunen, P.; et al. Effects of long-term whole-body cold exposures on plasma concentrations of ACTH, beta-endorphin, cortisol, catecholamines and cytokines in healthy females. Scand J Clin Lab Invest 2008, 68, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Janský, L.; Pospíšilová, D.; Honzová, S.; et al. Immune system of cold-exposed and cold-adapted humans. Eur J Appl Physiol 1996, 72, 445–450. [Google Scholar] [CrossRef]
- Wojtecka-Lukasik, E.; Ksiezopolska-Orlowska, K.; Gaszewska, E.; et al. Cryotherapy decreases histamine levels in the blood of patients with rheumatoid arthritis. Inflamm Res 2010, 59 Suppl. 2, 253–255. [Google Scholar] [CrossRef]
- Sattler, A.; Angermair, S.; Stockmann, H.; et al. SARS–CoV-2–specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest 2020, 130, 6477–6489. [Google Scholar] [CrossRef]
- Schulz, P.; Schlotz, W.; Becker, P. Trierer Inventar zur Erfassung von chronischem Stress (TICS): Skalenkonstruktion, teststatistische Überprüfung und Validierung der Skala Arbeitsüberlastung. Diagnostica 1999, 45, 8–19. [Google Scholar] [CrossRef]
- Kocalevent, R. D.; Klapp, B. F.; Albani, C.; et al. Zusammenhänge von Ressourcen, chronisch aktiviertem Distress und Erschöpfung in der deutschen Allgemeinbevölkerung. PPmP – Psychotherapie · Psychosomatik · Medizinische Psychologie 2013, 63, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Camm, A. J.; Bigger, J. T.; et al. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Porges, S. W. Respiratory sinus arrhythmia: physiological basis, quantitative methods, and clinical implications. In Cardiorespiratory and cardiosomatic psychophysiology, NATO ASI Series; Grossman, P., Janssen, K. H. L., Vaitl, D., Eds.; Springer: Boston, MA, 1986; vol 114, pp. 101–115. [Google Scholar] [CrossRef]
- Kleiger, R. E.; Stein, P. K.; Bigger, J. T., Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol 2016, 594, 5781–5790. [Google Scholar] [CrossRef]
- Hausswirth, C.; Schaal, K.; Le Meur, Y.; et al. Parasympathetic activity and blood catecholamine responses following a single partial-body cryostimulation and a whole-body cryostimulation. PLoS ONE 2013, 8, e72658. [Google Scholar] [CrossRef] [PubMed]
- Leppäluoto, J.; Westerlund, T.; Huttunen, P.; et al. Effects of long-term whole-body cold exposures on plasma concentrations of ACTH, beta-endorphin, cortisol, catecholamines and cytokines in healthy females. Scand J Clin Lab Invest 2008, 68, 145–153. [Google Scholar] [CrossRef]
- Swenson, C.; Swärd, L.; Karlsson, J. Cryotherapy in sports medicine. Scand J Med Sci Sports 1996, 6, 193–200. [Google Scholar] [CrossRef]
- Swärd, P.; Edsfeldt, A.; Reepalu, A.; et al. Age and sex differences in soluble ACE2 may give insights for COVID-19. Crit Care 2020, 24, 221. [Google Scholar] [CrossRef] [PubMed]
- Farshbafnadi, M.; Kamali Zonouzi, S.; Sabahi, M.; et al. Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: the role of entangled risk factors. Exp Gerontol 2021, 154, 111507. [Google Scholar] [CrossRef]
- Kragstrup, T. W.; Petersen, J.; Mikkelsen, S.; Søndergaard; et al. Plasma ACE2 predicts outcome of COVID-19 in hospitalized patients. PLoS ONE 2021, 16, e0252799. [Google Scholar] [CrossRef] [PubMed]
- Fagyas, M.; Fejes, Z.; Sütö, R.; et al. Circulating ACE2 activity predicts mortality and disease severity in hospitalized COVID-19 patients. Int J Infect Dis 2022, 115, 8–16. [Google Scholar] [CrossRef]
- Mariappan, V.; Ranganadin, P.; Shanmugam, L.; et al. Early shedding of membrane-bounded ACE2 could be an indicator for disease severity in SARS-CoV-2. Biochimie 2022, 201, 139–147. [Google Scholar] [CrossRef]
- Viveiros, A.; Rasmuson, J.; Vu, J.; et al. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am J Physiol Heart Circ Physiol 2021, 320, H296–H304. [Google Scholar] [CrossRef]
- Jia, H.; Neptune, E.; Cui, H. Targeting ACE2 for COVID-19 therapy: opportunities and challenges. Am J Respir Cell Mol Biol 2021, 64, 416–425. [Google Scholar] [CrossRef]
- Capodaglio, P.; Alito, A.; Duguè, B. M.; Bouzigon, R.; et al. Contraindications to Whole-Body Cryostimulation (WBC). A position paper from the WBC Working Group of the International Institute of Refrigeration and the multidisciplinary expert panel. Front Rehabil Sci 2025, 6, 1567402. [Google Scholar] [CrossRef]
- Bouzigon, R.; Dupuy, O.; Tiemessen, I.; et al. Cryostimulation for post-exercise recovery in athletes: a consensus and position paper. Front Sports Act Living 2021, 3, 688828. [Google Scholar] [CrossRef]
- Jdidi, H.; de Bisschop, C.; Dugué, B.; et al. Optimal duration of whole-body cryostimulation exposure to achieve target skin temperature: influence of body mass index — a randomized cross-over controlled trial. J Physiol Anthropol 2024, 43, 28. [Google Scholar] [CrossRef] [PubMed]
- Pournot, H.; Bieuzen, F.; Louis, J.; et al. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS ONE 2011, 6, e22748. [Google Scholar] [CrossRef]
- Bouzigon, R.; Grappe, F.; Ravier, G.; et al. Whole- and partial-body cryostimulation/cryotherapy: current technologies and practical applications. J Therm Biol 2016, 61, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Kusmierczyk, J.; Wiecek, M.; Wojciak, G.; et al. The effect of physical activity and repeated whole-body cryotherapy on the expression of modulators of the inflammatory response in mononuclear blood cells among young men. J Clin Med 2024, 13, 2724. [Google Scholar] [CrossRef]
- He, Y.; Fekete, G. The effect of cryotherapy on balance recovery at different moments after lower extremity muscle fatigue. Phys Act Health 2021, 5, 255–270. [Google Scholar] [CrossRef]
- Chun, E.; Joseph, R.; Pojednic, R. Whole-Body Cryotherapy Reduces Systemic Inflammation in Healthy Adults: Pilot Cohort Study. Interact J Med Res 2024, 13, e60942. [Google Scholar] [CrossRef]
- Petrowski, K.; Kliem, S.; Sadler, M.; et al. Factor structure and psychometric properties of the English version of the Trier Inventory for Chronic Stress (TICS-E). BMC Med Res Methodol 2018, 18, 18. [Google Scholar] [CrossRef]
- Petrowski, K.; Paul, S.; Albani, C.; et al. Factor structure and psychometric properties of the Trier Inventory for Chronic Stress (TICS) in a representative German sample. BMC Med Res Methodol 2012, 12, 42. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).