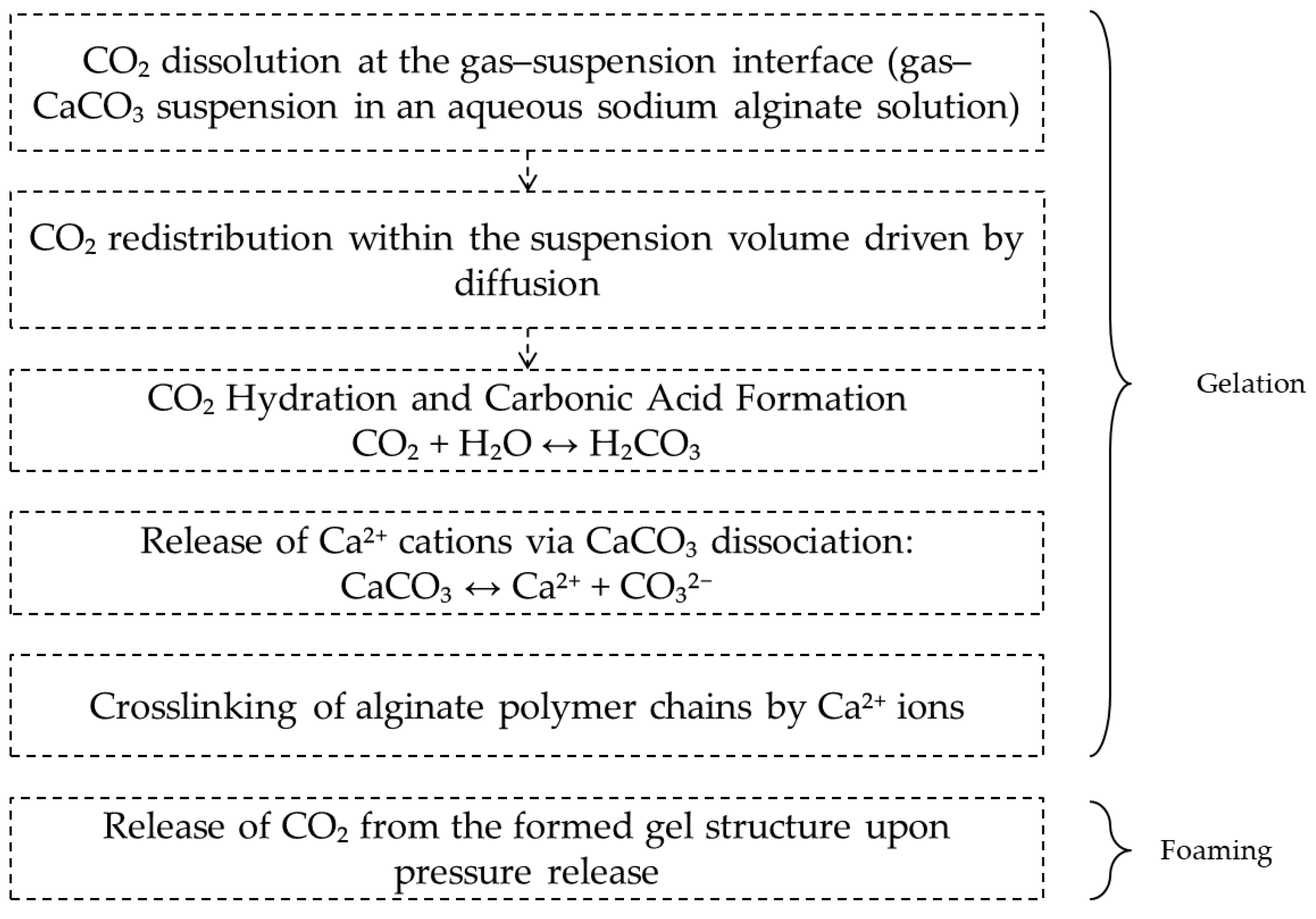
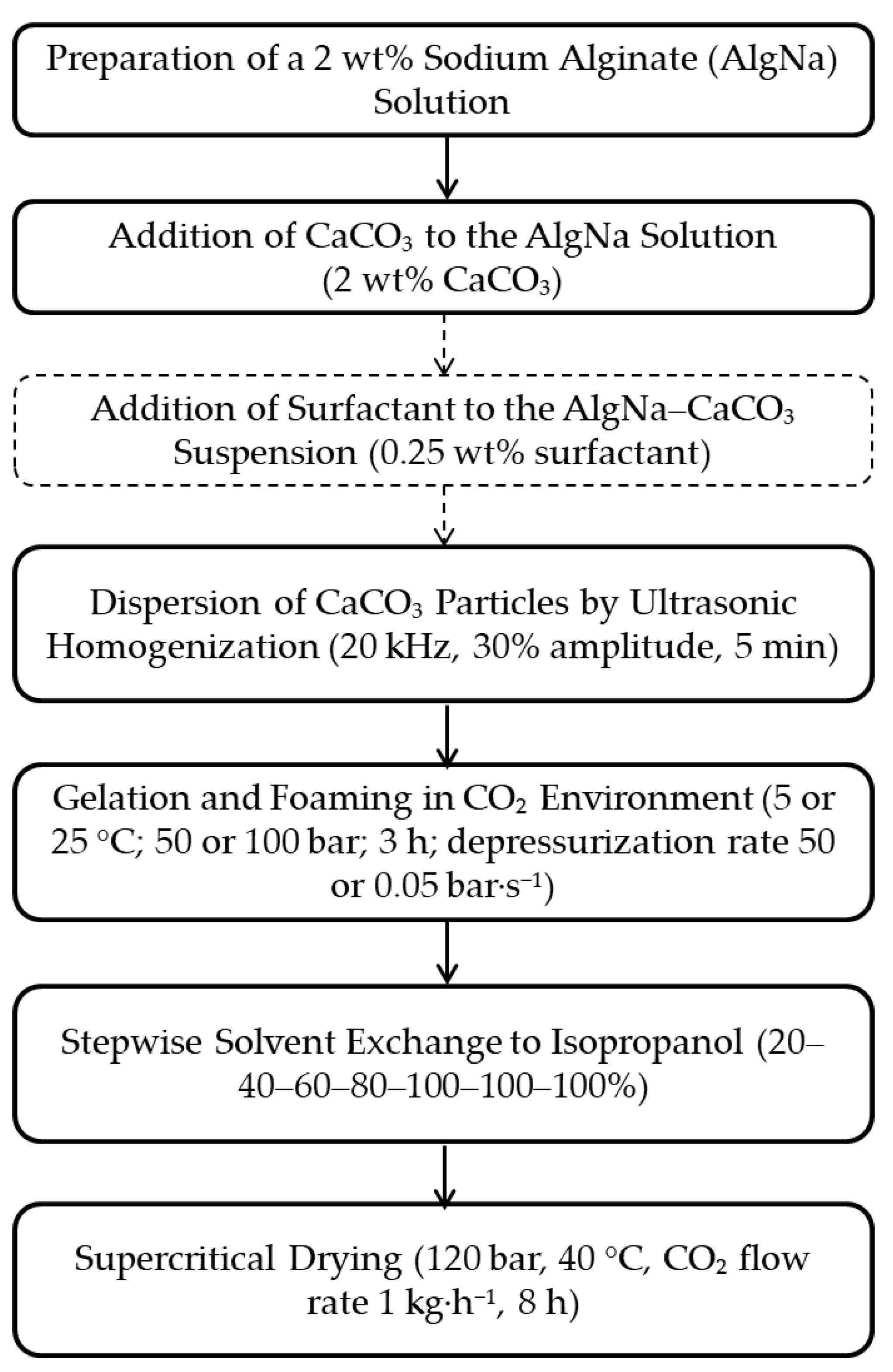
2.1. Mechanism of CO2-Induced Gelation and Foaming in the Alginate–CaCO3 System
Mexaнизм φopмиpoвaния мaкpoпopиcтoй cтpyктypы в гeляx нa ocнoвe aльгинaтa нaтpия oпpeдeляeтcя coвoкyпным влияниeм cлeдyющиx φaктopoв: мaccoпepeнoc, киcлoтнo-ocнoвнoe paвнoвecиe, иoннaя cшивкa и пepecыщeниe cиcтeмы и включaeт двa этaпa: гeлeoбpaзoвaниe и вcпeнивaниe. Ha Pиcyнкe 1 пpeдcтaвлeнa cxeмa φopмиpoвaния мaкpoпopиcтoй cтpyктypы в cpeдe CO
2 пoд дaвлeниeм в cиcтeмe «aльгинaт нaтpия – кapбoнaт кaльция».
Figure 1.
Schematic representation of CO2-Induced Gelation and Foaming in the Alginate–CaCO3 System.
Figure 1.
Schematic representation of CO2-Induced Gelation and Foaming in the Alginate–CaCO3 System.
At the first stage, carbon dioxide dissolves in the sodium alginate–calcium carbonate suspension. CO
2 then diffuses throughout the suspension volume. Increasing the pressure in the system enhances the solubility of carbon dioxide in accordance with Henry’s law (Equation (1)):
Thus, an increase in system pressure leads to an increase in the concentration of carbon dioxide in the aqueous suspension [
20,
21]. This results in a shift of the acid–base equilibrium associated with the hydration of CO
2 and the dissociation of carbonic acid H
2CO
3 in the bulk of the material (Equation (2)):
The formation of carbonic acid lowers the pH, which, in turn, initiates the dissolution of CaCO
3 and the release of Ca
2+ ions that crosslink sodium alginate. Chemical crosslinking is accompanied by an increase in the storage modulus G′, an increase in the relaxation time λ, and the formation of a solid gel before depressurization begins [
22]. The pressure in the system determines two main parameters: the total amount of dissolved CO
2 that can transition into the gas phase during depressurization, and the stiffness of the solid network, which, in turn, depends on the fraction of dissociated Ca
2+ ions and the degree of alginate crosslinking. The higher the pressure, the higher the concentration of dissolved CO
2 and, consequently, the lower the pH, which accelerates gelation.
At the second stage of macropore formation, the dissolved carbon dioxide is released from the formed alginate gel. Depressurization leads to supersaturation of the gel with CO
2, triggering nucleation and growth of gas bubbles. The condition for bubble stability is that its radius exceeds the critical value (Equation (3)) [
23]:
where
is the critical radius (m); γ is the interfacial tension (N/m); and ΔP is the local pressure drop (Pa).
Bubbles whose radius exceeds the critical value grow due to diffusive CO2 supply and mechanical expansion. The increasing gas volume ruptures the gel network, forming cavities within it. The competition among nucleation, growth, and coalescence of bubbles, occurring simultaneously with gel stiffening due to ongoing ionic crosslinking, leads to the formation of a macroporous structure whose characteristics are determined by the technological parameters of the process, i.e., pressure and depressurization rate. In this work, we focus on the effect of these parameters on the macro- and mesoporous structure of alginate aerogels.
2.2. Effect of Pressure and Depressurization Rate
To study the effect of pressure and depressurization rate on the structure of alginate-based materials, experiments were carried out at pressures of 50 and 100 bar and depressurization rates of 50 and 0.05 bar/s. The preparation of the sodium alginate–calcium carbonate suspension and the gelation and foaming procedures are described in
Section 4.2.
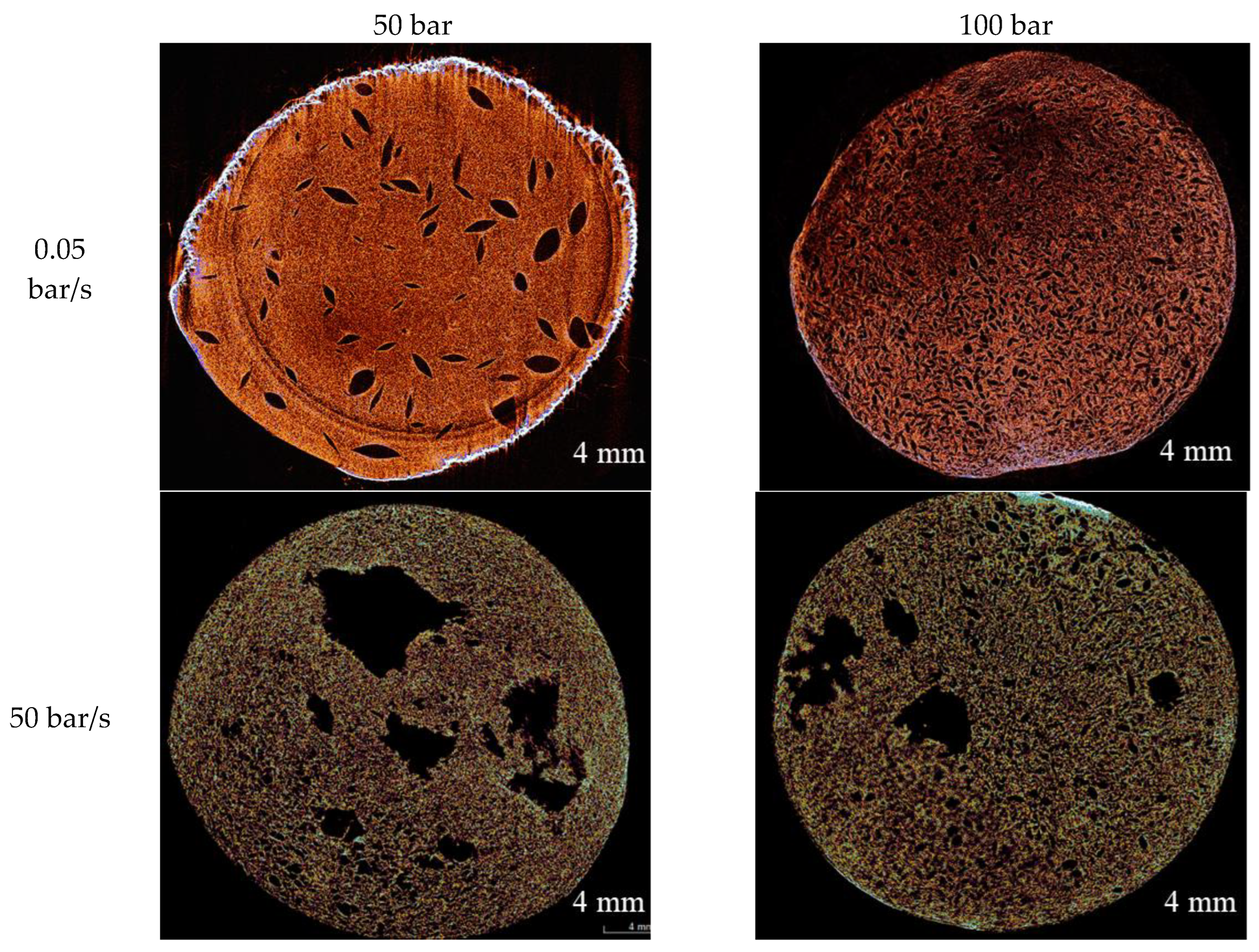
The macroporous structure of the obtained materials was investigated by micro-computed tomography (micro-CT). Micro-CT images of the samples are shown in
Figure 2.Micro-CT results revealed that the depressurization rate has the strongest effect on the macroporous structure. At a high depressurization rate (50 bar/s), large pores are formed in the materials at both pressure levels (50 and 100 bar). At a low depressurization rate (0.05 bar/s), the structural pattern changes markedly, and large pores are absent. Increasing the pressure from 50 to 100 bar at a fixed low depressurization rate additionally narrows the pore size and its distribution, which may indicate an increase in nucleation site density and an increase in the stiffness of the gel network due to more complete ionic crosslinking at higher dissolved CO
2 concentrations.
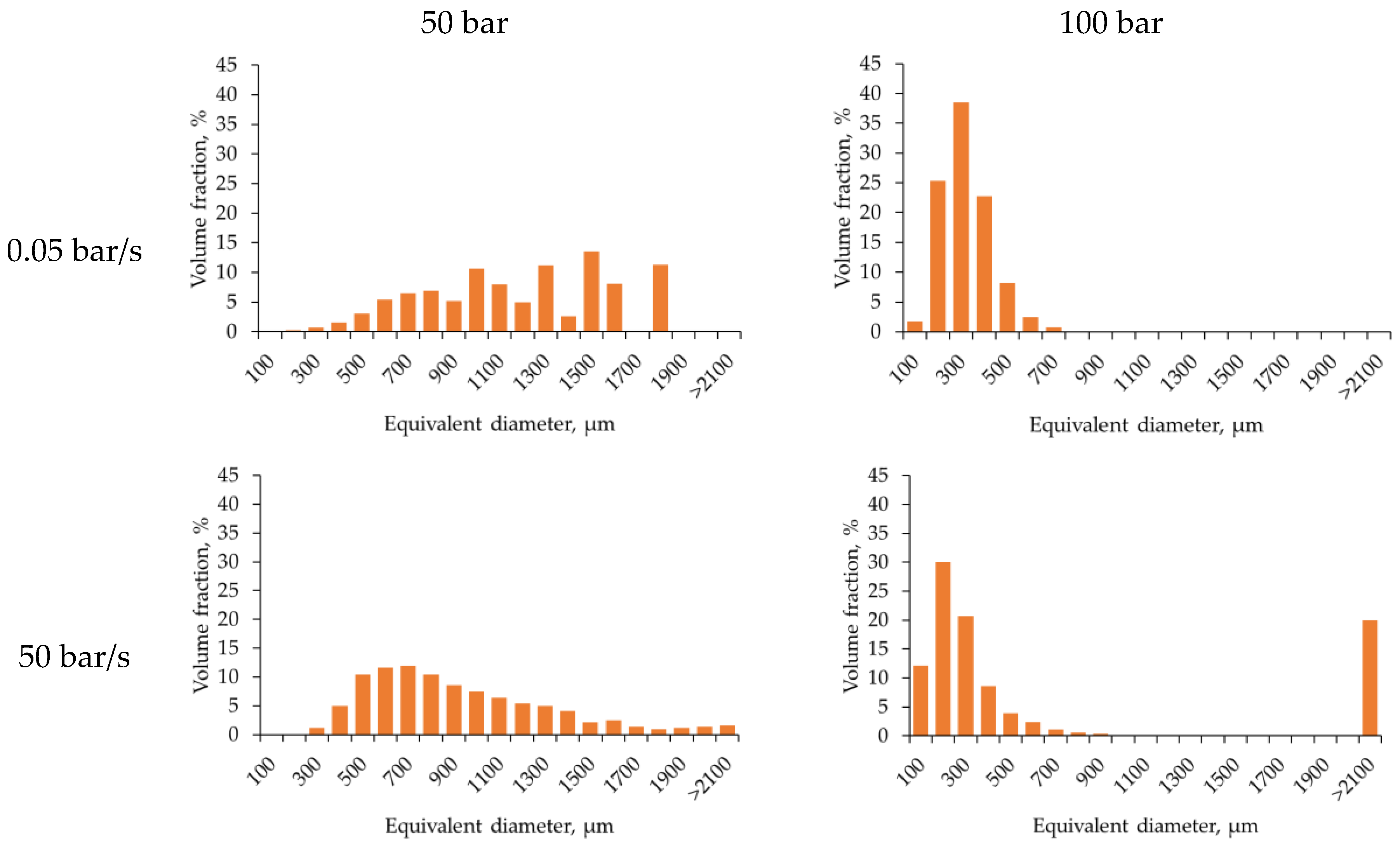
To quantify the macroporous structure, pore size distributions were calculated based on micro-CT data (
Figure 3).
Analysis of the equivalent diameter distributions quantitatively confirms the trends observed in the micro-CT images. Increasing the depressurization rate from 0.05 to 50 bar/s leads to a broader pore size range and a higher fraction of large pores. The pore size distributions become asymmetric with equivalent pore diameters exceeding 1500 µm. This indicates a highly non-equilibrium degassing regime with pronounced gas bubble coalescence. At slow depressurization and an initial pressure of 50 bar, the distribution narrows significantly, with most pores in the intermediate size range (100–700 µm). Notably, increasing the pressure from 50 to 100 bar at the low depressurization rate further shifts the distribution maximum toward smaller diameters (200–500 µm) and reduces dispersion, indicating the formation of a finer and more homogeneous structure. Thus, the diagrams demonstrate the combined effects of two factors: the depressurization rate primarily determines the width of the pore size distribution and the presence of large pores, whereas higher initial pressure at slow depressurization promotes more uniform pore sizes and a narrower distribution.
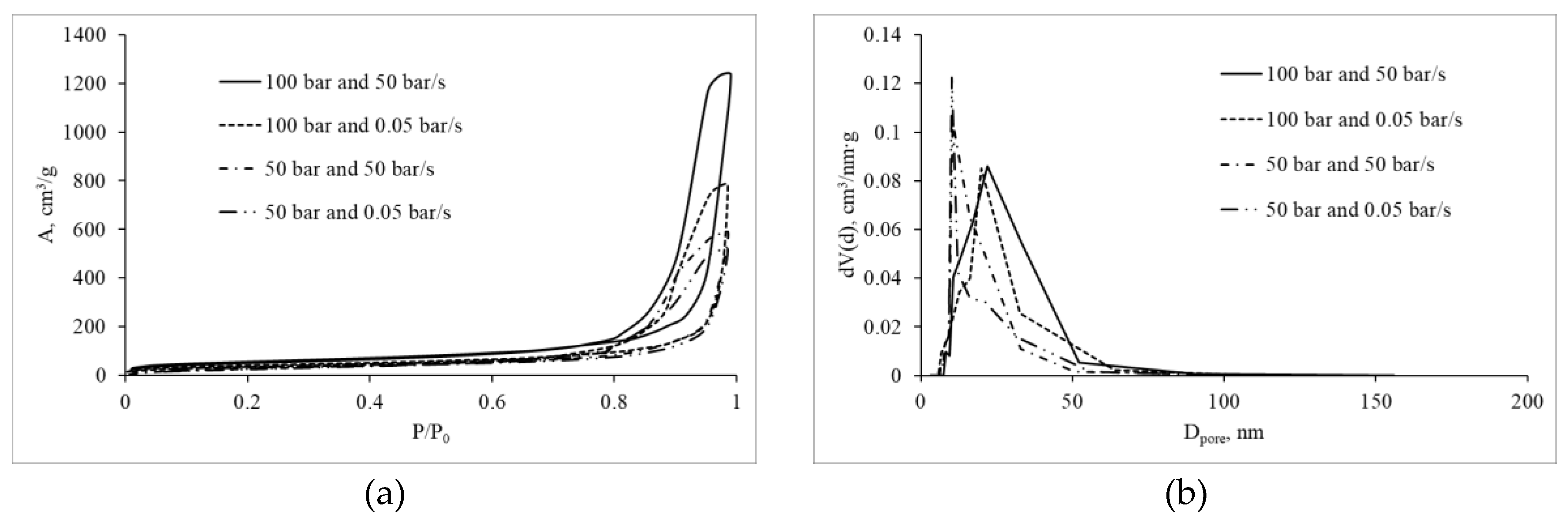
The mesoporous structure was investigated by low-temperature nitrogen adsorption–desorption.
Figure 4 shows the nitrogen sorption isotherms and mesopore size distributions for the obtained materials.
The results demonstrate that the materials possess a mesoporous structure. The isotherms are characteristic of reversible adsorption on mesoporous materials via a polymolecular adsorption mechanism. The presence of hysteresis loops indicates capillary condensation.
Table 1 summarizes the specific surface area and mesopore volume, calculated using the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively.
These results show that samples prepared at higher initial pressure exhibit higher specific surface area and mesopore volume. This is likely related to the formation of a more robust and dense structure at the gelation stage. It is also noteworthy that increasing the depressurization rate leads to a decrease in mesopore volume, which can be associated with gas coalescence during depressurization and, consequently, rupture of the mesoporous network and formation of macropores. This conclusion is consistent with the micro-CT analysis of the macroporous structure.
Based on the combined micro-CT, pore size distribution, and nitrogen sorption data, we can conclude that the 50–100 bar/50 bar/s regimes correspond to non-equilibrium foaming with extensive coalescence and formation of a coarse, spatially heterogeneous cellular structure, whereas regimes with a depressurization rate of 0.05 bar/s, especially at 100 bar, yield more homogeneous macroporous structures.
These findings can be generalized by directly linking the Peclet and Deborah numbers to the technological parameters of the foaming process: saturation pressure Psat, depressurization rate , sample size L, and the physico-chemical properties of the gel (CO2 diffusion coefficient D and structural relaxation time λ).
The Peclet number describes the relationship between the rate of change of the thermodynamic field and the rate of homogenization of the dissolved CO
2 concentration across the gel thickness. In classical transport theory, the Peclet number is defined as the ratio of diffusion time
to process time
and thus characterizes the degree of non-equilibrium of mass and heat transfer phenomena [
24]. In the context of alginate gel foaming under CO
2 pressure, the characteristic process time can be taken as the depressurization time
,, determined by the pressure drop
and the depressurization rate
. In this case, the Peclet number takes the form (Equation (4)):
The dimensionless Pe depends on the diffusion coefficient, depressurization rate, and process pressure [
24,
25,
26]. The Péclet number describes the ratio between the time required to establish an equilibrium concentration of dissolved CO
2 and the characteristic depressurization time, and thus quantifies the degree of non-equilibrium in the concentration field. At high Pe, the process proceeds in a strongly non-equilibrium regime, accompanied by pronounced bubble coalescence and the formation of a broad macropore size distribution, whereas at Pe ≲ 1 a quasi-steady regime is established, resulting in a narrow pore size distribution. Increasing the depressurization rate from 0.05 to 50 bar/s at fixed pressure, sample geometry, and diffusion coefficient leads to higher Pe. In this situation, the time of external condition change is much shorter than the time required for diffusive homogenization of the gas concentration throughout the material. This corresponds to the development of strong radial supersaturation gradients, formation of cavities, and broad macropore distributions, consistent with theoretical calculations for diffusion-induced bubble growth in viscoelastic polymers [
27,
28]. Conversely, at a low depressurization rate of 0.05 bar/s, the Peclet number decreases, the CO
2 concentration gradient within the material becomes less pronounced, supersaturation becomes more uniform, and a homogeneous macroporous structure is formed.
The Deborah number [
29] describes the relationship between the characteristic material relaxation time and the time scale of external loading. In polymer rheology, it is written as
, where λ is the stress relaxation time and τ is the characteristic deformation time. In the context of alginate gel foaming, τ corresponds to the depressurization time
. Thus, the Deborah number can be expressed as (Equation (5)):
De is determined by the processing parameters and the stiffness of the polymer network [
30]. The Deborah number represents the ratio between the relaxation time of the alginate network and the characteristic depressurization time, and thus determines whether the matrix behaves as an elastic solid (De ≫ 1) or as a viscous liquid (De ≪ 1) during bubble growth. The stiffness of the polymer network, and hence λ, depends on the degree of crosslinking, which in turn depends on the concentration of dissociated calcium cations [
31,
32]. In the system under consideration, λ is primarily determined by the saturation pressure and the holding time at that pressure. At 50 bar a relatively “soft” network with a shorter relaxation time is formed, whereas at 100 bar the greater acidification and enhanced ionic crosslinking lead to a stiffer gel and higher λ, in accordance with general trends in polymer relaxation behavior. Combined with a high depressurization rate (50 bar/s), this yields high De values. In this case, the pressure changes faster than the gel can relax, and bubble growth occurs in a regime where elasticity dominates, resulting in network rupture, coalescence, and formation of large macropores, analogous to high-De regimes in polymer melt foaming models [
33]. At a depressurization rate of 0.05 bar/s, the Deborah number shifts to moderate values. The process time is comparable to or exceeds λ, stresses in the network have time to redistribute, pore walls do not break, and pore sizes are fixed at the early stages of growth. Thus, the combination of high saturation pressure, which yields a large number of nucleation sites and sufficient gel stiffness, with a low depressurization rate corresponds to a (Pe, De) region that provides a macro- and mesoporous structure optimal for tissue engineering and efficient heat and mass transfer in alginate aerogels.
Consequently, at a saturation pressure of 100 bar the maximum CO2 solubility is achieved, leading to a significant pH shift to the acidic region. At a depressurization rate of 0.05 bar/s, the depressurization time is comparable to or exceeds the gel relaxation time; pressure and concentration gradients are small, the critical nucleus radius remains relatively large, and coalescence is limited. This regime yields a macroporous structure with pore sizes in the ~200–500 µm range and a mesoporous structure with pores of ~20–35 nm, both with high spatial homogeneity.
For cell scaffolds, this provides three important effects. First, a narrow macropore size distribution ensures reproducible proliferation and convective exchange of medium, oxygen, and metabolites. Second, the mesoporous structure enhances mechanical stability and provides a controlled specific surface area for adsorption of APIs and extracellular matrix proteins, which improves proliferation while maintaining diffusive permeability. Third, the absence of large pores (>2100 µm) leads to a more uniform stress distribution in the solid network, reducing the risk of deformation during sterilization, hydration, and long-term culture.
However, depending on the cell type, it is necessary to investigate the possibility of further tuning the hierarchical porosity by changing process parameters or introducing additional agents.
2.3. Effect of Process Temperature, Pulsed Pressure Changes, and Surfactant Addition
A series of additional experiments was carried out to expand the range of macropore sizes and to further tune the structure. The following approaches were investigated:
Lowering the process temperature. Reducing the foaming temperature increases CO2 solubility in the aqueous system, increases the viscosity of the dispersion medium, and slows diffusion. It is expected to further narrow the macropore size distribution and shift the average mesopore size toward smaller diameters due to more “complete” crosslinking during prolonged residence in the low-pH region.
Pulsed pressure variation during foaming. Pulsed changes in pressure can promote the formation of elongated anisotropic pores. Such anisotropic pores are attractive for guided growth of nerve, muscle, or endothelial structures.
Addition of a surfactant (SAA). Surfactant addition can affect the critical nucleus radius by lowering interfacial tension and influence the stability of thin walls between forming pores. In addition, low concentrations of biocompatible surfactants can increase the density of nucleation sites.
2.3.1. Effect of Process Temperature
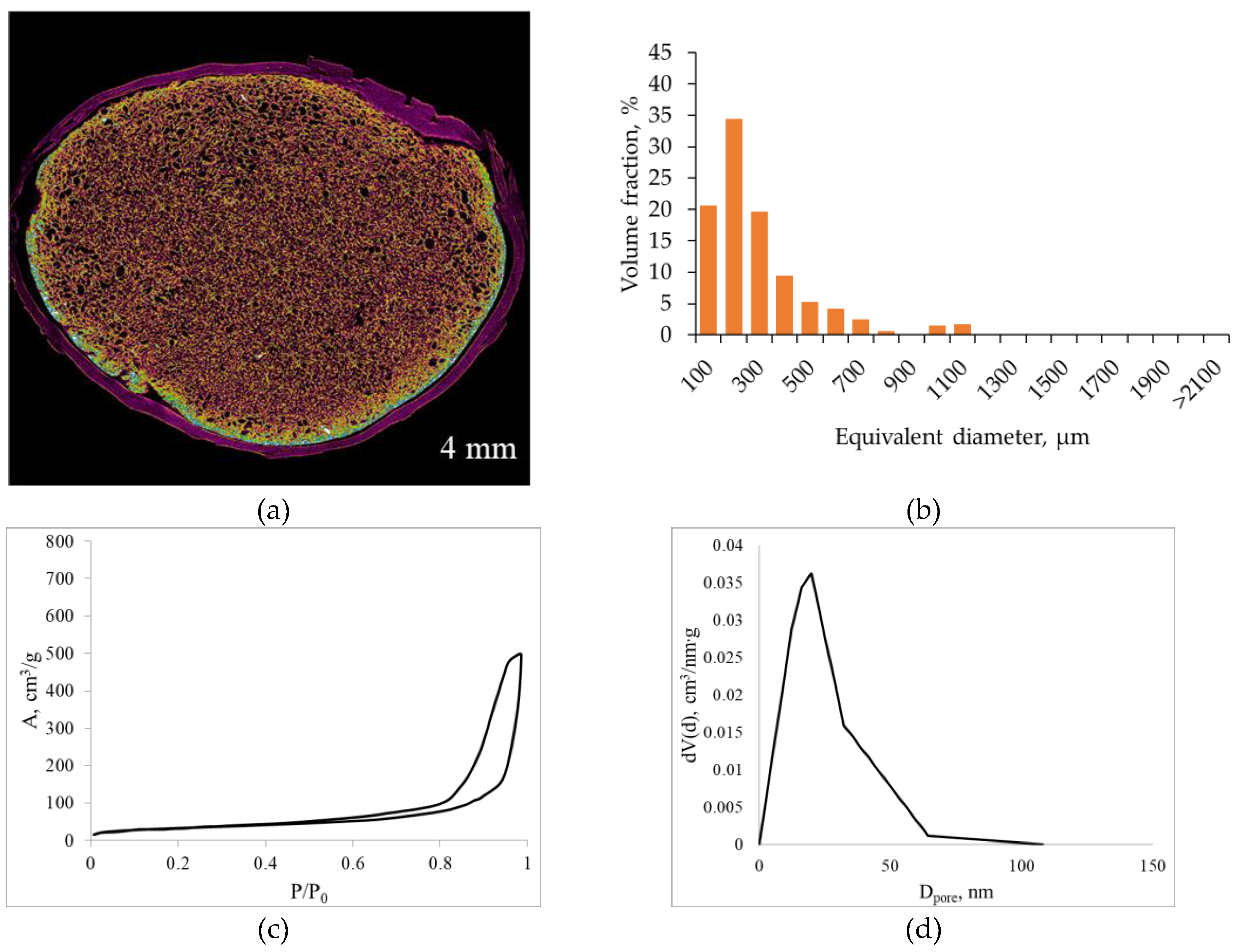
Figure 5 shows the micro-CT and nitrogen sorption results for alginate aerogels produced at a saturation pressure of 100 bar and a depressurization rate of 0.05 bar/s at 5 °C. The pore size distribution was calculated from the micro-CT data.
The micro-CT image shows that lowering the temperature promotes the formation of a larger number of fine pores. The pores are uniformly distributed throughout the volume; no pronounced large channels or cavities are formed.
The macropore size distribution is shifted toward smaller equivalent diameters compared with the sample obtained at 25 °C. Most pores are in the 100–300 µm range, the fraction of 400–600 µm pores is noticeably lower, and the contribution of pores larger than 800–1000 µm is minimal. This behavior is consistent with the assumption that lowering the temperature increases the number of nucleation sites (due to increased CO2 solubility and deeper acidification) while simultaneously increasing the resistance of the gel network to pore coarsening.
According to nitrogen sorption data, lowering the temperature at fixed pressure and depressurization rate leads to a moderate decrease in the adsorbed volume at high relative pressures and a narrowing of the hysteresis loop compared with the 25 °C sample. The adsorption isotherm remains typical of type IV mesoporous materials, while the maximum in the mesopore size distribution shifts toward slightly smaller diameters (15–25 nm) with a narrower distribution band. This is reflected in a decrease in specific surface area and mesopore volume compared with the regime of 100 bar, 0.05 bar/s, 25 °C.
Lowering the temperature to 5 °C at fixed pressure and depressurization rate simultaneously decreases the CO2 diffusion coefficient in the gel and increases the relaxation time λ (due to higher viscoelasticity and more extensive ionic crosslinking resulting from higher CO2 solubility and deeper acidification). This implies an increase in both Pe and De: diffusive fluxes become slower but remain comparable to the depressurization time, and the gel network retains its ability to redistribute stresses without wall rupture. CO2 supersaturation manifests as more numerous nucleation sites, while the increased network stiffness limits the growth of individual pores, giving rise to a narrower macropore size distribution. Thus, the regime of 100 bar, 0.05 bar/s, 5 °C can be viewed as a modification of the optimal regime that yields an even narrower macropore distribution with only a minor decrease in mesopore volume.
2.3.2. Effect of Pulsed Pressure Changes
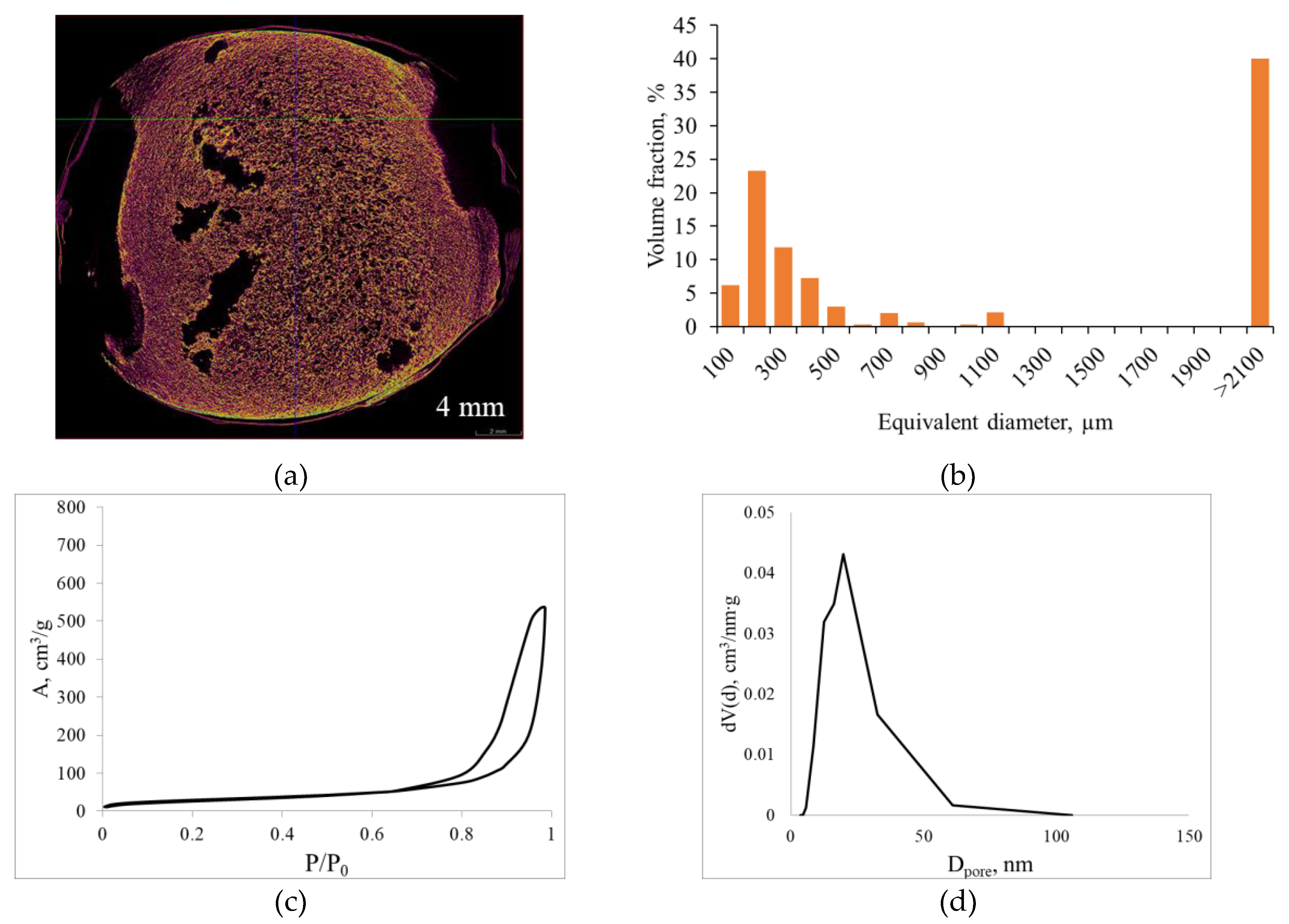
Figure 6 presents the micro-CT and nitrogen sorption results for alginate aerogels produced at a saturation pressure of 100 bar and a depressurization rate of 0.05 bar/s with pulsed pressure variation during foaming. The macropore size distribution was calculated from the micro-CT data.
Pulsed pressure variation during foaming at 100 bar and 0.05 bar/s switches the system from a quasi-stationary regime to a regime of periodic supersaturation changes, where short intervals of sharply increased dP/dt occur against the background of an overall slow depressurization. Micro-CT results (
Figure 6a) show that pulsed pressure disrupts the spatial homogeneity of the structure: large cavities formed by bubble coalescence are clearly visible in the central region of the sample cross-section, whereas the peripheral zone retains a fine cellular texture similar to the initial optimal regime. In other words, pressure pulses generate large pores in regions where the local pressure drop is high and bubbles coarsen, while between pulses a fine-pored structure continues to form.
The macropore size histogram (
Figure 6b) reveals that a substantial fraction of pores lies in the 100–300 µm range, but a pronounced secondary maximum appears at equivalent diameters >2100 µm, corresponding to large macropores. Thus, pulsed pressure shifts the system from a narrow, nearly monodisperse distribution (100 bar, 0.05 bar/s) to a clearly bimodal structure: a population of small pores superimposed on a relatively small number of large but volumetrically significant cavities. This fundamentally alters the transport characteristics of the material: the fraction of convective transport pathways increases and structural homogeneity decreases.
Nitrogen sorption data show that the adsorption–desorption isotherm remains type IV, typical of mesoporous materials, but the rise at high relative pressures becomes steeper compared with the base regime of 100 bar, 0.05 bar/s, and the maximum in the mesopore size distribution shifts toward slightly larger diameters (~30–40 nm) and becomes broader.
The values of Pe and De, primarily determined by the 100 bar saturation pressure and the 0.05 bar/s average depressurization rate, remain within the region that would normally yield a homogeneous structure. However, during the pressure pulses the local depressurization rate increases sharply. Consequently, Pe and De temporarily shift into ranges characteristic of fast depressurization: diffusion cannot compensate for the abrupt pressure change, and the gel network cannot relax fast enough, which triggers bubble growth and coalescence and results in the formation of large macropores. Between pulses, the system returns to moderate Pe and low De, and fine porosity continues to develop. Thus, pulsed pressure produces alternating “favorable” and “coalescent” structural regimes, leading to a hierarchical macroporous structure. This may be promising for applications requiring both a high surface area and the presence of large macropores serving as main transport pathways, but is less attractive for mechanically robust scaffolds for cell culture.
2.3.3. Effect of Surfactant Addition
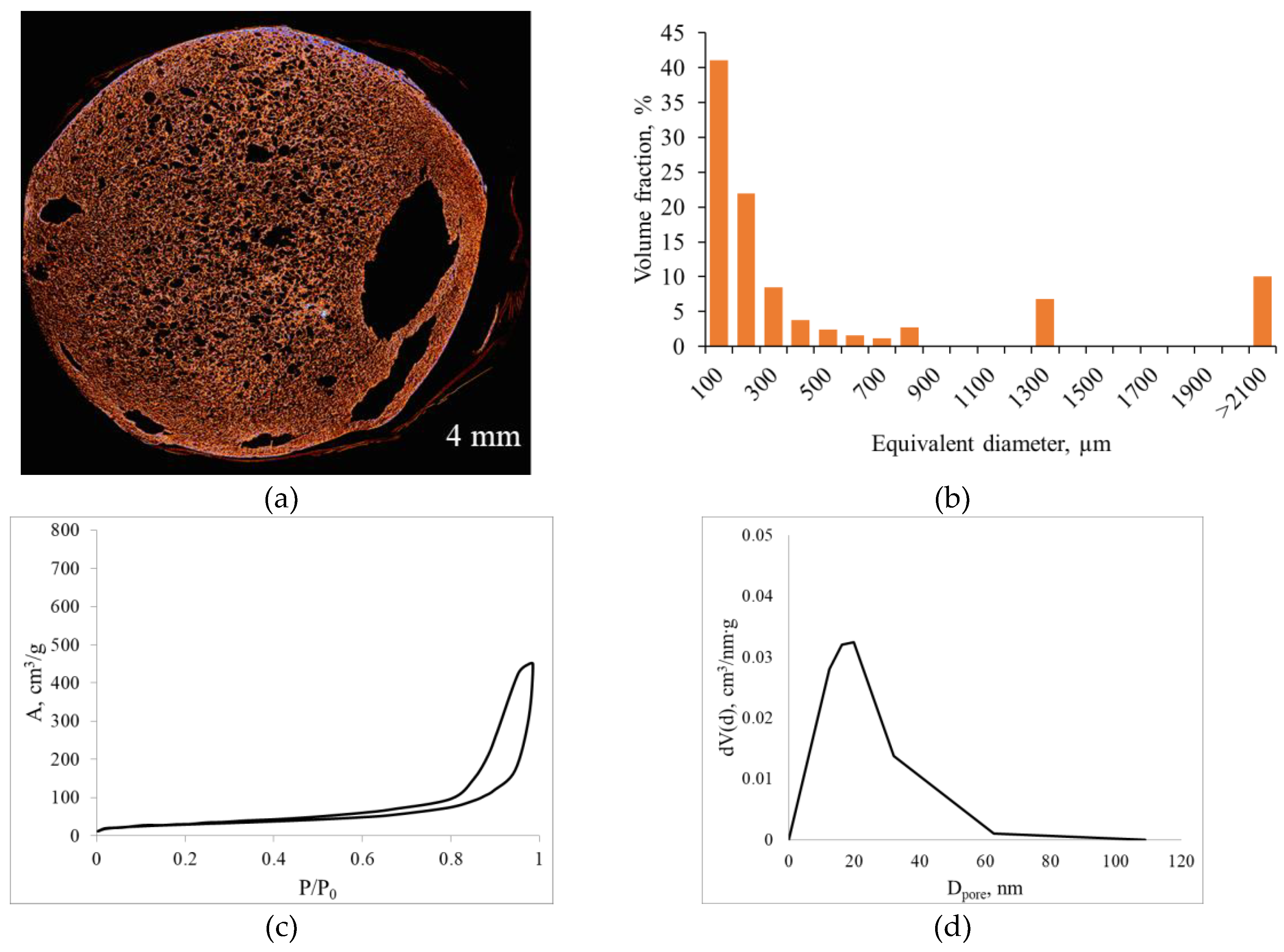
Figure 7 shows the micro-CT and nitrogen sorption results for alginate aerogels produced at a saturation pressure of 100 bar and a depressurization rate of 0.05 bar/s with added surfactant.
Micro-CT results demonstrate that surfactant addition still yields a macroporous structure throughout the volume, but extended large macropores appear at the periphery and in local regions. In the pore size histogram (
Figure 7b), most pores are in the 100–300 µm range, but there is a significant contribution from pores with equivalent diameters >2100 µm, indicating a combination of intense nucleation of small pores with localized coalescence. Nitrogen sorption data show that the adsorption isotherm remains type IV, typical of mesoporous materials, and the mesopore size distribution has a maximum in the ~20–30 nm range.
Surfactant addition reduces interfacial tension, decreasing the critical nucleus radius and facilitating the formation of a large number of small bubbles. At the same time, interface stabilization limits their coalescence, increasing the fraction of small macropores and preserving a well-developed mesoporous network. Under the same Pe and De (i.e., with unchanged depressurization rate and network relaxation time), this leads to a local transition into a “coalescent” regime and the formation of large macropores observed in the micro-CT images.
Thus, the considered modifications—lowering the process temperature, applying pulsed pressure variation, and adding surfactant—significantly affect macropore formation and, to a lesser extent, the mesoporous structure.