1. Introduction
The DU is an important digestive tract component, functionally closely connected with the stomach and the rest of the intestine. The most important link between the stomach and intestine is DRBP [
1]. This is confirmed by the data of a number of authors who described the important EC role in the DU submucosa in the IBS development, which quite fully explains the clinical gastrointestinal tract (GIT) expression [
2].
Current research based on circumstantial evidence suggests that the duodenal contents, including its microbiome, pathogens, neurohormones and allergies, act as triggering factors in IBS with FD [
3,
4]. The EC disfunction seems to explain motility changes, visceral hypersensitivity, and abnormal intestinal secretion in IBS with FD patients. The literature provides strong evidence that the underlying cause of both epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS) in FD is impaired gastroduodenal neurophysiology [
3,
4]. At the same time, EC and stem cells and their precursors demonstrate a high degree of variability in the small intestine proximal and distal parts in IBS patients [
2,
5]. Particular attention should be paid to the data on the density variability of stem cells and EC in IBS patients` DU in contrast to the group of healthy individuals [
6].
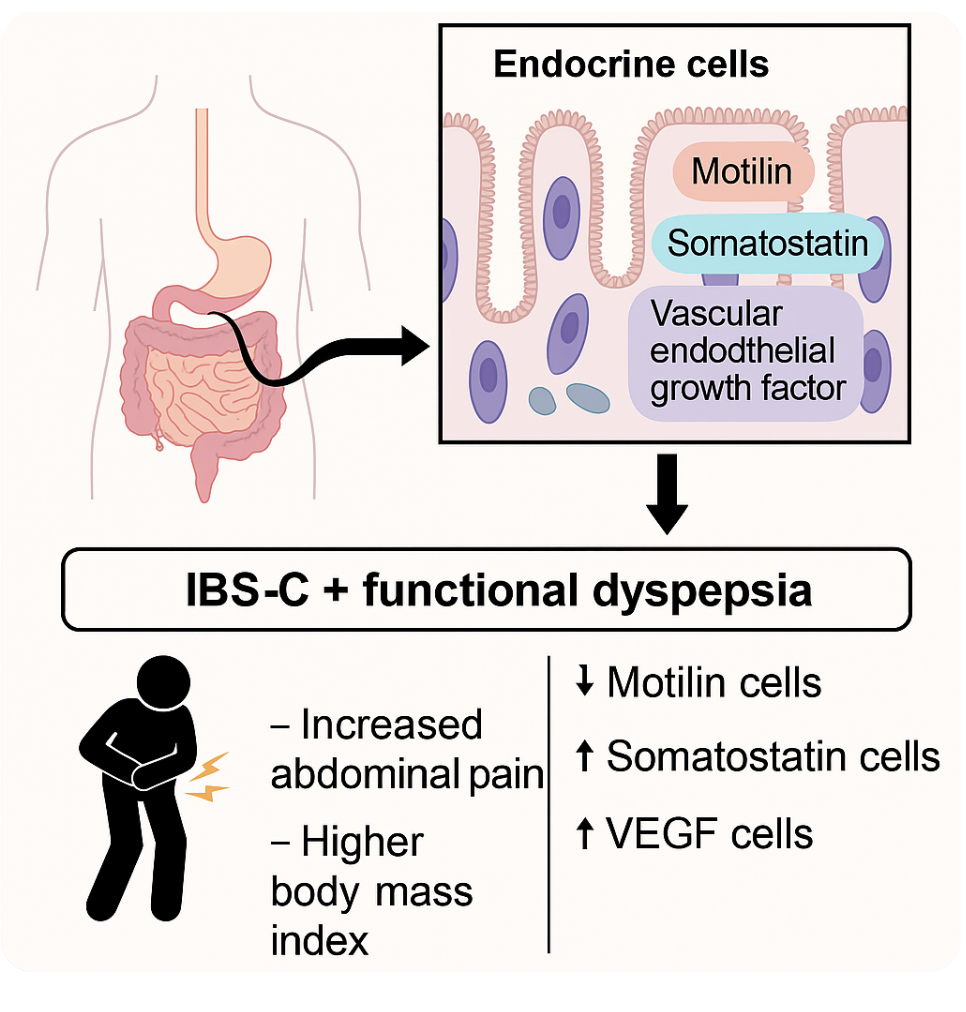
Among EC, cellular structures secreting MLN, SS and VEGF are of great importance [
Figure 1]. Thus, MLN is a GIT hormone that is produced during fasting to stimulate GIT motility using the migrating motor complex (MMC). Studies have shown that MLN is important for initiating MMC phase III contraction [
7]. SS is a key regulatory hormone in the gastrointestinal tract, synthesised and secreted by Delta- cells (DC) located in the stomach, small and large intestines, as well as in pancreas DC [
8]. SS suppresses other hormones and enzymes` secretion, thereby slowing down the digestive processes [
9].
VEGF is defined as a potent proangiogenic factor secreted by parenchymal, endothelial and activated immune cells [
10,
11]. This hormone belongs to the family of platelet-derived growth factors (PDGF). VEGF regulates various biochemical processes associated with cell growth, proliferation and survival, and maintains their structural integrity [
10]. VEGF plays an important role in angiogenesis, inflammatory response, and energy metabolism.
Aim of the study. To determine the role of the duodenal mucosa EC secreting MLN, SS and VEGF in the development of IBS-C and its combination with FD.
2. Material and Methods
The study included 35 IBS-C with FD patients and 35 ones without FD. The comparison group consisted of 30 practically healthy individuals. The age of patients and healthy individuals ranged from 18 to 45 years. Among the IBS patients, there were 42 women and 28 men. The patients included in the study met the Rome IV criteria for IBS and FD.
Both sick and healthy people were examined according to a single programme, including clinical and laboratory, instrumental and immunomorphological studies. To exclude organic intestinal pathology, all subjects underwent colonoscopy and a fecal calprotectin content analysis
The abdominal pain syndrome manifestation was determined on the Visual Analogue Scale (VAS) [
12]; the dynamics of the anxiety and/or depression manifestation – using the HADS (Hospital Anxiety and Depression Scale) [
13].
EGDS was performed before the start of therapy with subsequent DRBP mucous membrane targeted biopsy. H. pylori verification was based on histiobacillioscopy data with the study of imprints` smears of the antral part of the stomach mucous membrane, stained according to Romanovsky-Giemsa.
Tissue samples were fixed in 10% neutral buffered formalin (pH 7.2), then dehydrated in alcohols and embedded in paraffin according to the standard histological scheme. For histological examination, 4-6 μm thick sections were prepared, which were stained with hematoxylin and eosin.
For immunohistochemical DRBP mucosa staining serial 4-6 μm thick sections were prepared and placed on slides coated with poly-L-lysine. Studies were performed on deparaffinised and dehydrated sections using the avidin-biotin immunoperoxidase method.
To verify the expression of ECs secreting MT, SS and VEGF, the primary antibodies listed in
Table 1 were used.
To assess the immunohistochemical staining results, a morphometric study was performed using a computer-aided microscopic image analysis system consisting of an Olympus IX73 microscope, an Olympus DP80 digital camera, and CellSens software. In each case, at least 5 fields of view were analysed at a magnification of x40 with measurement of the studied marker relative expression area using ImageJ software.
3. Results
The distribution analysis of the IBS-C patients and those with FD did not reveal significant differences by gender, but in the overall morbidity structure a certain predominance of females was noted (
Table 2).
In the IBS-C patients` clinical picture the leading symptoms were pain along the colon, decreasing or disappearing after defecation, accompanied by flatulence, a feeling of incomplete bowel evacuation. In IBS-C with FD patients having classical IBS-C symptoms signs of heaviness and fullness after eating and pain in the epigastric region dominated. It should be emphasised that in IBS-C patients the FD subtype with PDS prevailed (
Table 3). Bloating in the upper abdomen was 2 times more common in IBS-C with FD patients than in individuals with IBS-C alone. In contrast to the above, bloating in the lower abdomen did not have statistically significant differences in frequency of occurrence in both groups of patients.
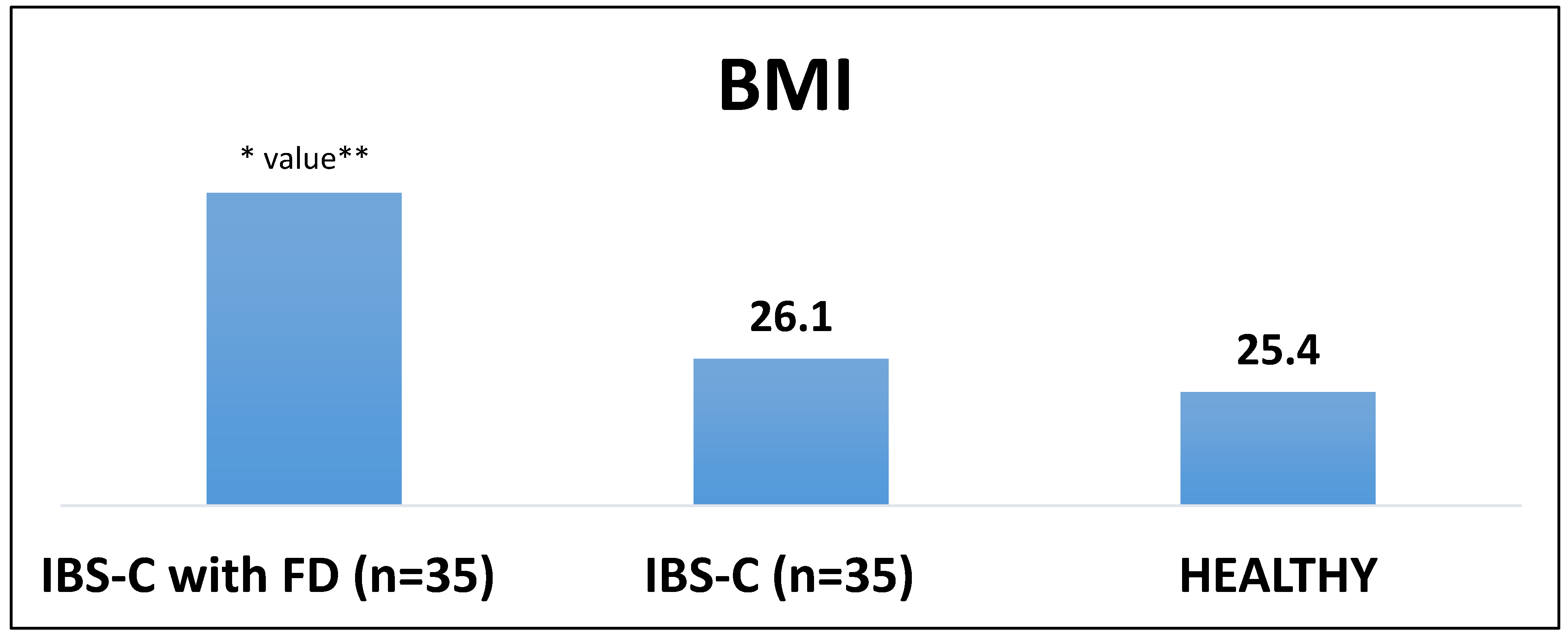
The studies conducted suggest a statistically significant BMI increase in IBS-C patients compared to the healthy group.It is more pronounced in IBS-C with FD patients (
Figure 2). The HADS data analysis testifies to a statistically significant dominance of IBS-C with FD data (
Table 4). It cannot be excluded that the anxiety and depression domination is largely due to the presence and severity of a painful abdominal syndrome affecting both the upper and lower floor of the digestive tract in IBS-C with FD patients (
Table 5).
Chronic gastritis associated with H. pylori was identified in 18 (51.4%) IBS-C with FD patients and in 16 (45.7%) IBS-C FD free ones, and its frequency had no statistically significant differences between both patients` groups.
Fecal calprotectin test shows that in IBS-C patients and in the ones with FD there is activation of the minimal statistically insignificant inflammatory process in the mucosal intestine ( in IBS-C with FD – 52.7; in IBS-C - 43.8 and in healthy patients- 39.9 μg/g calcium).
In DRBP, the morphological picture was presented by mucous membrane with preserved histoarchitectonics and signs of weakly expressed chronic inflammation.
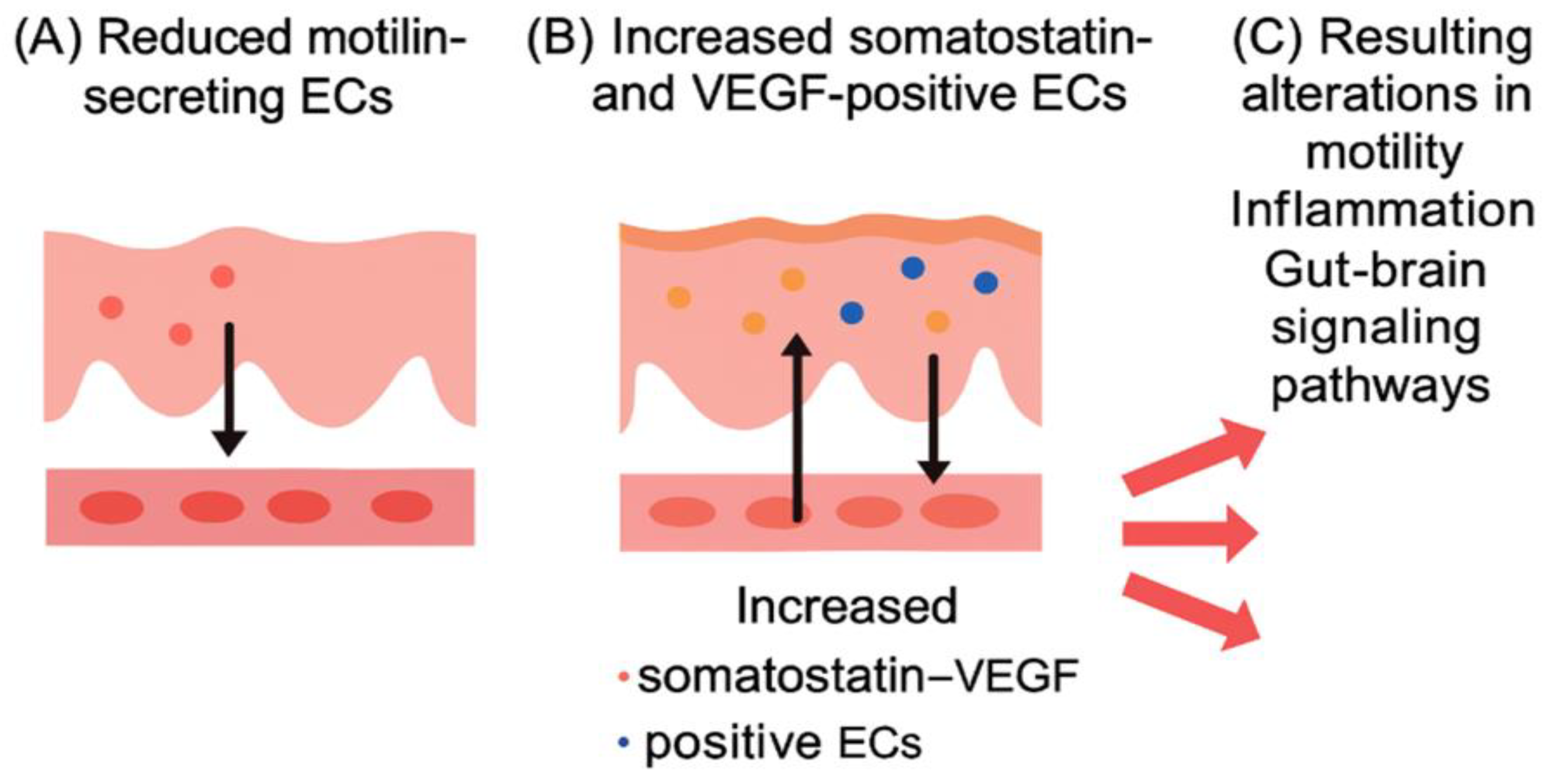
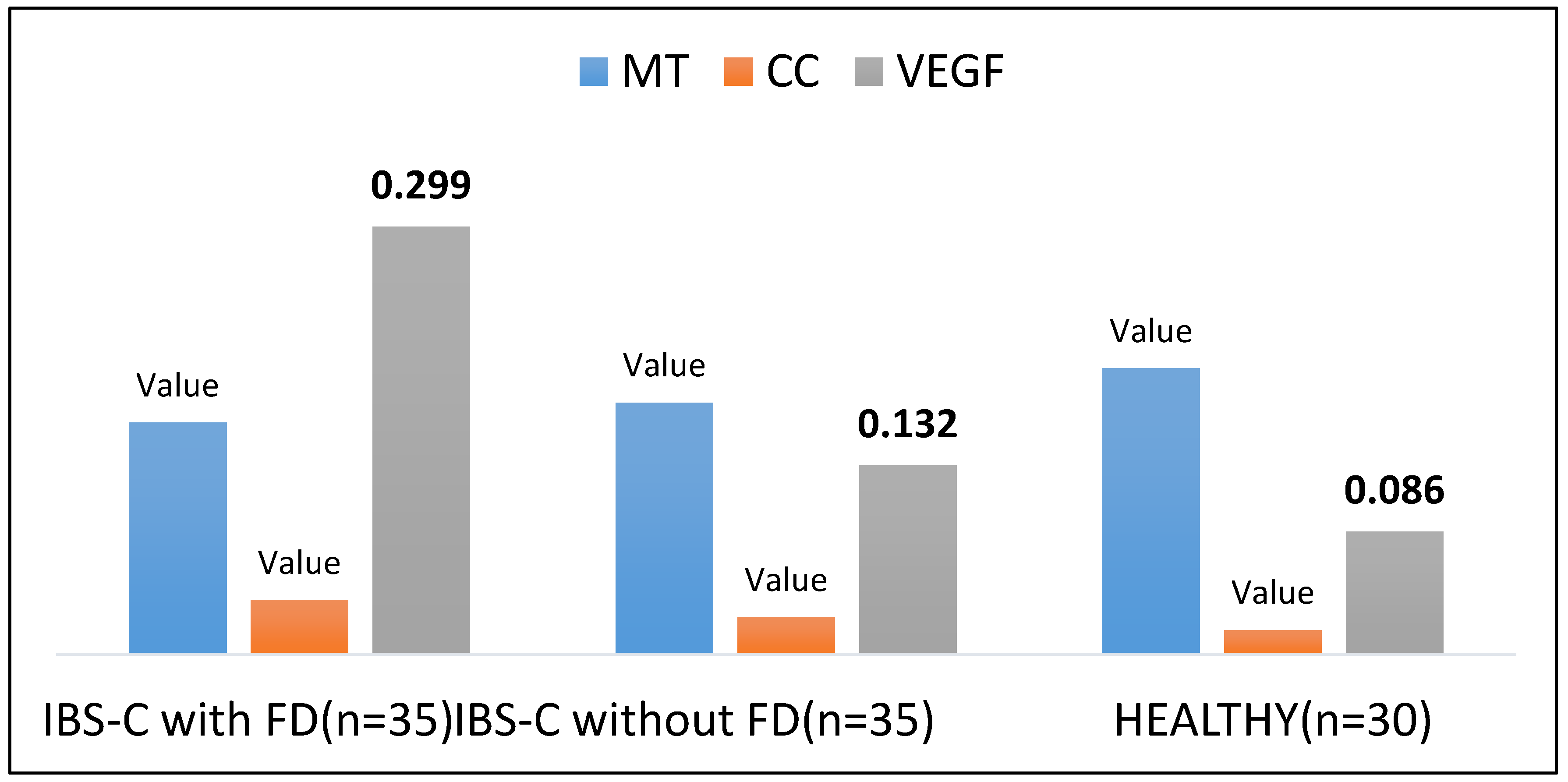
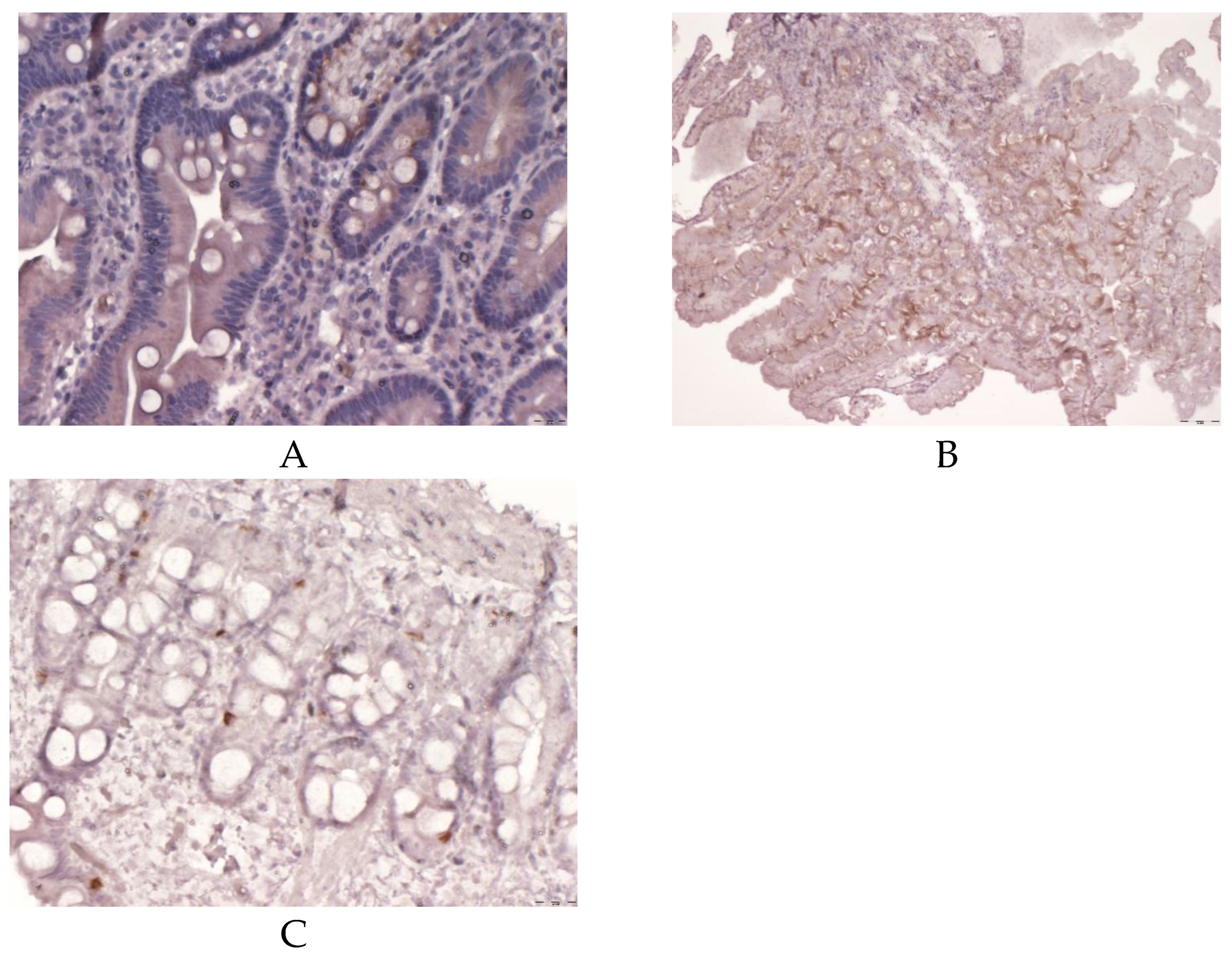
The obtained data evidence that the EC expression area in DRBP secreting VEGF, MLN and SS in IBS-C with FD patients was characterised by a significant decrease in the area of expression of EC secreting MLN and high expression of EC producing SS and VEGF. Less significant expression area figures were determined in individuals with IBS-C compared to IBS-C with FD patients (
Figure 3,
Figure 4 and
Figure 5 (A, B, C).
4. Discussion of the Results Obtained
The studies conducted point to the DU important role in the IBS and its combination with FD genesis. Apparently, there is a tight functional DRBP connection with the stomach and intestine, which may act as one of the major pathogenetic factors in the occurrence of not only IBS-C but also the formation of such combined pathology as IBS-C with FD.
The following data deserve attention : abdominal pain syndrome is more pronounced in IBS-C with FD patients than in the ones wihout FD. So, according to our data severe abdominal pain was found virtually 3 times more frequently in IBS with FD patients. Similar results are reported by other authors who state that more pronounced extraintestinal symptoms and psychological disturbances are associated with pain in IBS-C. [
14].
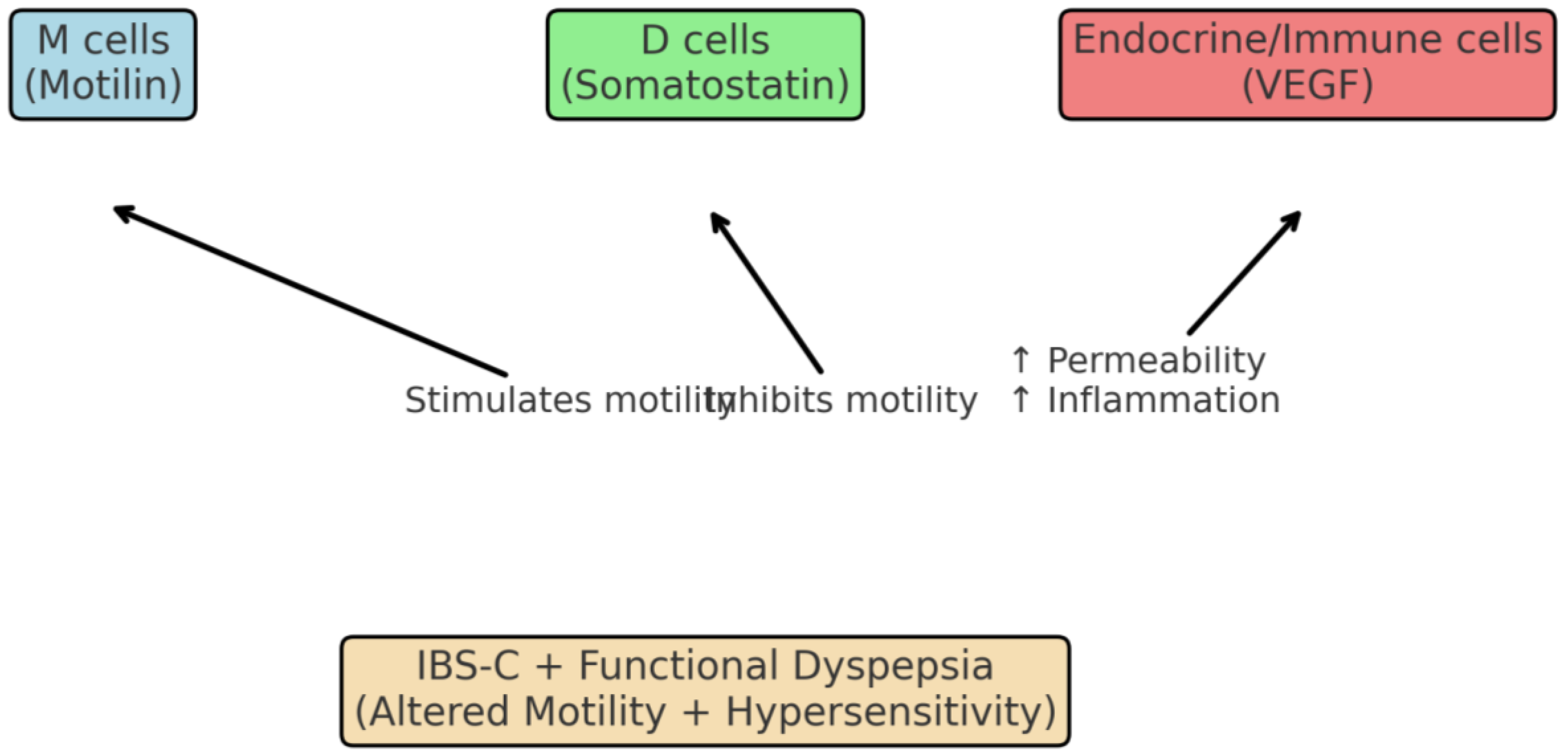
The duodenal mucosa contains specialized endocrine cells (ECs) that secrete motilin (MLN), somatostatin (SS), and vascular endothelial growth factor (VEGF), which are increasingly recognized as pivotal mediators in the pathogenesis of irritable bowel syndrome with constipation (IBS-C) and its overlap with functional dyspepsia (FD). Reduced expression of MLN-producing ECs may impair migrating motor complex activity, contributing to abnormal motility and constipation, while increased SS secretion exerts inhibitory effects on gastric emptying and intestinal peristalsis, exacerbating dyspeptic symptoms [
15,
16,
17,
18,
19]. Simultaneously, VEGF overexpression has been linked to enhanced angiogenesis and low-grade inflammation in the duodenum, processes that perpetuate visceral hypersensitivity and abdominal pain [
20,
21,
22,
23]. Recent studies suggest that the duodenal retrobulbar part (DRBP) functions as a critical neuroendocrine hub within the gut–brain axis, where EC dysfunction alters gastrointestinal homeostasis and promotes comorbid anxiety and depression [
24,
25]. These findings indicate that EC-derived mediators not only regulate gastrointestinal physiology but also represent potential biomarkers and therapeutic targets for IBS-C with FD.
5. Conclusions
The conducted studies imply that DU, namely its retrobulbar part, plays an important role not only in the genesis of IBS-C but also in its combination with FD. In IBS-C with FD, there is pronounced pain syndrome and abdominal bloating, both in the upper and lower digestive tract. At the same time, there is an impression that DRBP is an important part of the neuro-endocrine gut-brain axis functioning, where a significant role is played by MLN, SS and VEGF, providing their involvement in shaping the main clinical IBS-C manifestations and when it is combined with FD. There is a better understanding of the role of a number of clinical and biochemical parameters such as excess body mass and hypertriglyceridemia as potential risk factors in the IBS manifestation. In the current situation, purely functional GIT pathology begins to be considered in the minimal inflammation phase. This is a breakthrough in understanding that a functional disease does not necessarily require a complete absence of minor manifestations typical of organic pathology. This can further increase therapeutic possibilities aimed at eliminating the underlying cause.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI |
body mass index |
| DC |
Delta Cell |
| DRBP |
duodenum retrobulbar part |
| DU |
duodenum |
| EC |
endocrine cells |
| EGDS |
esophagogastroduodenoscopy |
| EPS |
epigastric pain syndrome |
| GIT |
gastrointestinal tract |
| FD |
functional dispepsia |
| HADS |
Hospital Anxiety and Depression Scale |
| H. pylory |
Helicobacter pylory |
| IBS |
irritable bowel syndrome |
| IBS -C |
irritable bowel syndrome with constipation |
| MLN |
motilin |
| MLN-R |
receptor |
| MMC |
gastric migrating motor complex |
| PDS |
postprandial distress syndrome |
| PDGF |
platelet-derived growth factor |
| SS |
somatostatin |
| VAS |
visual Analogue scale |
| VEGF |
vascular endothelial growth factor |
References
- Han, Y.; Jung, H.-K.; Chang, J.Y.; Moon, C.M.; Kim, S.-E.; Shim, K.-N.; Jung, S.-A.; Kim, J.-Y.; Bae, J.-Y.; Kim, S.-I.; et al. Identification of distinctive clinical significance in hospitalized patients with endoscopic duodenal mucosal lesions. Korean J. Intern. Med. 2017, 32, 827–835. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gilja, O.H.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 2383–91. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.M.; Talley, N.J. The Role of Duodenal Inflammation in Functional Dyspepsia. J. Clin. Gastroenterol. 2017, 51, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.; Colombo, J.M.; Deacy, A.; Schurman, J.V. An Update on the Assessment and Management of Pediatric Abdominal Pain. Pediatr. Heal. Med. Ther. 2021, ume 12, 373–393. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gilja, O.H. Abnormalities in ileal stem, neurogenin 3, and enteroendocrine cells in patients with irritable bowel syndrome. BMC Gastroenterol. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; El-Salhy, M.; Lied, G.A.; Hausken, T. The Effects of Fecal Microbiota Transplantation on the Symptoms and the Duodenal Neurogenin 3, Musashi 1, and Enteroendocrine Cells in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Posovszky, C.; Wabitsch, M. Regulation of Appetite, Satiation, and Body Weight by Enteroendocrine Cells. Part 2: Therapeutic Potential of Enteroendocrine Cells in the Treatment of Obesity. Horm. Res. Paediatr. 2015, 83, 11–18. [Google Scholar] [CrossRef]
- Shamsi, B.H.; Chatoo, M.; Xu, X.K.; Xu, X.; Chen, X.Q. Versatile Functions of Somatostatin and Somatostatin Receptors in the Gastrointestinal System. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- La Mendola, D.; Trincavelli, M.L.; Martini, C. Angiogenesis in Disease. Int. J. Mol. Sci. 2022, 23, 10962. [Google Scholar] [CrossRef]
- Chidlow, J.H.; Langston, W.; Greer, J.J.; Ostanin, D.; Abdelbaqi, M.; Houghton, J.; Senthilkumar, A.; Shukla, D.; Mazar, A.P.; Grisham, M.B.; et al. Differential Angiogenic Regulation of Experimental Colitis. Am. J. Pathol. 2006, 169, 2014–2030. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, J.; Wang, A.; Zhang, R.; Wang, L. Vascular Endothelial Growth Factor A (VEGFA) Regulates Hepatic Lipid and Glycogen Metabolism in Schizothorax prenanti. Int. J. Mol. Sci. 2023, 24, 15171. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, O.; Koyama, T.; Ahrentløv, N.; Jensen, L.; Malita, A.; Naseem, M.T.; Lassen, M.; Nagy, S.; Texada, M.J.; Halberg, K.V.; et al. The gut hormone Allatostatin C/Somatostatin regulates food intake and metabolic homeostasis under nutrient stress. Nat. Commun. 2022, 13, 1–17. [Google Scholar] [CrossRef]
- Folestad, E.; Mehlem, A.; Ning, F.C.; Oosterveld, T.; Palombo, I.; Singh, J.; Olauson, H.; Witasp, A.; Thorell, A.; Stenvinkel, P.; et al. Vascular endothelial growth factor B-mediated fatty acid flux in the adipose-kidney axis contributes to lipotoxicity in diabetic kidney disease. Kidney Int. 2024, 107, 492–507. [Google Scholar] [CrossRef]
- Shamsi, B.H.; Chatoo, M.; Xu, X.K.; Xu, X.; Chen, X.Q. Versatile Functions of Somatostatin and Somatostatin Receptors in the Gastrointestinal System. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Al-Missri, MZ; Jialal, I. Physiology, Motilin. In StatPearls; StatPearls Publishing, 2025; Available online: http://www.ncbi.nlm.nih.gov/books/NBK545309/ (accessed on 28 June 2025).
- Svistunov, A.A.; Osadchuk, M.A.; Mironova, E.D.; Guliaev, P.V.; Vasileva, I.N. The role of the main risk factors and endocrine cells of the antrum of the stomach producing motilin in the occurrence of cholelithiasis. Ter. arkhiv 2022, 94, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Maev, I.V.; Osadchuk, M.A.; Burdina, V.O.; Mironova, E.D. The Role of Endocrine Cells of the Colon, Secreting Vasoactive Intestinal Polypeptide, Somatostatin and Motilin, in Irritable Bowel Syndrome, Occurring with Diarrhea and Constipation. Ann. Russ. Acad. Med Sci. 2022, 77, 79–86. [Google Scholar] [CrossRef]
- Maev, I.; Krylova, Y.; Osadchuk, M.; Mironova, E.; Hudarova, A. Motilin, vascular endothelial growth factor, and somatostatin expression in the gastric mucosa of patients with NSAID-gastropathy. Dokazatel'naya Gastroenterol. 2022, 11. [Google Scholar] [CrossRef]
- La Mendola, D.; Trincavelli, M.L.; Martini, C. Angiogenesis in Disease. Int. J. Mol. Sci. 2022, 23, 10962. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, J.; Wang, A.; Zhang, R.; Wang, L. Vascular Endothelial Growth Factor A (VEGFA) Regulates Hepatic Lipid and Glycogen Metabolism in Schizothorax prenanti. Int. J. Mol. Sci. 2023, 24, 15171. [Google Scholar] [CrossRef]
- Maev, I.; Krylova, Y.; Osadchuk, M.; Mironova, E.; Hudarova, A. Motilin, vascular endothelial growth factor, and somatostatin expression in the gastric mucosa of patients with NSAID-gastropathy. Dokazatel'naya Gastroenterol. 2022, 11. [Google Scholar] [CrossRef]
- Suda, K.; Yamada, S.; Miyahara, K.; Fujiwara, N.; Kosaka, S.; Abe, K.; Seo, S.; Nakamura, S.; Lane, G.J.; Yamataka, A. High intestinal vascular permeability in a murine model for Hirschsprung’s disease: implications for postoperative Hirschsprung-associated enterocolitis. Pediatr. Surg. Int. 2022, 39, 1–8. [Google Scholar] [CrossRef]
- Kubrak, O.; Koyama, T.; Ahrentløv, N.; Jensen, L.; Malita, A.; Naseem, M.T.; Lassen, M.; Nagy, S.; Texada, M.J.; Halberg, K.V.; et al. The gut hormone Allatostatin C/Somatostatin regulates food intake and metabolic homeostasis under nutrient stress. Nat. Commun. 2022, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Folestad, E.; Mehlem, A.; Ning, F.C.; Oosterveld, T.; Palombo, I.; Singh, J.; Olauson, H.; Witasp, A.; Thorell, A.; Stenvinkel, P.; et al. Vascular endothelial growth factor B-mediated fatty acid flux in the adipose-kidney axis contributes to lipotoxicity in diabetic kidney disease. Kidney Int. 2024, 107, 492–507. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).