1. Introduction
Molten alkali carbonates—both pure and mixed—are regarded as highly promising fluids for high-temperature applications, including concentrated solar power (CSP), molten salt oxidation (MSO), and advanced nuclear reactor technologies. Aneke and Wang (2016) [
1] provided an excellent review of energy storage technologies and their real-life applications, comparing various storage approaches in terms of technical performance, deployment scale, number of operational projects, and technological maturity. Their analysis concluded that molten salts (MS) will continue to dominate the thermal energy storage sector for large-scale applications.
In a comprehensive two-part review, Frangini and Masi (2016) [
2,
3], the properties and applications of molten carbonates were examined in detail. Owing to their unique physical and physicochemical characteristics, molten carbonates were shown to be highly attractive for a wide range of energy technologies operating under diverse conditions. Key advantages highlighted in that analysis include high thermal and moisture stability, reusability, elevated electrical conductivity, and relatively low corrosiveness toward metals under controlled atmospheres. Their eutectic mixtures also exhibit reduced melting points, lowering the risk of freezing in pipelines and equipment.
Among these materials, the ternary eutectic mixture Li₂CO₃–Na₂CO₃–K₂CO₃ has been selected as an alternative high-temperature heat transfer fluid (HTF) and energy storage medium for a molten salt solar-thermal pilot plant, Prieto et al. (2020) [
4]. Its adoption enabled an increase in operational temperatures from the conventional limit of 565 °C (typical of alkali nitrates) up to 700 °C. For proper equipment design and reliable process modelling, accurate thermophysical property data are essential. However, a survey of the literature reveals significant scatter among published measurements, as shown in a previous review by our group, Nunes et al. (2019) [
5]. This situation has not improved substantially since then, despite the publication of some new datasets. Consequently, there remains a strong need for high-quality experimental measurements of the most relevant thermophysical properties for heat transfer and thermal storage—namely density, heat capacity, thermal conductivity, and viscosity—and for the establishment of recommended reference values for these properties.
Measuring thermophysical properties of molten carbonates presents several challenges. Some arise from their intrinsic behavior at high temperatures, while others stem from limitations of the experimental techniques themselves, including high measurement uncertainties and material incompatibilities between molten samples and instrument components, often metallic. These factors contribute to inconsistencies and reduce confidence in the available data.
In a recent study, Tasidou et al. (2019) [
6] proposed reference correlations for the viscosity of 13 inorganic molten salts, reporting expected uncertainties between 2% and 7%. However, none of the carbonate salts or their eutectic mixtures were included due to the poor quality of the available viscosity measurements. More recently, the same group [
7] proposed a viscosity correlation for the ternary carbonate eutectic, although it relied on Arrhenius modelling of experimental data that had not been reported by the original authors.
The aim of this paper is to provide a critical assessment of the existing thermophysical property data for the ternary eutectic molten carbonate system. Our approach follows a strategy inspired by methodologies pioneered under IUPAC for common liquids, such as hydrocarbons and water [
8,
9,
10]. Historical recommendations for the thermophysical properties of molten salts date back to the work of George Janz and co-workers in the 1960s and 1970s [
11]. The present work does not replace the need for new, accurate measurements of the heat capacity, viscosity, and thermal conductivity of the Li₂CO₃–Na₂CO₃–K₂CO₃ eutectic—a task currently underway in our laboratory—but aims to clarify the state of the literature and identify critical gaps.
2. Ternary Eutectic of Molten Carbonates
The ternary lithium carbonate, sodium carbonate, and potassium carbonate (Li
2CO
3-Na
2CO
3-K
2CO
3) is a mixture with the composition 43.5-31.5-25.0 mol% respectively (or 32.12-33.36-34.52 wt%) and with a melting point of
Tm = 397 °C (670 K), determined by DSC [
15]. Along the text, it will be denoted as (LiNaK)
2CO
3, or LiNaK (in equations).
3. Data Analysis
3.1. Density
Table 1 summarizes the available works for the molten ternary eutectic LiNaK)
2CO
3 density. Included are the reference data of Janz et al (1979) [
11].
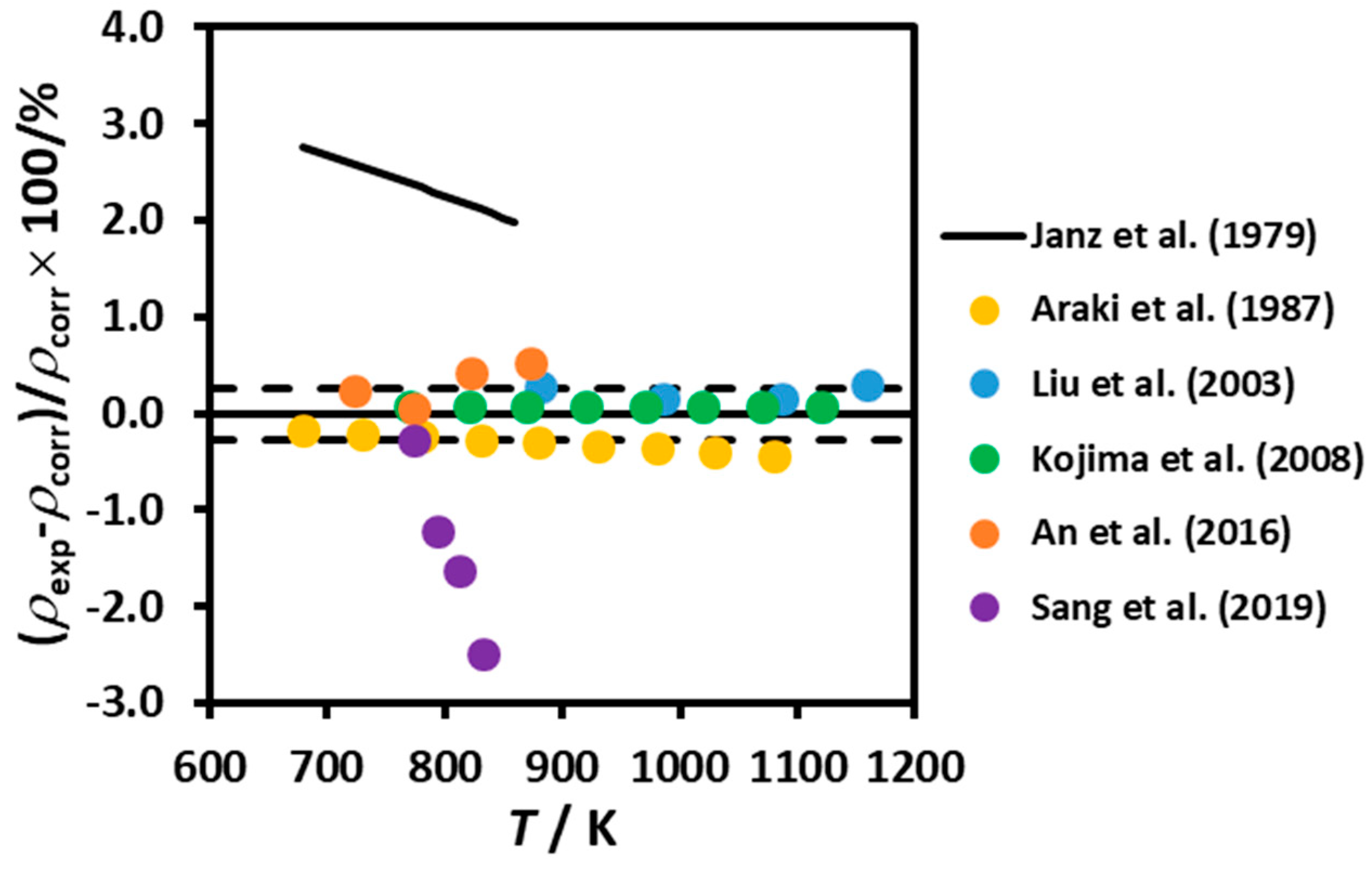
Figure 1 shows the plotted data. For comparison purposes, to show the effect of composition on the density of the melt, Sang et al. (2019) [
16] data for a ternary carbonate mixture with a different composition (4-4-2 mass ratio) have been included.
Based on the plotted data, we have chosen the data of Araki et al (1987) [
12], Liu et al. (2003) [
13], Kojima et al. (2008) [
14], and An et al. (2016) [
15], to establish a correlation for the density of the (LiNaK)
2CO
3 system. For data sets where only regression lines were available, a total of eight points were selected to cover the temperature range. The obtained equation is (1):
The correlation has a root mean square deviation of 5.29 kg·m
-3, can accommodate the data used within ± 0.3 %, and can be applied in the temperature interval 650 <
T/K < 1150.
Figure 2 shows the deviation of the different data sets relative to this correlation. It can be seen that the older recommendations of Janz et al. (1979) [
11] are significantly higher than this correlation.
In
Table 2, we propose recommended values for the density of molten ternary eutectic (LiNaK)
2CO
3, obtained from Eq. (1), with an uncertainty of 0.5%.
It is interesting to quote a result obtained by Kojima et al. (2008) [
14] that the molar volume of the eutectic can be calculated to within 0.1 cm
3·mol
-1 from the pure component molar volumes at the same temperature, using the molar additivity rule, at 1073 K. This means that the excess volume on the formation of the eutectic is almost negligible. However, this is not a real physical situation, as pure salts are solid at the temperatures of the eutectic, melting at temperatures above the eutectic (996 K for Li
2CO
3; 1124 K for Na
2CO
3 and 1164 K for K
2CO
3), and liquid metastable states of molten salts at temperatures 500 K below the melting point are not known to exist. To try to understand if this “pseudo” ideal solution was valid at the full liquid temperature range of the eutectic, for which there are available density data of the pure components, we have calculated the “pseudo” molar volume of the pure components, by extrapolating linearly the pure carbonates density data given by Kojima et al. (1999) [
17] to the molten eutectic temperature, and calculating the molar volume of the eutectic mixture using the additivity rule for the ideal solution (no differences in interionic interactions between the three cations and between these and the carbonate ions), as explained in Eq. (2) to (4).
where
xi (
i = Li
2CO
3, Na
2CO
3, and K
2CO
3) is the mole fraction of each salt in the eutectic mixture and
is the average molar molecular weight of the eutectic salt. The difference between the molar volume of the molten eutectic and that of an “pseudo” ideal solution of the individual molten salts at the same temperature is the excess volume of the mixture, defined in Eq. (5):
Using the recommended values from
Table 1 for the density of (LiNaK)
2CO
3 and the densities for the pure salts obtained from [
17], it can be shown that the excess volume is negligible at all temperatures, smaller than 0.001 cm
3·mol
-1. Therefore, Eqs. (4) and (5) can also be used to calculate the density of LiNaK, with
. This result also confirms the similarity of the interactions of alkali metal ions between them and with carbonate ions. However, care must be taken not to make any conclusions from this result, as, as said above, we are not dealing with an ideal solution in thermodynamic terms, where the components must be liquid at the same temperature than the mixture.
3.2. Heat Capacity
Like for density, the available data for the heat capacity of the ternary eutectic is scarce.
Table 3 resumes the available data.
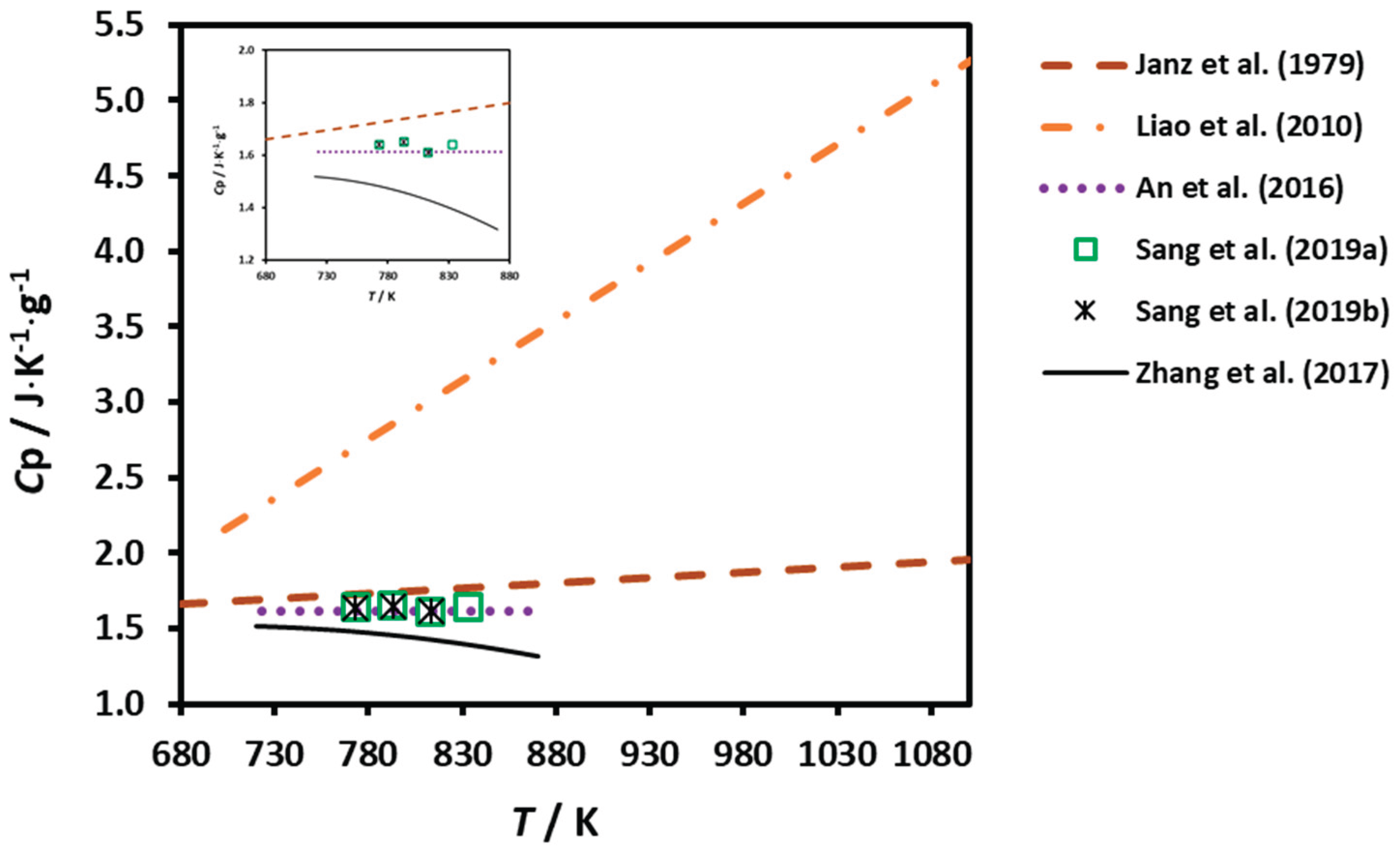
Figure 3 shows the plotted data. Included are data of Sang et al. (2017, 2019) [
16,
20] for a different composition (as stated before). The scarcity of data, especially in extended temperature intervals, does not allow the establishment of a correlation for the heat capacity, and further measurements are in progress in our laboratory. There are three sets of data in the temperature interval from 723 to 873 K, but unfortunately, with different temperature slopes. An et al. (2016) [
15] suggested a constant value of Cp = 1.61 J·g
-1·K
-1.
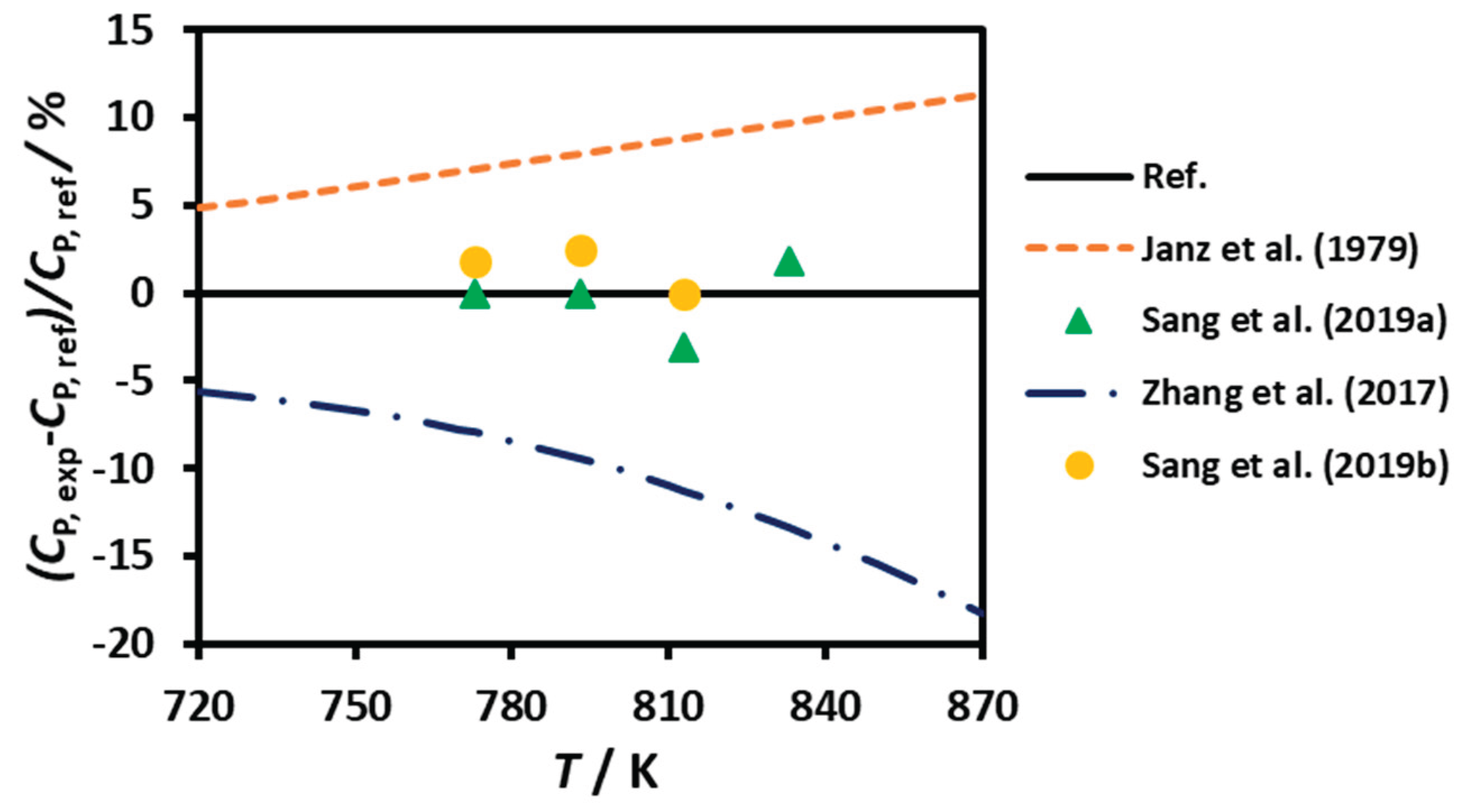
Figure 4 shows the deviations of the other data from this reference value.
The data of Liao et al. (2010) [
18] is far higher than all the other. Even for the other sets, the percent deviation can only be accommodated within ± 20% of the chosen reference point. It means that improved measurements of this property are a task that must be pursued.
3.3. Thermal Conductivity
Contrary to other properties, Janz et al. (1979) do not provide a reference correlation for the thermal conductivity of the (LiNaK)
2CO
3 system.
Table 4 lists the available data.
Figure 5 shows the thermal conductivity as a function of temperature for the different sets of data. As can be seen, the differences between different sets of data are too large, an obvious consequence of not so well-designed experimental apparatus or not accounting for additional methods of heat transfer present in the measurements, namely, radiation. There are 3 sets of data with similar trends, those of Araki et al. (1987) [
12], Sang et al. (2019) [
16], and Grosu et al. (2021) [
24], all obtained with the Laser Flash technique, but new data, preferably obtained with a direct method to measure the thermal conductivity, is necessary. This technique measures directly the thermal diffusivity, and if density,
ρ and specific heat capacity,
Cp are available, the thermal conductivity,
λ, can be obtained from the definition through Eq. (6):
In our opinion, it is premature to suggest any reference correlation for this property at this time, as further measurements are necessary. Measurements based on the transient hot-strip method [
25,
26], using a special designed platinum thin-film sensor, are under way in our laboratory and we hope to present data soon.
3.3. Viscosity
Viscosity was first measured using an oscillating cylinder viscometer by Janz and Saegusa (1963) [
27], and these measurements were the basis for the first reference correlation proposed by Janz et al. (1979) [
11]. However, these measurements were later revised, using an improved instrument, and found to have serious errors.
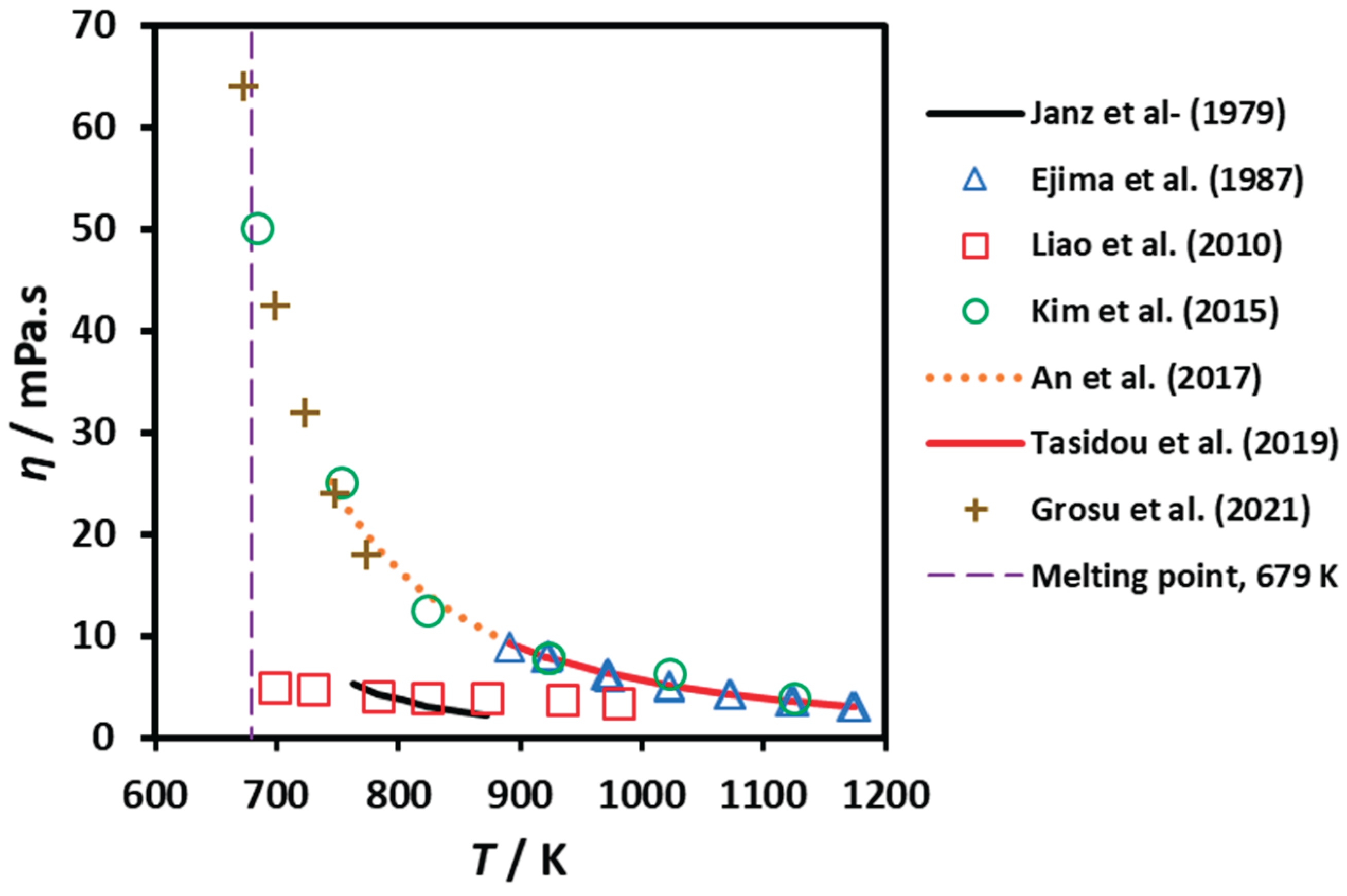
Table 5 summarizes the available data for the viscosity of the ternary melt, and
Figure 6 displays the variation of viscosity with temperature for all data sets.
It is clear from
Figure 6 that the data sets of Janz et al. (1979) [
11] and Liao et al. (2010) [
18] deviate very much from the other data, namely in the lower temperatures of the melt. Recently, as mentioned in the introduction, Tasidou et al. (2019) [
7] proposed a reference correlation for the viscosity of the ternary melt for 740 <
T/K< 1140. They considered the data of Ejima et al. [
28,
29] and the data of An et al. (2017) [
31] as primary data, although data points used were taken from the correlation. The proposed reference, Eq. (7), was:
An uncertainty of 3% was claimed. An et al. (2016) [
15] also published the viscosity of this melt early, and a slightly different equation was presented, not differing more than 0.5 % from the previous equation.
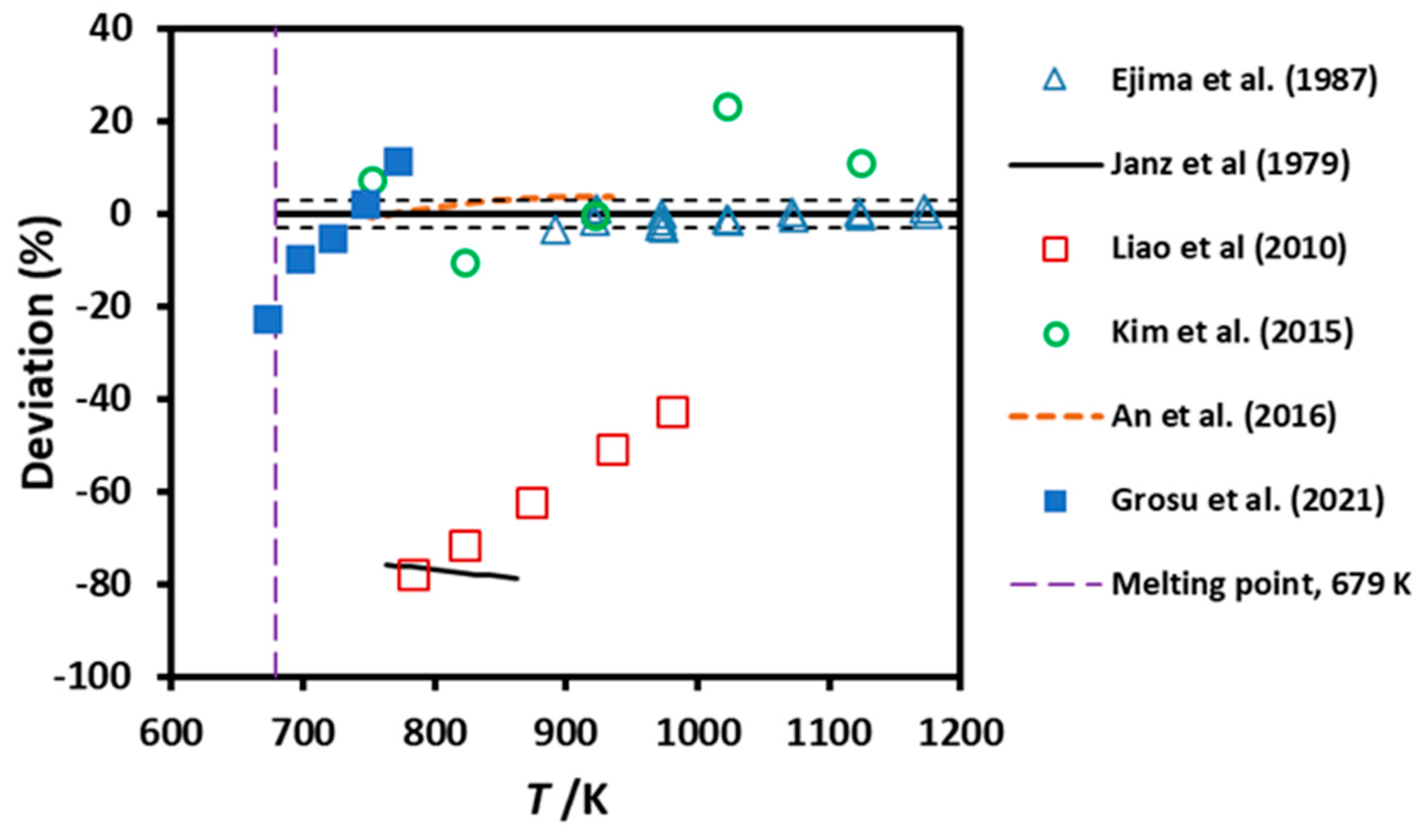
Figure 7 shows the percent deviation of the other data from the reference correlation proposed by Tasidou et al. (2019) [
7], in the temperature interval considered. It includes the other existing experimental points. Deviations of the data used in the correlation never amount to more than 3 %. However, the data of Janz et al. (1979) [
11] and Liao et al. (2010) [
18] deviate considerably from the proposed equation (- 80 to - 40%), probably due to deficient measurements. Data of Kim et al. (2015) [
30] must have an uncertainty greater than the claimed 5%, but it follows the temperature variation trend. The data of Grosu et al. (2021) [
24] also deviates very much, showing a completely different slope with temperature (and consequently a wrong activation energy for flow). However, it must be said that most of this data was obtained at temperatures below the correlation limit, recommending new experimental data with an absolute viscometer, currently undertaken in our laboratories, with an estimated expanded uncertainty of 1.2 %, with the oscillating cup viscometer.
The equation presented by Ejima et al. [
28] is in the form of the common Arrhenius equation, normally obeyed by fluids with Newtonian behavior. The equation obtained, Eq. (8), using a reagent and commercial-grade eutectic, was:
However, the equation of Tasidou et al. (2019) [
7] extends the applicability of Eq. (4) to much lower temperatures, although with the graphical data of An et al. (2017) [
30]. Since the method used by Ejima et al. (1987) [
28,
29] is an absolute method and experimental data are available, it can be considered as the reference for the viscosity in the interval of temperatures from 890 to 1175 K. New data on the viscosity of molten ternary eutectic (LiNaK)
2CO
3 is necessary, namely for temperatures between the melting point and 920 K, obtained with an absolute method. These data are currently being obtained in our laboratory and will be published soon.
4. Conclusions
This report provides a critical assessment of the available thermophysical property data for the ternary molten carbonate eutectic (LiNaK)₂CO₃, a material of growing relevance for high-temperature heat transfer and thermal energy storage in concentrated solar power, molten salt oxidation, and advanced nuclear reactor technologies. Despite its technological importance, the quality and consistency of published data have not improved substantially since our previous review (Nunes et al., 2019) [
5], although some new datasets have appeared.
Among the key properties evaluated,
density remains the only one for which a reliable reference correlation can be proposed. Because density is an equilibrium thermodynamic property, the literature includes sufficiently consistent measurements, making Eq. (1) suitable for estimating its temperature dependence. For the
specific heat capacity at constant pressure, a constant value of Cp = 1.61 J·g⁻¹·K⁻¹ can be suggested, although this does not differ significantly from the classical recommendation of Janz et al. (1979) [
11]. In contrast, no meaningful reference data can be established for the
thermal conductivity, as most measurements are derived from thermal diffusivity experiments that yield contradictory temperature trends (d
λ/dT either positive or negative), as observed previously for conductive liquids [
5]. Regarding
viscosity, the correlation recently proposed by Tasidou et al. (2019) [
6] is applicable only within the range 890–1175 K, corresponding to the absolute measurements of Ejima et al. (1987) [
27,
28], obtained using the oscillating-cylinder method. Older recommendations by Janz et al. (1979) [
11] are significantly lower and inconsistent with these more reliable data.
Overall, there is a clear need for new high-quality measurements of heat capacity, thermal conductivity, and viscosity for the molten ternary eutectic (LiNaK)2CO3, ideally extending up to at least 1100 K (827 °C), to enable the development of robust standard reference correlations essential for accurate process design in CSP and MSO applications.
Nevertheless, following the insights of Starke et al. (2024) [
32], it appears increasingly likely that operating CSP plants above 650 °C may not yield net economic benefits. Although higher temperatures improve the thermal efficiency of Rankine cycles, this gain is offset by the substantially higher cost of the required high-temperature materials - such as nickel-based superalloys (Inconel 625 or Haynes 230) used in receivers, piping, and storage tanks. This economic constraint may ultimately limit the operating temperature range in which advanced molten carbonate systems can be deployed.
Author Contributions
Conceptualization, V.N and C.A.N.C.; Methodology, V.N. and M.J.V.L.; Software, P.R.; Validation, J.F.C., M.J.V.L and P.R.; Formal analysis, J.F.C., V.N and C.A.N.C.; Investigation, J.F.C., M.J.V.L. and V.N.; Writing original draft, V.N. and C.A.N.C.; Visualization, C.A.N.C.; Supervision, M.J.V.L. and C.A.N.C. All authors have reviewed and agreed to the published version of the manuscript.
Funding
This work was partially funded by Fundação para a Ciência e Tecnologia, Portugal, through Projects UID/QUI/00100/2019 and UIDB/00100/2020 (DOI: 10.54499/UIDP/00100/2020) of Centro de Química Estrutural, Project LA/P/0056/2020 of Institute of Molecular Sciences, and Project NEWS4CSP, 2022.05021.PTDC (DOI: 10.54499/2022.05021.PTDC).
Data Availability Statement
The data are contained within the article.
Acknowledgments
During the preparation of this manuscript, the authors used the open-access ChatGPT - Free version for superficial text editing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CSP |
Concentrated solar power |
| MSO |
Molten salts oxidation |
| A |
Archimedean |
| DBA |
Double-bob Archimedean |
| MBP |
Maximum bubble pressure |
| DC |
Drop calorimetry |
| DSC |
Differential scanning calorimetry |
| STA |
Simultaneous thermal analysis |
| LF (Calc) |
Laser flash, calculated from thermal diffusivity, heat capacity, and density |
| FRS (Calc) |
Forced Rayleigh scattering, calculated from thermal diffusivity, heat capacity, and density |
| THW |
Transient hot wire |
| SSCC |
Steady state coaxial cylinders |
| OCY |
Oscillating cylinder |
| RC |
Rotating cylinder |
| RPP |
Rheometer, parallel plate |
| NA |
Not available |
References
- Aneke, M.; Wang, M. Energy storage technologies and real-life applications – a state of the art review. Appl Energy 2016, 179, 350-77. [CrossRef]
- Frangini, S.; Masi, A. Molten carbonates for advanced and sustainable energy applications: Part I. Revisiting molten carbonate properties from a sustainable viewpoint. Int J. Hydrogen Energy 2016, 41, 18739-18746. [CrossRef]
- Frangini, S.; Masi, A. Molten carbonates for advanced and sustainable energy applications: Part II. Review of recent literature. Int. J. Hydrogen Energy 2016, 41, 18971-18994. [CrossRef]
- Prieto, C.; Fereres, S.; Ruiz-Cabañas, F.J.; Rodriguez, A.; Montero, C. Carbonate molten salt solar thermal pilot facility: Plant design, commissioning and operation up to 700ºC. Renewable Energy 2020, 151, 528-541. [CrossRef]
- Nunes, V.M.B.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A. Molten alkali carbonates as alternative engineering fluids for high temperature applications. Applied Energy 2019, 242, 1626-1633. [CrossRef]
- Tasidou, K.A.; Chliatzou, Ch.D.; Assael, M.J.; Antoniadis, K.D.; Mylona, S.K.; Huber, M.L.; Wakeham, W.A. Reference correlations for the viscosity of 13 inorganic molten salts. J. Phys. Chem. Ref. Data 2019, 48, 013101. [CrossRef]
- Tasidou, K.A.; Magnusson, J.M.; Munro, T.; Assael, M.J. Reference correlations for the viscosity of LiF-NaF-KF, LiF-BeF2, and Li2CO3-Na2CO3-K2CO3. J. Phys. Chem. Ref. Data 2019, 48, 043102.
- Ramires, M.L.V.; Nieto de Castro, C.A.; Nagasaka, Y.; Nagashima, A.; Assael M.J.; Wakeham, W.A. Standard reference data for the thermal conductivity of water. J. Phys. Chem. Ref. Data 1995, 24, 1377-1381. [CrossRef]
- Nieto de Castro, C.A.; Li, S.F.Y.; Nagashima, A.; Trengove, R.D.; Wakeham, W.A. Standard Reference Data for the Thermal Conductivity of Liquids. J. Phys. Chem. Ref. Data 1986, 15, 1073-1086. [CrossRef]
- Santos, F.J.V.; Nieto de Castro, C.A.; Dymond, J.H.; Dalaouti, N.K.; Assael, M.J.; Nagashima, A. Standard Reference Data for the Viscosity of Toluene. J. Phys. Chem. Ref. Data 2006, 35, 1-8. [CrossRef]
- Janz, G.J.; Allen, C.B.; Bansal, N.P.; Murphy, R.M.; Tomkins, R.P.T. Physical Properties Data Compilations. II. Molten Salts: Data on Single and Multicomponent Salt Systems, NSRDS-NBS 61, Part II, 1979.
- Araki, N.; Matsuura, M. Measurements of Thermophysical Properties of Molten Salts (Mixtures of Alkaline Carbonate Salts). Proceedings of the Eight Japan Symposium on Thermophysical Properties 1987, 1-4. [CrossRef]
- Liu, Q.; Lange, R.A. New density measurements on carbonate liquids and the partial molar volume of the CaCO3 component. Contrib. Mineral. Petrol. 2003, 146, 370-381. [CrossRef]
- Kojima, T.; Miyazaki, Y.; Nomura, K.; Tanimoto, K. Density, Surface Tension, and Electrical Conductivity of Ternary Molten Carbonate System Li2CO3–Na2CO3–K2CO3 and Methods for Their Estimation. J. Electrochem. Soc. 2008, 155(7), F150-F156.
- An, X.; Cheng, J.; Zhang, P.; Tang, Z.; Wang, J. Determination and evaluation of the thermophysical properties of an alkali carbonate eutectic molten salt. Farad. Disc. 2016, 190: 327-338. [CrossRef]
- Sang, L.; Ai, W.; Wu, Y.; Ma, C. Enhanced specific heat and thermal conductivity of ternary carbonate nanofluids with carbon nanotubes for solar power applications. Int. J. Energy Res. 2019, 1-10. [CrossRef]
- Kojima, T.; Yanagida, M.; Tanimoto, K.; Tamiya, Y.; Matsumoto, H.; Miyazaki, Y. The surface tension and the density of molten binary alkali carbonate systems. Electrochemistry (Tokyo, Jpn.) 1999, 67, 593-602. [CrossRef]
- Liao, M.; Wei, X.L.; Ding, J.; Hu, B.H.; Peng, Q. Preparation and experimental investigation for LNK carbonate molten salts. Acta Energ. Solar Sin. 2010, 31, 863-867.
- Zhang, Z.; Yuan, Y.; Zhang, N.; Sun, Q.; Cao, X.; Sun, L. Thermal properties enforcement of carbonate ternary via lithium fluoride: A heat transfer fluid for concentrating solar power systems. Renew. Energy 2017, 111, 523-531. [CrossRef]
- Sang, L.; Ai, W.; Liu, T.; Wu, Y.; Ma, C. Insights into the specific heat capacity enhancement of ternary carbonate nanofluids with SiO2 nanoparticles: the effect of change in the composition ratio. RSC Adv. 2019, 9, 5288-5294. [CrossRef]
- Otsubo, S.; Nagasaka, Y.; Nagashima, A. Experimental study on the forced Rayleigh scattering method using CO2 laser (3rd report, measurements of molten simple carbonates and their binary and ternary mixtures). Trans. Japan Soc. Mech. Engrs. Part B 1998, 64, 806-813. [CrossRef]
- Zhang, X.; Wicaksono, H.; Fujiwara, S.; Fujii, M. Accurate measurements of thermal conductivity and thermal diffusivity of molten carbonates. High Temp.-High Press. 2002, 34, 617-625. [CrossRef]
- Dokutovich, V.N.; Khokhlov, V.A.; Zakir’yanova, I.D. Thermal conductivity of composite materials: Alkali carbonate-based melts filled with fine α-Al2O3. Int. J. Heat Mass Transf. 2018, 119, 365-371. [CrossRef]
- Grosu, Y.; Anagnostopoulos, A.; Balakin, B.; Krupanek, J.; Navarro, M.E.; González-Fernández, L.; Ding, Y.; Faik, A. Nanofluids based on molten carbonate salts for high-temperature thermal energy storage: Thermophysical properties, stability, compatibility and life cycle analysis, Sol. Energy Mater. Sol. Cells. 2021, 220, 110838. [CrossRef]
- Nieto de Castro, C.A.; Lourenço, M.J.V. Towards the Correct Measurement of Thermal Conductivity of Ionic Melts and Nanofluids, Energies 2020, 13(1), 99.
- Lourenço, M.J.V.; Alves, M.; Serra, J.M.; Nieto de Castro, C.A.; Buschmann, M.H. The Thermal Conductivity of Near-Eutectic Galinstan (Ga68.4In21.5Sn10) Molten Alloy, Int. J. Thermophys. 2024, 45, 6. [CrossRef]
- Janz, G.J.; Saegusa, F. Molten carbonates as electrolytes: viscosity and transport properties. J. Electrochem. Soc. 1963, 110, 452-456. [CrossRef]
- Ejima, T.; Sato, Y.; Yamamura, T; Tamal, K.; Hasebe, M.; Bohn, M.S.; Janz, G.J. Viscosity of the Eutectic Li2CO3-Na2CO3-K2CO3 Melt. J. Chem. Eng. Data 1987, 32, 180-182.
- Ejima, T.; Sato, Y.; Yamamura, T.; Tamal, K.; Hasebe, M.; Bohn, M.S.; Janz, G.J. Viscosity Measurements: Molten Ternary Carbonate Eutectic. Proceedings of The Electrochemical Society, 1987, PV1987-7, 317-323. [CrossRef]
- Kim S.W.; Uematsu, K.; Toda, K.; Sato, M. Viscosity analysis of alkali metal carbonate molten salts at high temperature. J. Ceram. Soc. Jpn. 2015, 123, 355-358. [CrossRef]
- An, X; Cheng, J.; Su, T.; Zhang, P. Determination of thermal physical properties of alkali fluoride/carbonate eutectic molten salt AIP Conf. Proc. 2017, 1850, 070001.
- Starke, A.R.; Cardemil, J.M.; Bonini, V.R.B.: Escobar, R.; Castro-Quijada, M.; Videla, A. Assessing the performance of novel molten salt mixtures on CSP applications. Applied Energy 2024, 359, 122689. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).