Submitted:
11 December 2025
Posted:
12 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
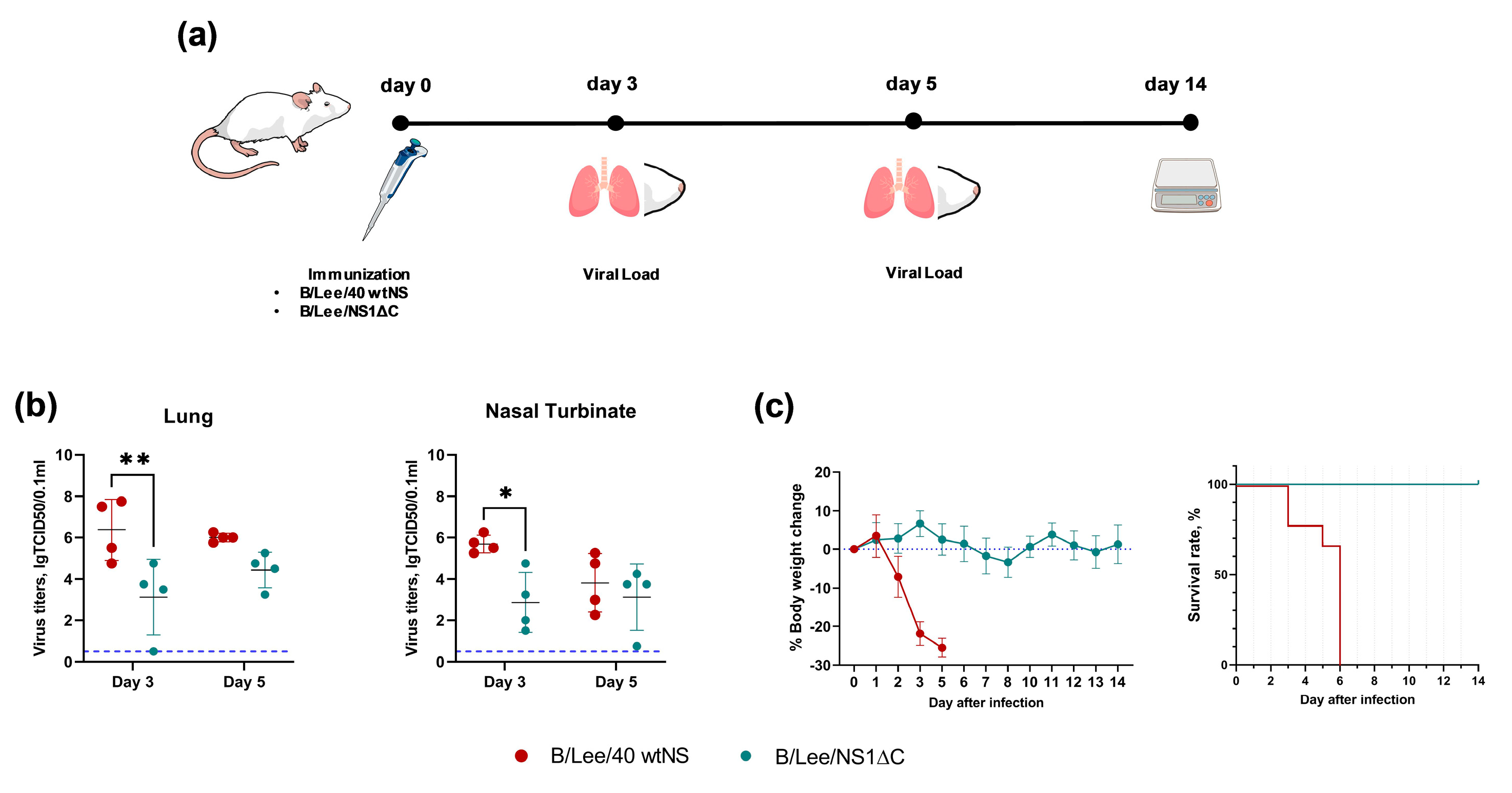
3.1. Generation and Safety Profile of Influenza B Virus with a Truncated NS1 Protein
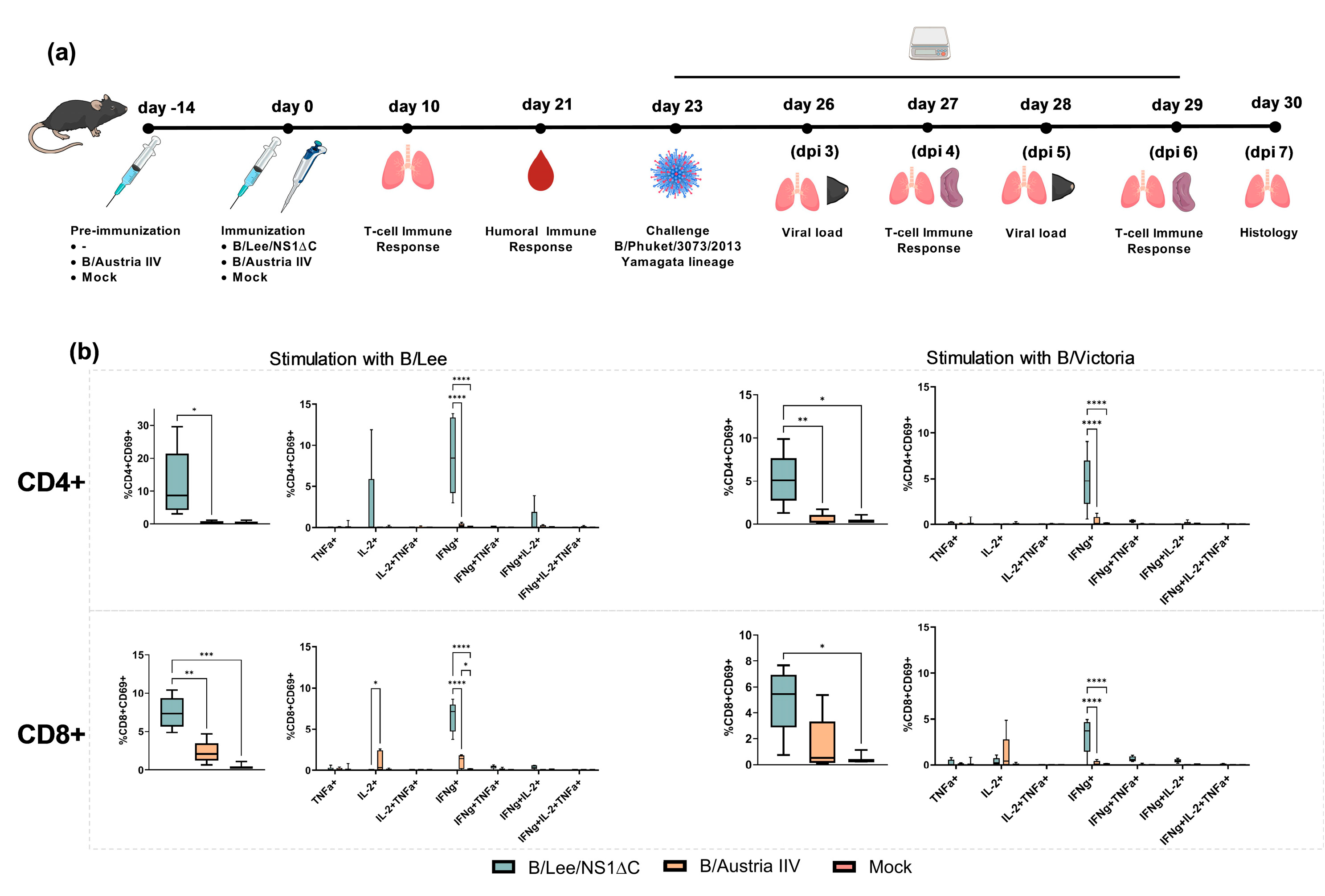
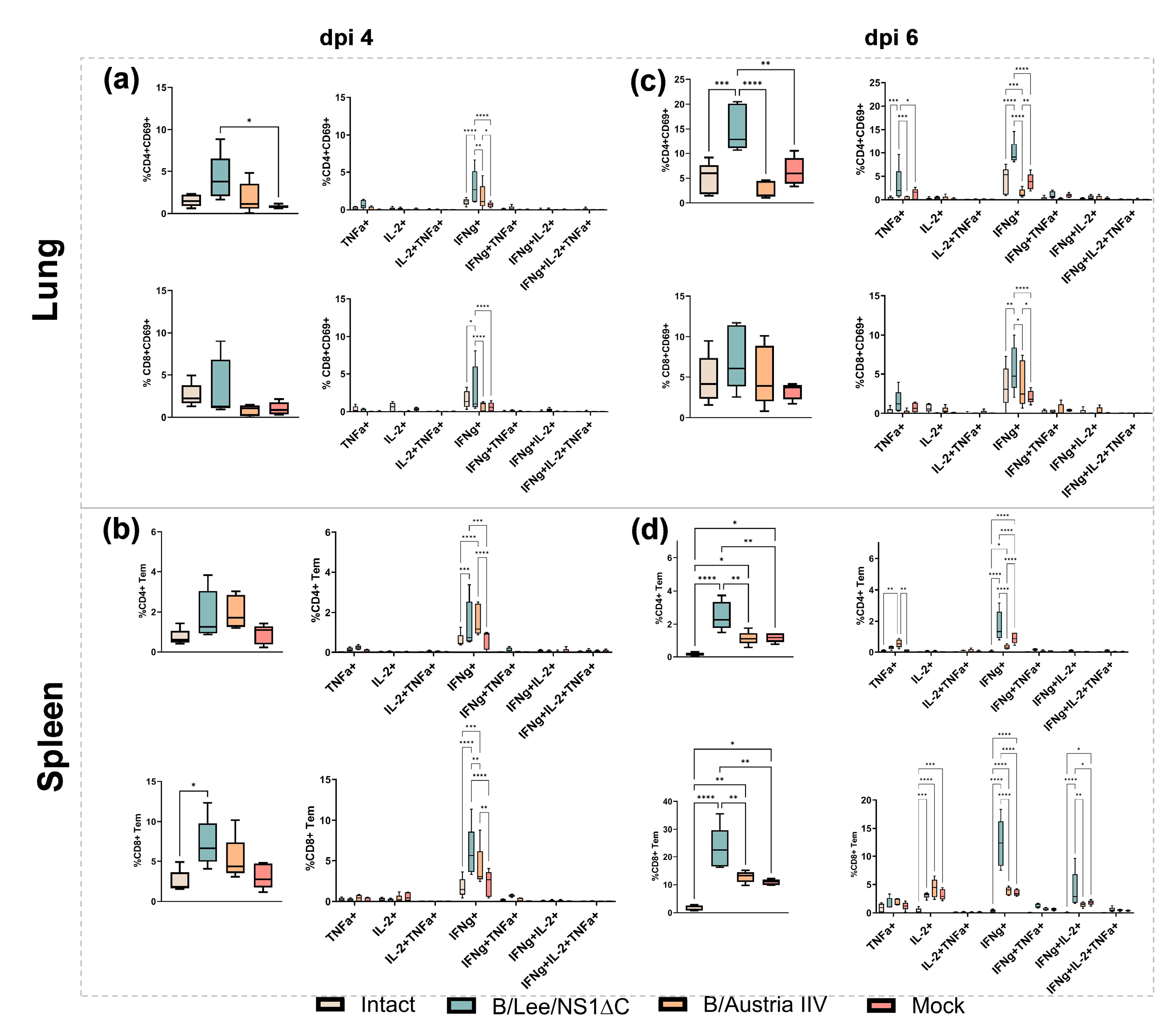
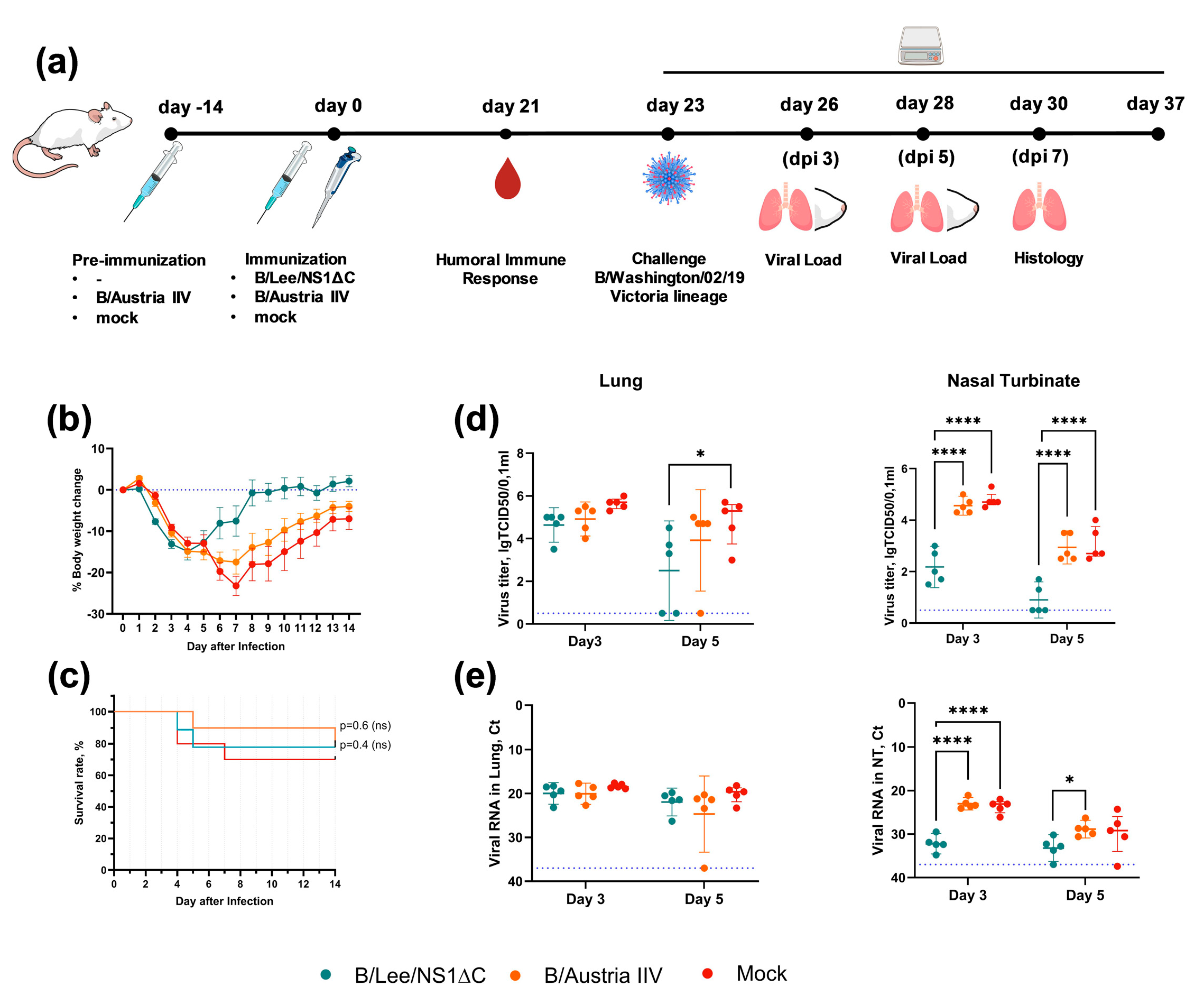
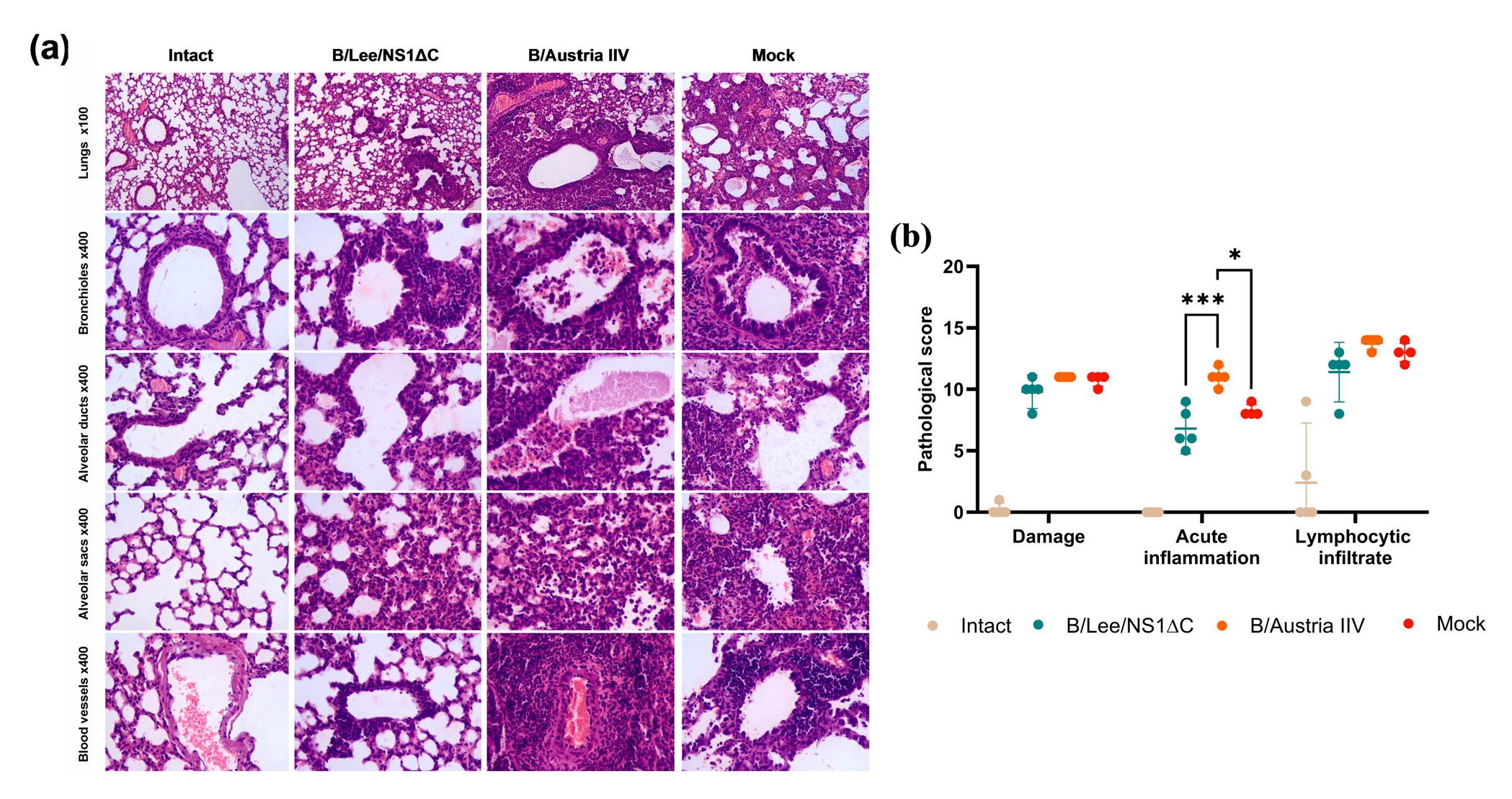
3.2. Immunogenicity of Influenza B Virus with a Truncated NS1 and Protection Against Influenza B/Yamagata Infection
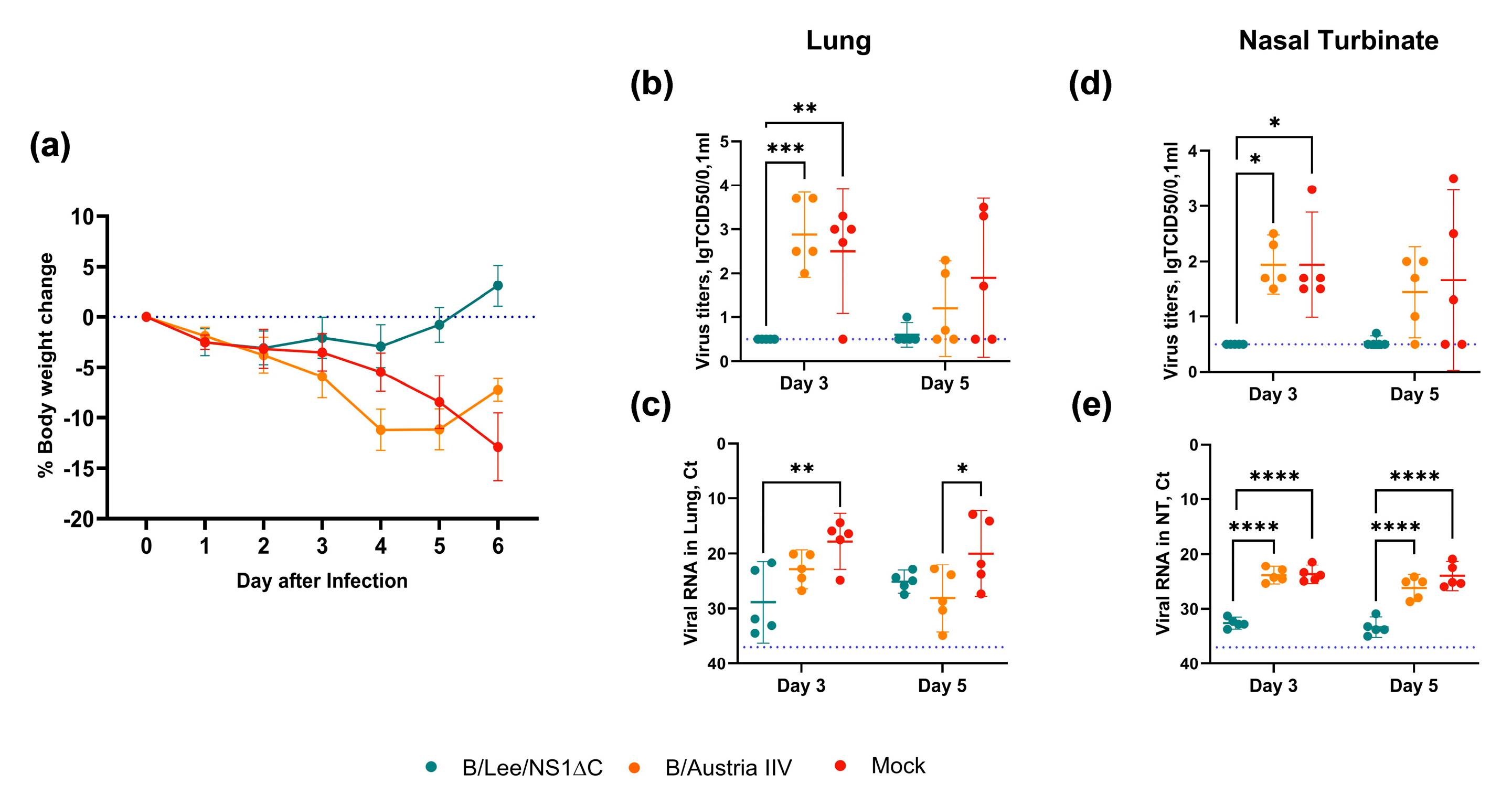
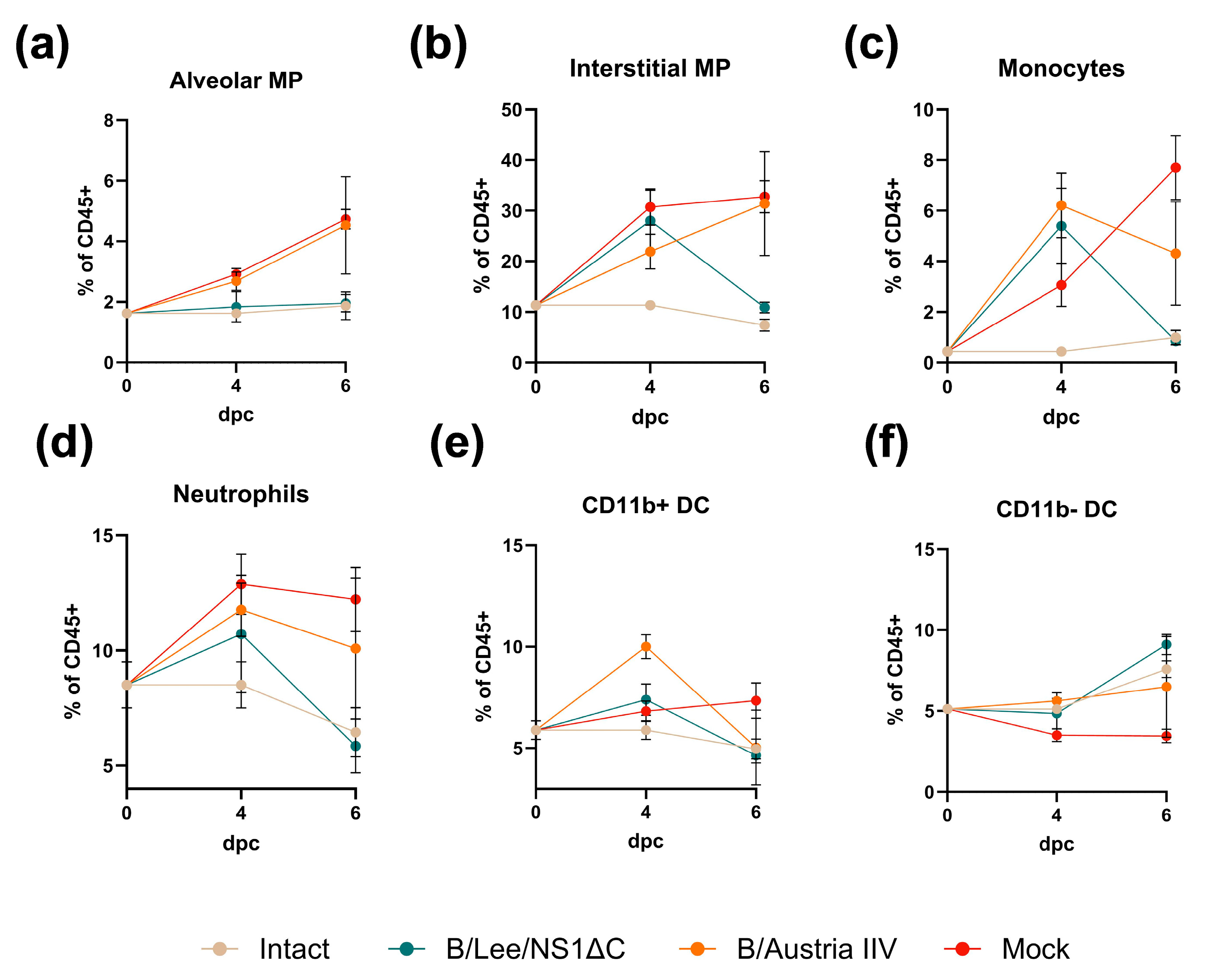
3.3. Protective Efficacy of Influenza B Virus with a Truncated NS1 Protein Against B/Victoria Challenge
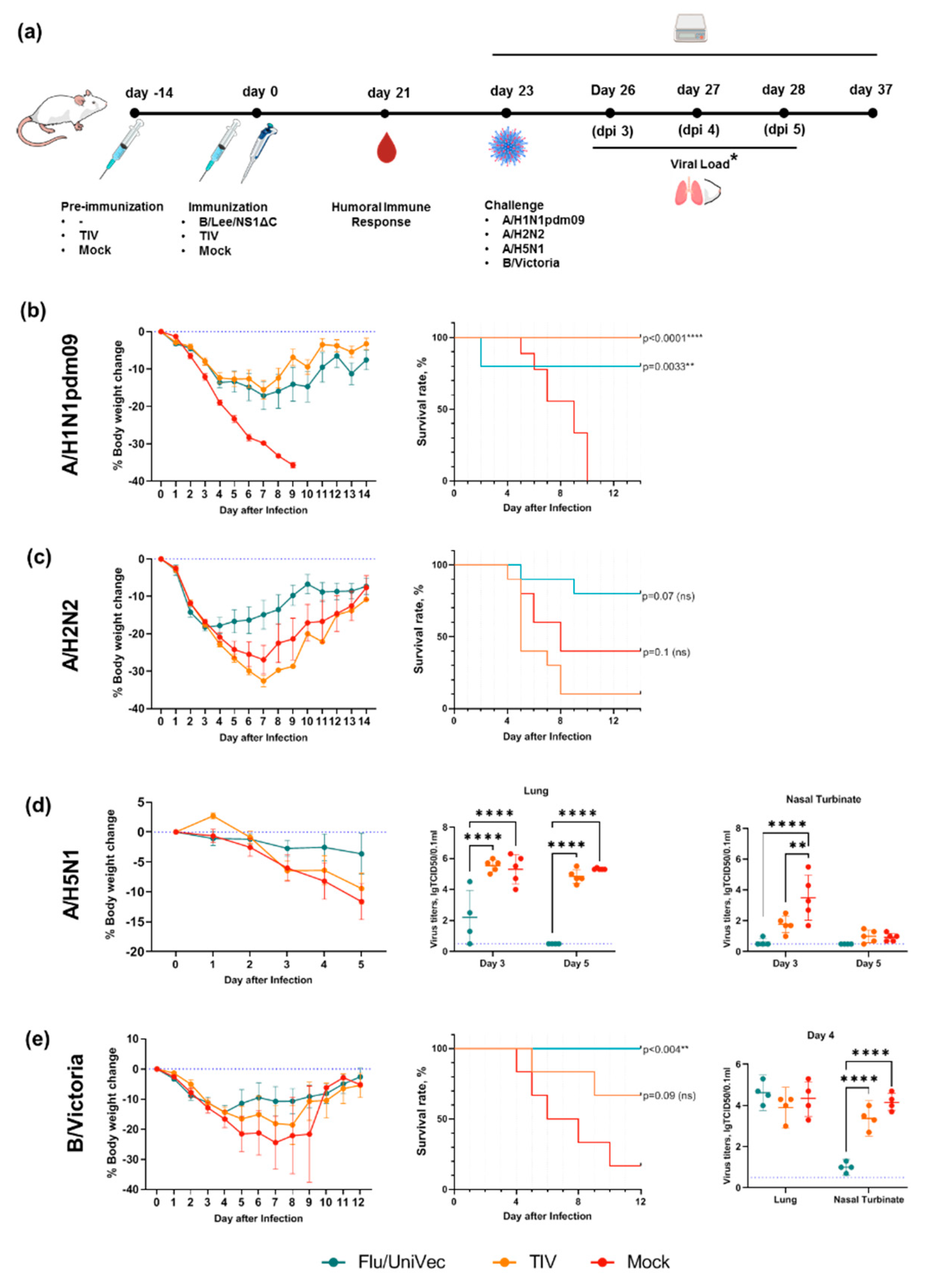
3.4. Cross-Protection by a Trivalent Formulation of NS1ΔС Influenza Viruses Against Divergent Influenza A and B Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IIV | Inactivated Influenza Vaccine |
| HA | Haemagglutinin |
| LAIV | Live Attenuated Influenza Vaccine |
| TIV | Trivalent Inactivated Influenza Vaccine |
| TCID | Tissue Culture Infective Dose |
| MLD | Mouse Lethal Dose |
| EID | Egg Infective Dose |
| ANOVA | Analysis Of Variance |
| Trm | Resident memory T cell |
| Tem | Effector memory T cell |
References
- Hoft, D.F.; Lottenbach, K.R.; Blazevic, A.; Turan, A.; Blevins, T.P.; Pacatte, T.P.; Yu, Y.; Mitchell, M.C.; Hoft, S.G.; Belshe, R.B. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin Vaccine Immunol. 2017, 24, e00414-16. [Google Scholar] [CrossRef]
- Guo, J.; Chen, X.; Guo, Y.; Liu, M.; Li, P.; Tao, Y.; Liu, Z.; Yang, Z.; Zhan, S.; Sun, F. Real-world effectiveness of seasonal influenza vaccination and age as effect modifier: A systematic review, meta-analysis and meta-regression of test-negative design studies. Vaccine 2024, 42, 1883–1891. [Google Scholar] [CrossRef]
- Choi, Y.J.; Song, J.Y.; Wie, S.H.; Choi, W.S.; Lee, J.; Lee, J.S.; Kim, Y.K.; Kim, S.W.; Lee, S.H.; Park, K.H.; Jeong, H.W.; Yoon, J.G.; Seong, H.; Nham, E.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Real-world effectiveness of influenza vaccine over a decade during the 2011-2021 seasons-Implications of vaccine mismatch. Vaccine 2024, 42, 126381. [Google Scholar] [CrossRef]
- Thwaites, R.S.; Uruchurtu, A.S.S.; Negri, V.A.; Cole, M.E.; Singh, N.; Poshai, N.; Jackson, D.; Hoschler, K.; Baker, T.; Scott, I.C.; Ros, X.R.; Cohen, E.S.; Zambon, M.; Pollock, K.M.; Hansel, T.T.; Openshaw, P.J.M. Early mucosal events promote distinct mucosal and systemic antibody responses to live attenuated influenza vaccine. Nat Commun. 2023, 14, 8053. [Google Scholar] [CrossRef] [PubMed]
- Pizzolla, A.; Nguyen, T.H.O.; Smith, J.M.; Brooks, A.G.; Kedzieska, K.; Heath, W.R.; Reading, P.C.; Wakim, L.M. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol. 2017, 2, eaam6970. [Google Scholar] [CrossRef]
- Morimoto, N.; Takeishi, K. Change in the efficacy of influenza vaccination after repeated inoculation under antigenic mismatch: A systematic review and meta-analysis. Vaccine 2018, 36, 949–957. [Google Scholar] [CrossRef]
- Fernandez-Sesma, A.; Marukian, S.; Ebersole, B.J.; Kaminski, D.; Park, M.S.; Yuen, T.; Sealfon, S.C.; García-Sastre, A.; Moran, T.M. Influenza virus evades innate and adaptive immunity via the NS1 protein. J Virol. 2006, 80, 6295–304. [Google Scholar] [CrossRef] [PubMed]
- Shurygina, A.P.; Shuklina, M.; Ozhereleva, O.; Romanovskaya-Romanko, E.; Kovaleva, S.; Egorov, A.; Lioznov, D.; Stukova, M. Truncated NS1 Influenza A Virus Induces a Robust Antigen-Specific Tissue-Resident T-Cell Response and Promotes Inducible Bronchus-Associated Lymphoid Tissue Formation in Mice. Vaccines (Basel) 2025, 13, 58. [Google Scholar] [CrossRef]
- Baskin, C.R.; Bielefeldt-Ohmann, H.; García-Sastre, A.; Tumpey, T.M.; Van Hoeven, N.; Carter, V.S.; Thomas, M.J.; Proll, S.; Solórzano, A.; Billharz, R.; Fornek, J.L.; Thomas, S.; Chen, C.H.; Clark, E.A.; Murali-Krishna, K.; Katze, M.G. Functional genomic and serological analysis of the protective immune response resulting from vaccination of macaques with an NS1-truncated influenza virus. J Virol. 2007, 81, 11817–27. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, M.; Lau, S.Y.; et al. Generation of DelNS1 Influenza Viruses: a Strategy for Optimizing Live Attenuated Influenza Vaccines. mBio 2019, 10, e02180-19. [Google Scholar] [CrossRef]
- Wressnigg, N.; Shurygina, A.P.; Wolff, T.; Redlberger-Fritz, M.; Popow-Kraupp, T.; Muster, T.; Egorov, A.; Kittel, C. Influenza B mutant viruses with truncated NS1 proteins grow efficiently in Vero cells and are immunogenic in mice. J Gen Virol. 2009, 90 Pt 2, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Hai, R.; Martínez-Sobrido, L.; Fraser, K.A.; Ayllon, J.; García-Sastre, A.; Palese, P. Influenza B virus NS1-truncated mutants: live-attenuated vaccine approach. J Virol. 2008, 82, 10580–90. [Google Scholar] [CrossRef]
- Talon, J.; Salvatore, M.; O'Neill, R.E.; Nakaya, Y.; Zheng, H.; Muster, T.; García-Sastre, A.; Palese, P. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc Natl Acad Sci U S A 2000, 97, 4309–14. [Google Scholar] [CrossRef]
- Ma, L.C.; Guan, R.; Hamilton, K.; Aramini, J.M.; Mao, L.; Wang, S.; Krug, R.M.; Montelione, G.T. A Second RNA-Binding Site in the NS1 Protein of Influenza B Virus. Structure 2016, 24, 1562–72. [Google Scholar] [CrossRef]
- Hoffmann, E.; Neumann, G.; Kawaoka, Y.; Hobom, G.; Webster, R.G. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 2000, 97, 6108–13. [Google Scholar] [CrossRef]
- Hoffmann, E.; Mahmood, K.; Yang, C.F.; Webster, R.G.; Greenberg, H.B.; Kemble, G. Rescue of influenza B virus from eight plasmids. Proc Natl Acad Sci U S A 2002, 99, 11411–6. [Google Scholar] [CrossRef]
- Komissarov, A.; Fadeev, A.; Sergeeva, M.; et al. Rapid spread of influenza A(H1N1)pdm09 viruses with a new set of specific mutations in the internal genes in the beginning of 2015/2016 epidemic season in Moscow and Saint Petersburg (Russian Federation). Influenza Other Respir Viruses 2016, 10, 247–253. [Google Scholar] [CrossRef]
- Vasilyev, K.; Shurygina, A.-P.; Sergeeva, M.; Stukova, M.; Egorov, A. Intranasal Immunization with the Influenza A Virus Encoding Truncated NS1 Protein Protects Mice from Heterologous Challenge by Restraining the Inflammatory Response in the Lungs. Microorganisms 2021, 9, 690. [Google Scholar] [CrossRef]
- Sergeeva, M.V.; Vasilev, K.; Romanovskaya-Romanko, E.; Yolshin, N.; Pulkina, A.; Shamakova, D.; Shurygina, A.P.; Muzhikyan, A.; Lioznov, D.; Stukova, M. Mucosal Immunization with an Influenza Vector Carrying SARS-CoV-2 N Protein Protects Naïve Mice and Prevents Disease Enhancement in Seropositive Th2-Prone Mice. Vaccines (Basel) 2024, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Kirby, M.K.; Davis, W.G.; et al. Multiplex Real-Time Reverse Transcription PCR for Influenza A Virus, Influenza B Virus, and Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2021, 27, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.; Muench, H. A simple method of estimating fifty per cent endpoints. Am J of Hyg. 1938, Vol.27(№3.), 493–497. [Google Scholar]
- Potter, C.W.; Jennings, R.; Clark, A.; Ali, M. Interference following dual inoculation with influenza A (H3N2) and (H1N1) viruses in ferrets and volunteers. J Med Virol. 1983, 11, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Romanova, J.R.; Ermachenko, T.A.; Alexandrova, G.I.; Tannock, G.A. Interference between cold-adapted (ca) influenza A and B vaccine reassortants or between ca reassortants and wild-type strains in eggs and mice. Vaccine 1994, 12, 23–27. [Google Scholar] [CrossRef]
- Universal Influenza Vaccine Technology Landscape. Update Nov 25, 2025. The IVR initiative. University of Minnesota. Electronic resource. Available online: https://ivr.cidrap.umn.edu/universal-influenza-vaccine-technology-landscape (accessed on 27 November 2025).
- Ostrowsky, J.; Arpey, M.; Moore, K.; Osterholm, M.; Friede, M.; Gordon, J.; Higgins, D.; Molto-Lopez, J.; Seals, J.; Bresee, J. Tracking progress in universal influenza vaccine development. Curr Opin Virol. 2020, 40, 28–36. [Google Scholar] [CrossRef]
- Vasilyev, K.; Yukhneva, M.; Shurygina, A.-P.; Stukova, M.; Egorov, A. Enhancement of the Immunogenicity of Influenza A Virus by the Inhibition of Immunosuppressive Function of NS1 Protein. Microbiol. Indep. Res. J. 2018, 5, 48–58. [Google Scholar] [CrossRef]
- Pica, N.; Langlois, R.A.; Krammer, F.; Margine, I.; Palese, P. NS1-truncated live attenuated virus vaccine provides robust protection to aged mice from viral challenge. J Virol. 2012, 86, 10293–301. [Google Scholar] [CrossRef]
- Wacheck, V.; Egorov, A.; Groiss, F.; Pfeiffer, A.; Fuereder, T.; Hoeflmayer, D.; Kundi, M.; Popow-Kraupp, T.; Redlberger-Fritz, M.; Mueller, C.A.; Cinatl, J.; Michaelis, M.; Geiler, J.; Bergmann, M.; Romanova, J.; Roethl, E.; Morokutti, A.; Wolschek, M.; Ferko, B.; Seipelt, J.; Dick-Gudenus, R.; Muster, T. A novel type of influenza vaccine: safety and immunogenicity of replication-deficient influenza virus created by deletion of the interferon antagonist NS1. J Infect Dis. 2010, 201, 354–62. [Google Scholar] [CrossRef]
- Mössler, C.; Groiss, F.; Wolzt, M.; Wolschek, M.; Seipelt, J.; Muster, T. Phase I/II trial of a replication-deficient trivalent influenza virus vaccine lacking NS1. Vaccine 2013, 31, 6194–200. [Google Scholar] [CrossRef]
- Nicolodi, C.; Groiss, F.; Kiselev, O.; Wolschek, M.; Seipelt, J.; Muster, T. Safety and immunogenicity of a replication-deficient H5N1 influenza virus vaccine lacking NS1. Vaccine 2019, 37, 3722–3729. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; Zang, X.; Liu, D.; Pan, H.; Hu, Y.; Liu, X.; Chen, Q.; Song, Q.; Quan, J.; Huang, Z.; Zhong, G.; Chen, J.; Han, J.; Sun, H.; Cui, L.; Li, J.; Chen, Y.; Zhang, T.; Ye, X.; Li, C.; Wu, T.; Zhang, J.; Xia, N.S. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir Med. 2022, 10, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Huang, S.; Liu, X.; Chen, Q.; Zhuang, C.; Zhao, H.; Han, J.; Jaen, A.M.; Do, T.H.; Peter, J.G.; Dorado, A.G.; Tirador, L.S.; Zabat, G.M.A.; Villalobos, R.E.M.; Gueco, G.P.; Botha, L.L.G.; Iglesias Pertuz, S.P.; Tan, J.; Zhu, K.; Quan, J.; Lin, H.; Huang, Y.; Jia, J.; Chu, X.; Chen, J.; Chen, Y.; Zhang, T.; Su, Y.; Li, C.; Ye, X.; Wu, T.; Zhang, J.; Xia, N.; COVID-19-PRO-003 Study Team. Safety and efficacy of the intranasal spray SARS-CoV-2 vaccine dNS1-RBD: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2023, 11, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Quan, J.; Liu, X.; Chen, Q.; Zang, X.; Jiang, H.; Liu, D.; Chu, X.; Zhuang, C.; Han, J.; Ye, X.; Pan, H.; Huang, S.; Wu, T.; Zhang, J.; Xia, N. A randomized phase I trial of intranasal SARS-CoV-2 vaccine dNS1-RBD in children aged 3-17 years. NPJ Vaccines 2025, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Randomized open label phase 1 clinical trial of TB/FLU-01L tuberculosis vaccine administered intranasally or sublingual in BCG-vaccinated healthy adults, Marina Stukova, Global Forum on TB Vaccines, 20-23 Feb 2018, New Dehli, India. Electronic recourse. Available online: https://tbvaccinesforum.org/wp-content/uploads/2018/03/5GF-Breakout-2-Stukova.pdf (accessed on 1 December 2025).
- Prokopenko, P.; Matyushenko, V.; Rak, A.; Stepanova, E.; Chistyakova, A.; Goshina, A.; Kudryavtsev, I.; Rudenko, L.; Isakova-Sivak, I. Truncation of NS1 Protein Enhances T Cell-Mediated Cross-Protection of a Live Attenuated Influenza Vaccine Virus Expressing Wild-Type Nucleoprotein. Vaccines 2023, 11, 501. [Google Scholar] [CrossRef]
- Kappes, M.A.; Sandbulte, M.R.; Platt, R.; Wang, C.; Lager, K.M.; Henningson, J.N.; Lorusso, A.; Vincent, A.L.; Loving, C.L.; Roth, J.A.; Kehrli, M.E., Jr. Vaccination with NS1-truncated H3N2 swine influenza virus primes T cells and confers cross-protection against an H1N1 heterosubtypic challenge in pigs. Vaccine 2012, 30, 280–8. [Google Scholar] [CrossRef]
- Dauber, B.; Heins, G.; Wolff, T. The influenza B virus nonstructural NS1 protein is essential for efficient viral growth and antagonizes beta interferon induction. J Virol. 2004, 78, 1865–72. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.; Brandt, S.; Sereinig, S.; Romanova, J.; Ferko, B.; Katinger, D.; Grassauer, A.; Alexandrova, G.; Katinger, H.; Muster, T. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J Virol. 1998, 72, 6437–41. [Google Scholar] [CrossRef]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 2016, 1, e85832. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; He, J.; et al. Intranasal influenza-vectored COVID-19 vaccine restrains the SARS-CoV-2 inflammatory response in hamsters. Nat Commun. Published. 2023, 14, 4117. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Salvatore, M.; Zheng, H.; Jangra, S.; Kehrer, T.; Mena, I.; Schotsaert, M.; Muster, T.; Palese, P.; García-Sastre, A. Interferon mediated prophylactic protection against respiratory viruses conferred by a prototype live attenuated influenza virus vaccine lacking non-structural protein 1. Sci Rep. 2021, 11, 22164. [Google Scholar] [CrossRef]
- Worldwide Influenza Centre: Annual and interim reports. Report prepared for the WHO annual consultation on the composition of influenza vaccine for the Southern Hemisphere 2022. 13th – 23rd September 2021. Available online: https://www.crick.ac.uk/research/platforms-and-facilities/worldwide-influenza-centre/annual-and-interim-reports (accessed on 1 December 2025).
- Pekarek, M.J.; Madapong, A.; Wiggins, J.; Weaver, E.A. Adenoviral-Vectored Multivalent Vaccine Provides Durable Protection Against Influenza B Viruses from Victoria-like and Yamagata-like Lineages. Int J Mol Sci. 2025, 26, 1538. [Google Scholar] [CrossRef]
- Carlock, M.A.; Pierce, S.R.; Ross, T.M. Breadth of antibody activity elicited by an influenza B hemagglutinin vaccine is influenced by pre-existing immune responses to influenza B viruses. J Virol. 2025, 99, e0070525. [Google Scholar] [CrossRef]
- Su, S.; Chaves, S.S.; Perez, A.; D'Mello, T.; Kirley, P.D.; Yousey-Hindes, K.; Farley, M.M.; Harris, M.; Sharangpani, R.; Lynfield, R.; Morin, C.; Hancock, E.B.; Zansky, S.; Hollick, G.E.; Fowler, B.; McDonald-Hamm, C.; Thomas, A.; Horan, V.; Lindegren, M.L.; Schaffner, W.; Price, A.; Bandyopadhyay, A.; Fry, A.M. Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin Infect Dis. 2014, 59, 252–5. [Google Scholar] [CrossRef]
- Tran, D.; Vaudry, W.; Moore, D.; Bettinger, J.A.; Halperin, S.A.; Scheifele, D.W.; Jadvji, T.; Lee, L.; Mersereau, T. members of the Canadian Immunization Monitoring Program Active. Hospitalization for Influenza A Versus B. Pediatrics 2016, 138, e20154643. [Google Scholar] [CrossRef] [PubMed]
- Mattila, J.M.; Vuorinen, T.; Heikkinen, T. Comparative Severity of Influenza A and B Infections in Hospitalized Children. Pediatr Infect Dis J. 2020, 39, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Davido, B.; Lemarie, B.; Gault, E.; et al. Comparison between clinical outcomes in influenza A and B Infections: a multicenter retrospective cohort study. CMI Communications 2025, 2, 105072. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




