Submitted:
17 December 2025
Posted:
18 December 2025
You are already at the latest version
Abstract
Keywords:
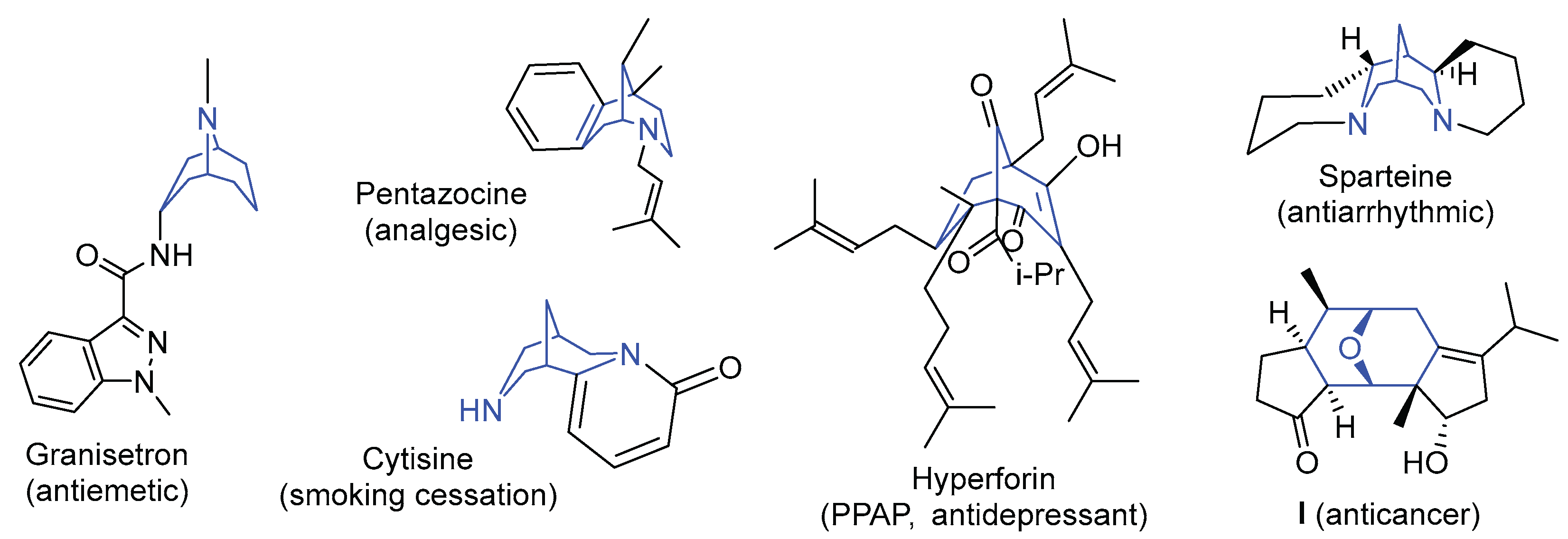
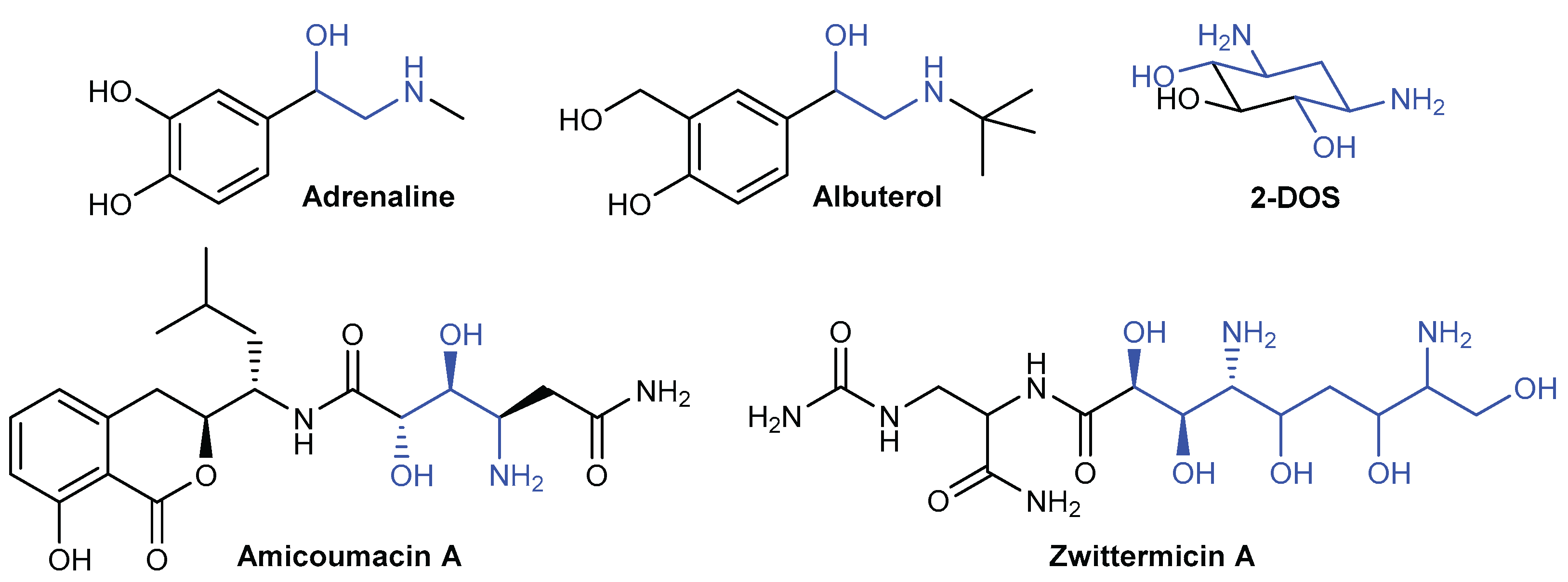
1. Introduction
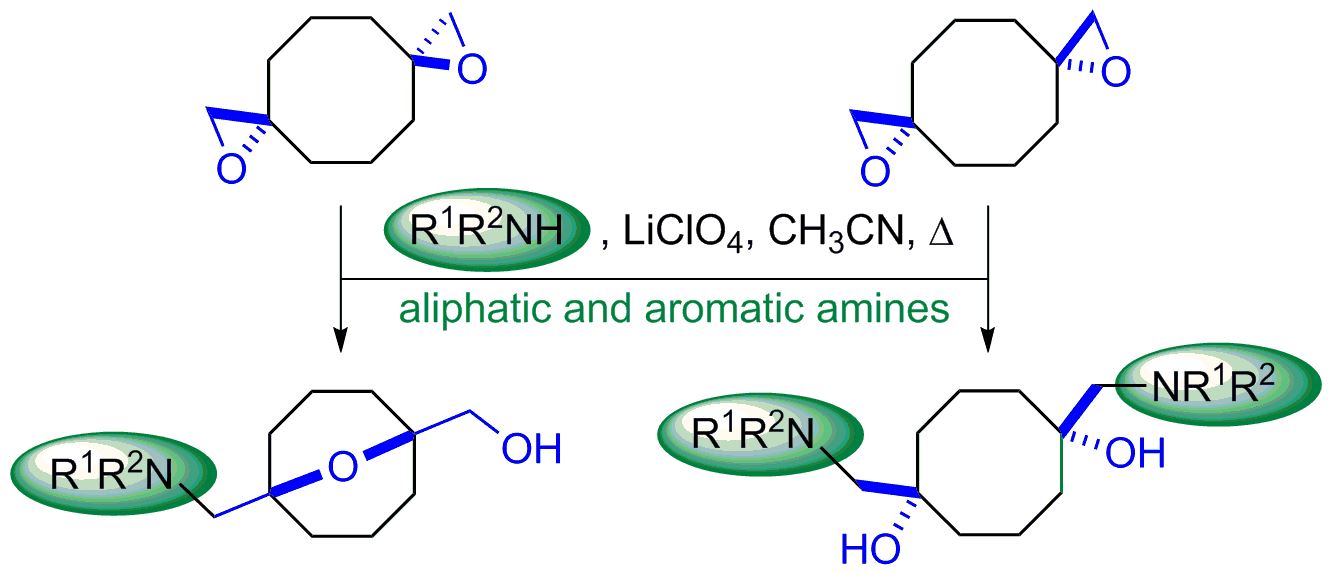
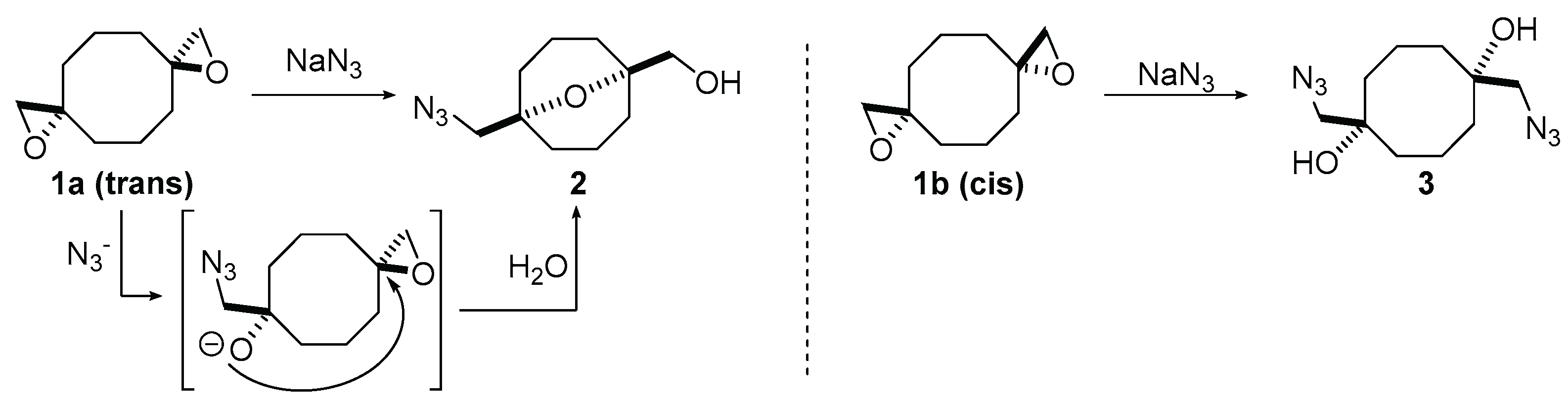
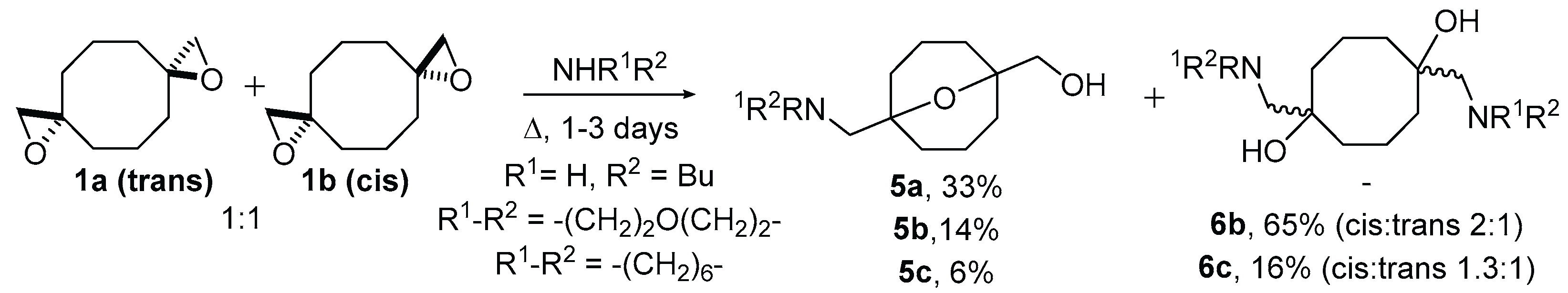
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Reaction of Bis(oxiranes) 1a,b with Amines (General Method)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamedova, V. L.; Khikmatova, G. Z.; Korshin, D. E.; Mamedova, S. V.; Gavrilova, E. L.; Mamedov, V. A. Epoxides: methods of synthesis, reactivity, practical significance. Russ. Chem. Rev. 2022, 91, RCR5049. [Google Scholar] [CrossRef]
- Meninno, S.; Lattanzi, A. Epoxides: Small Rings to Play with under Asymmetric Organocatalysis. ACS Org. Inorg. Au 2022, 2, 289−305. [Google Scholar] [CrossRef]
- Moschona, F.; Savvopoulou, I.; Tsitopoulou, M.; Tataraki, D.; Rassias, G. Epoxide syntheses and ring-opening reactions in drug development. Catalysts 2020, 10, 1117. [Google Scholar] [CrossRef]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F. R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef]
- Yang, G.-W.; Xie, R.; Zhang, Y.-Y.; Xu, C.-K.; Wu, G.-P. Evolution of Copolymers of Epoxides and CO2: Catalysts, Monomers, Architectures, and Applications. Chem. Rev. 2024, 124, 12305−12380. [Google Scholar] [CrossRef]
- Saddique, F. A.; Zahoor, A. F.; Faiz, S.; Ali, S.; Naqvi, R.; Usman, M.; Ahmad, M. Recent trends in ring opening of epoxides by amines as nucleophiles. Synth. Commun. 2016, 46, 831–868. [Google Scholar] [CrossRef]
- Meninno, S.; Lattanzi, A. Organocatalytic Asymmetric Reactions of Epoxides: Recent Progress. Chem. A Eur. J. 2016, 22, 3632–3642. [Google Scholar] [CrossRef] [PubMed]
- Faiz, S.; Zahoor, A. F. Ring opening of epoxides with C-nucleophiles. Mol. Divers. 2016, 20, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Nasr, S. M.; Kazemi, M.; Mohammadi, M. A Mini-Review: Achievements in the Thiolysis of Epoxides. Mini. Rev. Org. Chem. 2020, 17, 352–362. [Google Scholar] [CrossRef]
- Talukdar, R. Synthetically important ring opening reactions by alkoxybenzenes and alkoxynaphthalenes. RSC Adv. 2020, 10, 31363–31376. [Google Scholar] [CrossRef]
- Kumar, A.B.; Anderson, J.M.; Melendez, A.L.; Manetsch, R. Synthesis and Structure–Activity Relationship Studies of 1,3-Disubstituted 2-Propanols as BACE-1 Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4740–4744. [Google Scholar] [CrossRef]
- Brediļņina, J.; Villo, P.; Andersons, K.; Toom, L.; Vares, L. Hydrolytic and Aminolytic Kinetic Resolution of Terminal Bis-Epoxides. J. Org. Chem. 2013, 78, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Zolfigol, M.A.; Moosavi-Zare, A.R.; Zarei, M.; Zare, A.; Noroozizadeh, E.; Karamian, R.; Asadbegy, M. Synthesis of β-Phthalimido-Alcohols via Regioselective Ring Opening of Epoxide by Using Reusable Basic Magnetic Nano Particles and Their Biological Investigation. RSC Adv. 2016, 6, 62460–62466. [Google Scholar] [CrossRef]
- Veidenberg, I.; Toom, L.; Villo, P.; Vares, L. An Efficient and Highly Stereoselective Approach to 2,5-Disubstituted-Tetrahydrofuran and 2,6-Disubstituted-Tetrahydropyran Derivatives. Tetrahedron Lett. 2014, 55, 3569–3571. [Google Scholar] [CrossRef]
- Qayed, W.S.; Luzzio, F.A. Chiral Pool/Henry/Enzymatic Routes to Acetogenin Synthons. Lett. Org. Chem. 2015, 12, 622–630. [Google Scholar] [CrossRef]
- Cope, A.C.; Martin, M.M.; McKervey, M.A. Transannular reactions in medium-sized rings. Q. Rev. Chem. Soc. 1966, 20, 119–152. [Google Scholar] [CrossRef]
- Reyes, E.; Uria, U.; Carrillo, L.; Vicario, J.L. Transannular reactions in asymmetric total synthesis. Tetrahedron 2014, 70, 9461–9484. [Google Scholar] [CrossRef]
- Sedenkova, K.N.; Ryzhikova, O.V.; Stepanova, S.A.; Averin, A.D.; Kositov, S.V.; Grishin, Y.K.; Gloriozov, I.P.; Averina, E.B. Bis(oxiranes) containing cyclooctane core: Synthesis and reactivity towards NaN3. Molecules 2022, 27, 6889. [Google Scholar] [CrossRef] [PubMed]
- Sedenkova, K.N.; Savchenkova, D.V.; Ryzhikova, O.V.; Grishin, Y.K.; Averina, E.B. Stereo-Dependent Nucleophilic Ring-Opening of 1,6,10,14-Tetraoxatetraspiro[2.1.25.1.29.1.213.13]Hexadecane upon Treatment with Sodium Azide. Russ. Chem. Bull. 2024, 73, 2105–2109. [Google Scholar] [CrossRef]
- Roy, N.; Das, R.; Paira, R.; Paira, P. Different Routes for the Construction of Biologically Active Diversely Functionalized Bicyclo[3.3.1]nonanes: An Exploration of New Perspectives for Anticancer Chemotherapeutics. RSC Adv. 2023, 13, 22389–22480. [Google Scholar] [CrossRef]
- Seynaeve, C.; Verweij, J.; de Mulder, P.H.M. 5-HT₃ Receptor Antagonists, a New Approach in Emesis: A Review of Ondansetron, Granisetron and Tropisetron. Anti-Cancer Drugs 1991, 2, 343–355. [Google Scholar] [CrossRef]
- Brogden, R.N.; Speight, T.M.; Avery, G.S. Pentazocine: A Review of its Pharmacological Properties, Therapeutic Efficacy and Dependence Liability. Drugs 1973, 5, 6–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Huang, P.; Wang, D.; Zhang, Z.; Zhou, Z.; Liang, L.; Yao, R.; Yang, L. Cytisine: State of the art in pharmacological activities and pharmacokinetics. Biomed. Pharmacother. 2024, 171, 116210. [Google Scholar] [CrossRef] [PubMed]
- Ciochina, R.; Grossman, R.B. Polycyclic Polyprenylated Acylphloroglucinols. Chem. Rev. 2006, 106, 3963–3986. [Google Scholar] [CrossRef]
- Phang, Y.; Wang, X.; Lu, Y.; Fu, W.; Zheng, C.; Xu, H. Bicyclic polyprenylated acylphloroglucinols and their derivatives: structural modification, structure-activity relationship, biological activity and mechanism of action. Eur. J. Med. Chem. 2020, 205, 112646. [Google Scholar] [CrossRef]
- Bonjoch, J.; Diaba, F.; Bradshaw, B. Synthesis of 2-Azabicyclo[3.3.1]nonanes. Synthesis 2011, 2011, 993–1018. [Google Scholar] [CrossRef]
- Kim, N.; Estrada, O.; Chavez, B.; Stewart, C., Jr.; D’Auria, J.C. Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules 2016, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.P.; Rahman, M.T.; Cook, J.M. Bisindole Alkaloids from the Alstonia Species: Recent Isolation, Bioactivity, Biosynthesis, and Synthesis. Molecules 2021, 26, 3459. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, A.; Rossetti, A. Synthesis of Natural Compounds Based on the [3,7]-Diazabicyclo[3.3.1]nonane (Bispidine) Core. Eur. J. Org. Chem. 2021, 1491–1507. [Google Scholar] [CrossRef]
- Nurieva, E.V.; Zefirov, N.A.; Mamaeva, A.V.; Grishin, Y.K.; Kuznetsov, S.A.; Zefirova, O.N. Synthesis of Non-Steroidal 2-Methoxyestradiol Mimetics Based on the Bicyclo[3.3.1]nonane Structural Motif. Mendeleev Commun. 2017, 27, 240–242. [Google Scholar] [CrossRef]
- Nurieva, E.V.; Semenova, I.S.; Nuriev, V.N.; Shishov, D.V.; Baskin, I.I.; Zefirova, O.N.; Zefirov, N.S. The Diels–Alder Reaction as an Approach to the Synthesis of Bicyclo[3.3.1]nonane Analogues of Colchicine. Russ. J. Org. Chem. 2010, 46, 1877–1880. [Google Scholar] [CrossRef]
- Abate, C.; Perrone, R.; Berardi, F. Classes of Sigma₂ (σ₂) Receptor Ligands: Structure Affinity Relationship (SAfiR) Studies and Antiproliferative Activity. Curr. Pharm. Des. 2012, 18, 938–949. [Google Scholar] [CrossRef]
- Fallica, A.N.; Pittalà, V.; Modica, M.N.; Salerno, L.; Romeo, G.; Marrazzo, A.; Helal, M.A.; Intagliata, S. Recent Advances in the Development of Sigma Receptor Ligands as Cytotoxic Agents: A Medicinal Chemistry Perspective. J. Med. Chem. 2021, 64, 7926–7962. [Google Scholar] [CrossRef]
- Li, F.; Lin, S.; Zhang, S.; Pan, L.; Chai, C.; Su, J.-C.; Yang, B.; Liu, J.; Wang, J.; Hu, Z.; Zhang, Y. Modified Fusicoccane-Type Diterpenoids from Alternaria brassicicola. J. Nat. Prod. 2020, 83, 1931–1938. [Google Scholar] [CrossRef]
- Tsyrenova, B.D.; Lemport, P.S.; Nenajdenko, V.G. Di- and Polyazides: Synthesis, Chemical Transformations and Practical Applications. Russ. Chem. Rev. 2023, 92, RCR5066. [Google Scholar] [CrossRef]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjugate Chem. 2021, 32, 2049–2069. [Google Scholar] [CrossRef]
- Ryzhikova, O.V.; Sedenkova, K.N.; Kositov, S.V.; Tafeenko, V.A.; Grishin, Y.K.; Averina, E.B. Stereoselective approach to hydroxyalkyl-1,2,3-triazoles containing cyclooctane core and their use for CuAAC catalysis. Catalysts 2023, 13, 835. [Google Scholar] [CrossRef]
- Ryzhikova, O.V.; Churkina, A.S.; Sedenkova, K.N.; Savchenkova, D.V.; Shakhov, A.S.; Lavrushkina, S.V.; Grishin, Y.K.; Zefirov, N.A.; Zefirova, O.N.; Gracheva, Y.A.; Milaeva, E.R.; Alieva, I. B.; Averina, E. B. Mono- and bis(steroids) containing a cyclooctane core: Synthesis, antiproliferative activity, and action on cell cytoskeleton microtubules. Arch. Pharm. 2024, 357, 2400483. [Google Scholar] [CrossRef]
- Vardanyan, R. Adrenergic (Sympathomimetic) Drugs. In Synthesis of Best-Seller Drugs; Vardanyan, R., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 189–196. [Google Scholar] [CrossRef]
- Takahashi, Y.; Igarashi, M. Destination of Aminoglycoside Antibiotics in the ‘Post-Antibiotic Era’. J. Antibiot. 2018, 71, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, I.V.; Akulich, K.A.; Makeeva, D.S.; Osterman, I.A.; Skvortsov, D.A.; Sergiev, P.V.; Dontsova, O.A.; Yusupova, G.; Yusupov, M.M.; Dmitriev, S.E. Amicoumacin A Induces Cancer Cell Death by Targeting the Eukaryotic Ribosome. Sci. Rep. 2016, 6, 27720. [Google Scholar] [CrossRef]
- Sansinenea, E.; Ortiz, A. Zwittermicin A: A Promising Aminopolyol Antibiotic from Biocontrol Bacteria. Curr. Org. Chem. 2012, 16, 978–987. [Google Scholar] [CrossRef]
- Hansen, T.; Vermeeren, P.; Yoshisada, R.; Filippov, D. V.; van der Marel, G. A.; Codée, J. D. C.; Hamlin, T. A. How Lewis Acids Catalyze Ring-Openings of Cyclohexene Oxide. J. Org. Chem. 2021, 86, 3565−3573. [Google Scholar] [CrossRef] [PubMed]
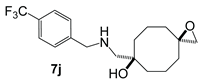
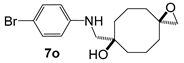
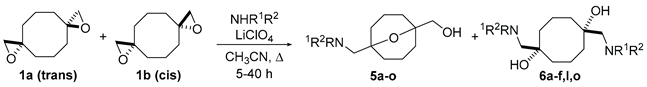 | ||||
| NHR1R2 | product | Yield,%1 | product | Yield,%1 |
| NH2Bu | 5a | 81 | 6a | 652 |
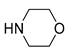 |
5b | 88 | 6b | 97 |
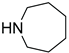 |
5c | 54 | 6c | 19 |
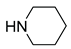 |
5d | 83 | 6d | 972 |
 |
5e | 83 | 6e | 612 |
| NHBu2 | 5f | 832 | 6f | 652 |
| NH2CH2C≡CH | 5g | 70 | 6g | – |
| NH2CH2Ph | 5h | 80 | 6h | – |
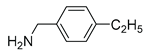 |
5i | 65 | 6i | – |
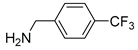 |
5j | 402 | 6j | –3 |
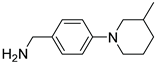 |
5k | 16 | 6k | – |
| NH2Ph | 5l | 36 | 6l | 6 |
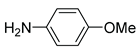 |
5m | 95 | 6m | – |
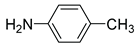 |
5n | 26 | 6n | – |
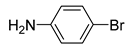 |
5o | 17 | 6o | 22,3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





