1. Hardware in Context
Respiratory and cardiovascular diseases are nowadays among the most prevalent ones [
1,
2], and their high morbidity and mortality are expected to increase further because of the worldwide current epidemics of obesity [
3,
4] and the rise in life expectancy [
5,
6]. Owing to either poor air oxygenation in the lungs or inadequate blood circulation and distribution, these diseases usually result in chronic hypoxia, a state of poor oxygenation of the cells in tissues and organs. Hypoxia severely affects normal cell function, resulting in negative consequences in multiple organs, such as myocardial ischemia, metabolic diseases, chronic heart and kidney diseases, reproductive diseases, and cancer [
7]. Hence, hypoxia plays a relevant pathophysiological role in human health and is a subject of intense investigation [
8].
Specifications table
| Hardware name |
Device for subjecting rodents to chronic hypoxia with minimal nitrogen supply |
| Subject area |
Experimental biology and biomedicine |
| Hardware type |
Device for biomedical animal research |
Closest commercial analog |
No commercial analog is available |
| Open source license |
GPL v3 |
| Cost of hardware |
The total cost of the material for the device building is ≈ 500 US$. |
| Source file repository |
https://data.mendeley.com/datasets/t7dk933sjm/1 |
Research on the mechanisms involved in the multiorgan consequences of normobaric hypoxia requires animal models based on chronically breathing air with O
2 concentration below the usual 21% of atmospheric air. The most common experimental setting for achieving hypoxia in animal models is to place them into a chamber where the O
2 concentration in the ambient air is reduced by injecting a flow of N
2 [
9,
10]. Indeed, regulating the flow of room air and of N
2 entering the chamber is the simplest way to control the concentration of O
2 the animals breathe. However, a relatively high flow of N
2 is required since, to prevent hypercapnia, the injected gas must sufficiently wash out the CO
2 exhaled by the animals.
In most cases, the source of N2 required for the most conventional setting to continously subject animals to hypoxia cannot be based on conventional bottles of compressed gas because of the high consumption required. A possible option would be an N2 generator (based on N2 extraction from room air by a pressure swing adsorption concentrator). However, these devices are expensive, limiting their use for this application. The most common alternative is a centralized N2 gas pipeline system installed in the building to provide gas to different points of use. However, this infrastructure, which is commonly available in hospitals and cell biology laboratories, is usually unavailable in most animal laboratory facilities. Such requirements for a continuous N2 source limit the widespread extension of hypoxia-related in vivo research.
To facilitate the research employing hypoxic animal models in facilities not having access to a continuous high-flow N2 source, we aimed to design, build, and test an open-source, low-cost device requiring minimal N2 provision.
2. Hardware Description
2.1. Principle of Functioning
Contrary to most conventional settings for producing hypoxic air by continuous N
2 injection, the device described here aims to minimize the supply of N
2 when subjecting mice to controlled chronic hypoxia. The setting is based on ensuring the balance of the gases involved in mice metabolism, thus providing the O
2 consumed and eliminating the exhaled CO
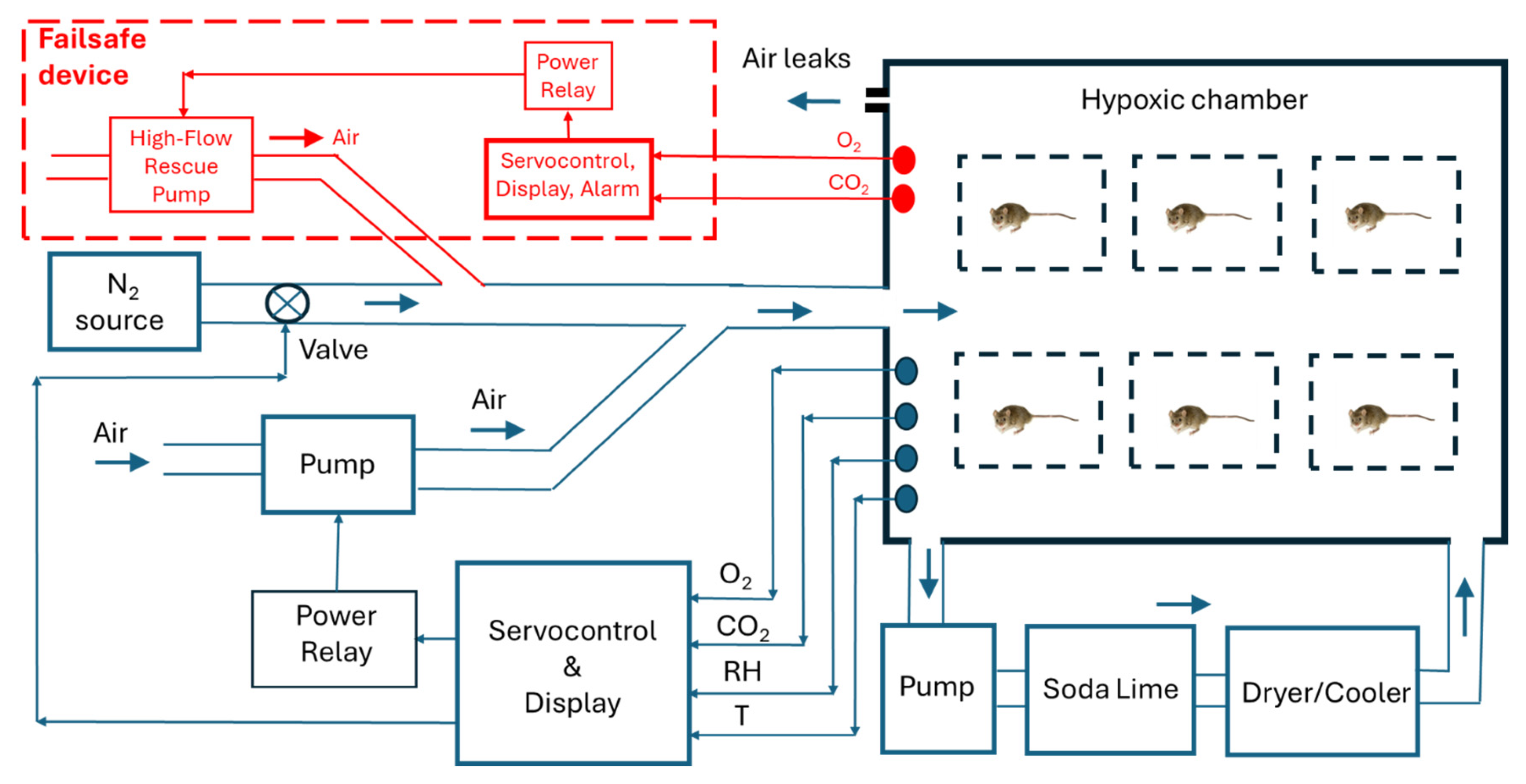
2 and water vapor. A schematic description of the setting is presented in
Figure 1: The O
2 and CO
2 concentrations, the relative humidity (RH), and temperature (T) inside the hypoxic chamber are continuously measured by sensors, and processed by the control and display unit, based on an Arduino microcontroller. A high-resistance air leak communicates the hypoxic chamber with the atmospheric air. The air is continuously recirculated within the chamber by the use of a domestic aquarium membrane-based pump (60 l/min). A soda lime filter is used to absorb the generated CO
2. Medical grade soda lime should be used since it contains ethyl violet, a pH-sensitive dye which changes from colorless to violet as the soda lime absorbs CO
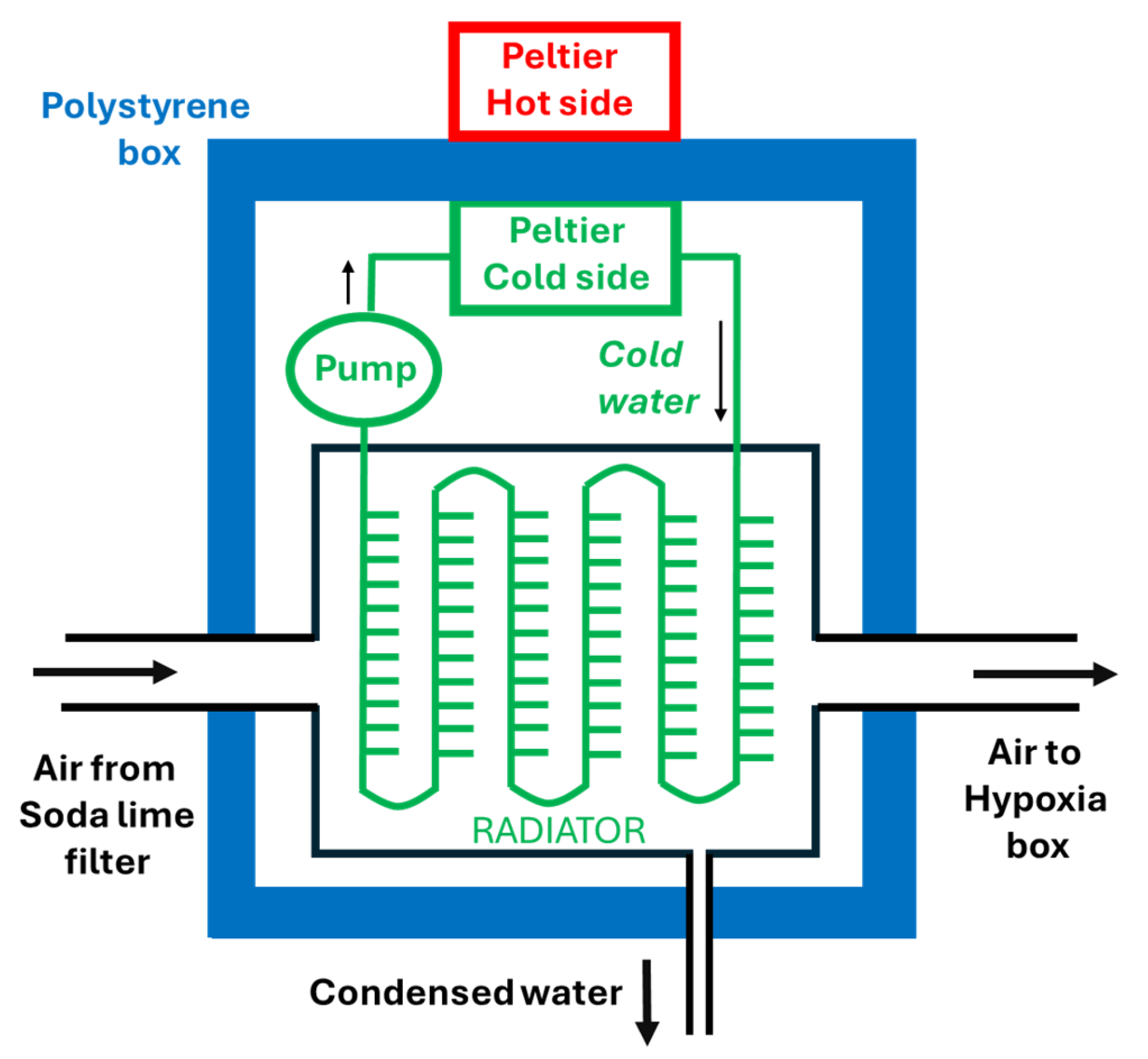
2). A dryer/cooler (based on an AC 12V 120W liquid-cooling thermoelectric Peltier-based unit providing cool water that circulates through an air radiator) is used to regulate the temperature and to condense water vapor (
Figure 1, bottom). For this purpose, the dryer/cooler section is enclosed into a thermally isolating box made with polystyrene walls including an outlet to extract the condensed water (
Figure 1, bottom).
A second domestic aquarium pump (30 l/min) is used to introduce room air into the hypoxic chamber to supply the O
2 consumed by the mice. Ideally, the setting would not require any additional N
2 injection to keep a target level of hypoxia since the consumption of O
2 and the production of CO
2 and water vapor are balanced by the components of the setting (
Figure 1). However, the control unit allows the injection of N
2 into the chamber from a gas bottle through an electrically controlled valve.
Figure 1 includes (in red) a completely independent (and optional) failsafe device to protect the animals against accidental life-threatening hypoxia or hypercapnia occurred in case any component (e.g., power, pumps, sensors, microprocessors) of the hypoxia device fails. The failsafe device has its own power source, O
2 and CO
2 sensors and Arduino control loop. The gas concentrations are continuously sensed and displayed, and in case concentrations of O
2 is below 8% or CO
2 is above 2000 ppm, both light and sound alarms are activated, and a high-flow (60 l/min) air pump (similar to the ones described for the hypoxic device) is activated to flush room air into the chamber. Using the failsafe device is not strictly required for the normal function of the hypoxic setting but it is strongly recommended. To avoid confusions, the technical description of the independent failsafe device is provided in a specific folder of Supplementary materials.
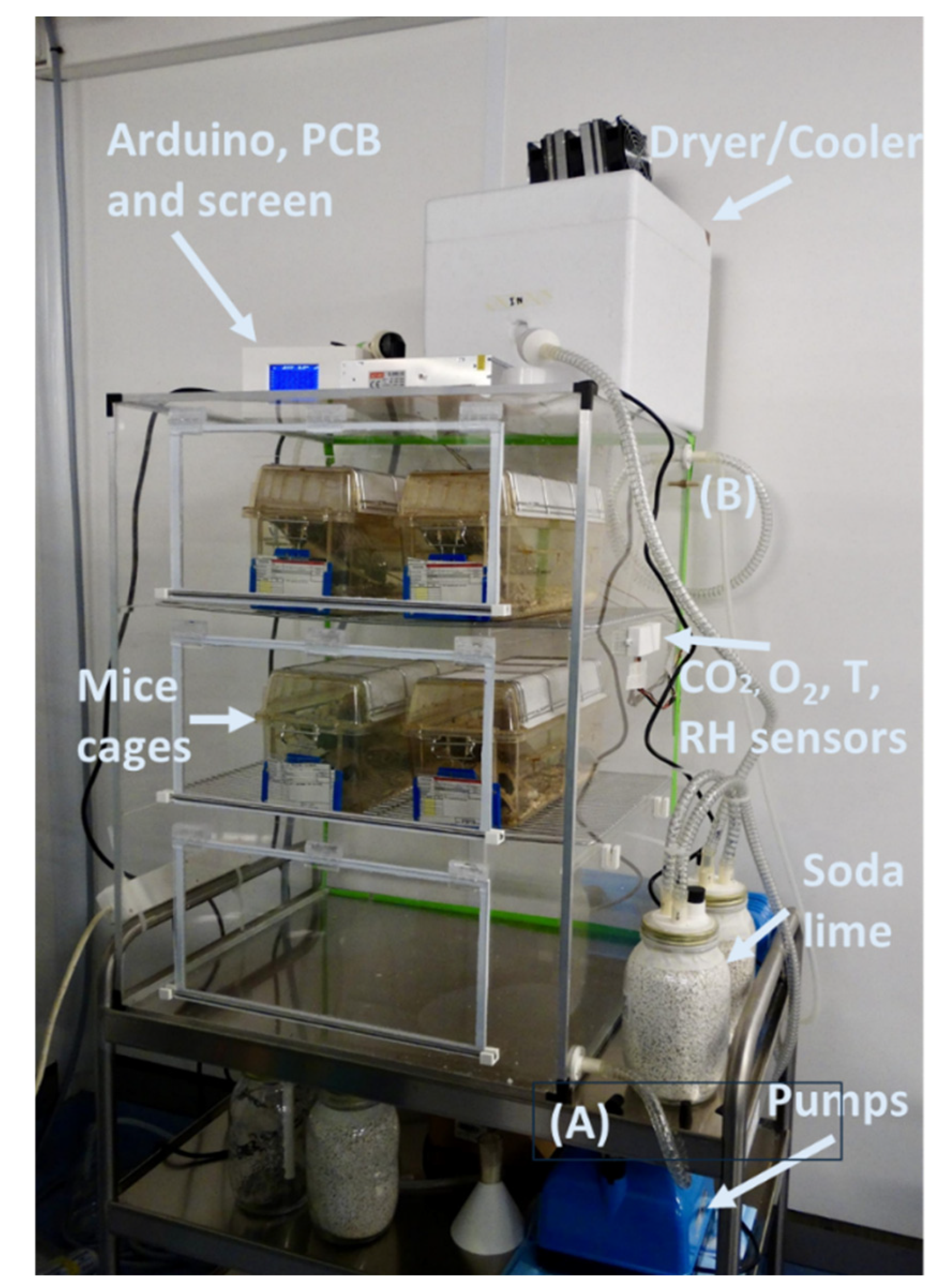
The specific device we implemented (
Figure 2) was designed to expose up to 30 adult mice to chronic normobaric hypoxia. The hypoxic chamber made with transparent methacrylate walls (4 mm width; dimensions 50 x 61 x 84 cm allowed to easily accommodate 6 conventional mice cages (17 x 20 x 39 cm) for 5 animals each. The design of the setting was based on the analysis of the physiological variables corresponding to the demanding conditions of 30 mice with the highest possible adult weight of 40 g each, as explained in the following subsections. The dimensions and specifications of the different components can be modified as required if changing the number of mice or the animal species (e.g., rats or guinea pigs).
2.2. Oxygen Balance
The typical rate of O
2 consumption in a mouse is 0.06 ml·(min·g)
-1) [
11]. Therefore, the total oxygen flow consumed (
V’O2) by the mice is ≈ 72 ml·min
-1. The value of oxygen fraction (
FiO2) in the hypoxic chamber that the user sets on the front panel of the control unit is achieved by mixing the flow of room air (
V’air) and the N
2 flow (
V’N2) entering from the N
2 source. The O
2 flow entering the chamber is the amount introduced by the air pump (21%·
V’air). The total flow of O
2 leaving the chamber is the addition of metabolic consumption (
V’O2) and the O
2 contained in the total gas leaving the chamber (
V’N2+
V’air) which is at
FiO2. Thus, 0.21·
V’air =
V’O2 +
FiO2 · (
V’air +
V’N2), and hence
V’air = (
V’O2 +
V’N2 ·
FiO2) / (0.21 –
FiO2). After an initial injection of N
2 to achieve the desired level of hypoxia, e.g., a target
FiO2 = 0.11, minimization of further N
2 consumption (
V’N2 ≈ 0) during steady-state hypoxia would require
V’air = 720 ml·min
-1 for
V’O2 = 72 ml·min
-1. This
FiO2 value is, in practice, ensured by using the O
2 sensor signal to control the power of the pump injecting the airflow
V’air into the chamber.
2.3. CO2 Balance
A critical condition to be achieved inside the hypoxic chamber is normocapnia since the setting would tend to induce hypercapnia by the potential accumulation of the CO
2 produced by mice metabolism. Assuming that the ratio between CO
2 production and O
2 consumption, commonly known as respiratory exchange rate (
RER), is equal to 1 in mice [
11], the total flow of CO
2 metabolically produced (
V’CO2 ) is ≈ 72 ml·min
-1. In case the CO
2 fraction in the chamber (
FiCO2) was simply the result of the washout induced by the total circulating air (
V’air +
V’N2), i.e., no CO
2 absorption by soda lime, the CO
2 entering the chamber by mice metabolism would be balanced by the CO
2 leaving the chamber. Under a steady state with minimal N
2 supply (
V’N2 ≈ 0,
V’air = 720 ml·min
-1), CO
2 washout would be negligible. Accordingly, the only effective way to reduce CO
2 accumulation in the chamber is by continuously pumping a flow (
V’sl) of the chamber air through a soda lime canister acting as a CO
2 absorber. Then, the CO
2 metabolically produced (
V’CO2 = 72 ml·min
-1) is balanced by the CO
2 absorbed by the soda lime (
V’sl ·
FiCO2) and the negligible amount of CO
2 leaving the chamber (
V’air ·
FiCO2). Hence, for a pump flow
V’sl= 60 l·min
-1, the increase in
FiCO2 would be of only ≈ 72 ml·min
-1/60000 ml·min
-1 = 12·10
-4 or 1200 ppm (0.12%) above the typical
FiCO2 of room air, hence a safe value [
12]. Since 1 kg of soda lime can absorb up to 260 l of CO
2 [
13], the soda lime required to drain the 72 ml·min
-1 of CO
2 production is 520 g·day
-1.
2.4. Water Vapor Balance
Typical ventilation in mice is 1.46 ml·g
-1 [
14], thus the total respiratory minute volume (
V’min) is ≈ 1752 ml·min
-1. Water vapor is released by mice metabolism, mainly through breathing, thus tending to increase the relative humidity (
RH) in the chamber air. Expiratory air (at 37 °C and water vapor saturated) and inspiratory air (at chamber temperature and
RHb) contain different amounts of water vapor. Taking into account that the partial pressure of water vapor (
PH2O,sat) at 37 °C is 47 mmHg, for
V’min = 1752 ml·min
-1, the water vapor content in the exhaled air is 1752 ml·min
-1 · (47 / 760) = 108.3 ml·min
-1. As
PH2O,sat(25 °C) = 23.8 mmHg, the water vapor in the inhaled air content is 1752 ml·min
-1·(23.8 / 760) ·
RHb = 54.9 ·
RHb ml·min
-1. Hence, the net water vapor produced by breathing is the difference content in expired and inspired air (108.3 − 54.9 ·
RHb) ml·min
-1. Thus, to keep
RHb at a reasonable value of 60%, a common value in lab animal facilities, the water vapor flow to be eliminated is 75.4 ml/min. Adding the water vapor released by mice transepithelial evaporation (which amounts to ≈ 50% of water vapor loss by breathing [
15], ≈ 113 ml/min of water vapor should be eliminated by the air dryer to avoid excessive humidification of the hypoxic chamber air. This amount of water vapor is eliminated by condensation in the cool dryer, specifically by cooling the airflow (
V’sl =60000 l/min) that is already circulated through soda lime to maintain normocapnia. Indeed, if a flow (
V’sl) of chamber air (
RHb = 60%, 25 °C, thus containing 1128 ml/min of water vapor) is circulated through a refrigerated element that cools the air to a temperature (
Tc), the maximum content of water vapor in the refrigerated air will be reduced and thus the exceeding amount will condensate on the inner walls of the cooler. The maximum flow of water vapor contained by saturated cooled airflow
V’sl at
Tc is 60000 ml/min ·
PH2O,sat(
Tc) / 760 = 79 ml/min ·
PH2O,sat(
Tc). Hence, the liquid water condensed by cooling
V’sl from 25 °C to
Tc is 1128 – 79 ·
PH2O,sat(
Tc). Accordingly, to eliminate the ≈ 113 ml/min of metabolically-produced water vapor, it is required that
PH2O,sat(
Tc) = 12.8 mmHg, corresponding to Tc ≈ 15 °C. Hence, a relatively mild ≈ 10 °C refrigeration from 25 °C would be enough.
2.5. Head Transfer Balance
The metabolic heat dissipation by 25-g mice at common ambient temperature (20-25 °C) is 0.5 Kcal/h [
11], and thus the heat dissipated by the 30 mice (40 g each) would be (
Q’met) is ≈ 30 W. The net heat balance in the hypoxic chamber is determined by the positive 30 W metabolically dissipated by the mice, the heat dissipated by the soda lime in the absorption process of CO
2 (13.7 kcal/mol
CO2) [
13], which in the setting amounts 2.8 W (for CO
2 density 1.8 g·l
-1 at 20 °C), and the negative heat transfer required for heating the previously cooled airflow
V’sl entering the chamber from 15 °C to 25 °C, which amounts –12.0 W (as computed for air density 1.2 g·l
-1 and specific heat capacity 1 J·g
-1·K
-1). Hence, the net heat balance is ≈ 21 W. Taking into account that the chamber (50 x 61 x 84 cm) has a surface (A) of 2.47 m
2, that the chamber walls are made of 4-mm width (
d) transparent polymethyl methacrylate (thermal conductivity:
K = 0.18 W·m
-1·K
-1), and that the basic equation for heat transfer is
Q’ =
K·
A·
ΔT ·
d-1, it results that passive dissipation of
Q’ = 21 W through the chamber walls would be achieved for a
ΔT ≤
0.2 °C. Hence, heat transfer balance is achieved for a hypoxic chamber temperature that minimally differs from room temperature.
3. Design Files Summary
All the design and software files necessary to build the device presented in this work are distributed under the GPL v3 license and they can be found in the supplementary materials of this manuscript at the following public repositories (DOI: 10.17632/t7dk933sjm.1)
https://data.mendeley.com/datasets/t7dk933sjm/1
Table 1.
Files summary.
| Design file name |
File type |
Open source license |
Location of the file |
| Enclosures and lids |
STL |
GPL v3 |
STL files folder |
| Code |
ino file |
GPL v3 |
Arduino Code folder |
| PCB Layout |
pdf and jpg file |
GPL v3 |
Electronics folder |
4. Bill of Materials Summary
The total cost of the materials for building the device is 633,77€. Materials such as resistors, LEDs, pin connectors, capacitors, PCB, ICs, and fuse holders were purchased as a kit, however, not all materials available in the set were used when building a single device. Most of them can be also easily reused from obsolete/damaged consumer electronic devices or household appliances.
| Component |
Quantity |
Cost per unity € |
Total Cost currency € |
Source of materials |
| Sensor O2 |
1 |
62,30 |
62,30 |
https://es.farnell.com/dfrobot/sen0322/i2c-oxygen-sensor-module-arduino/dp/3879708?gad_source=1&CMP=KNC-GES-GEN-SHOPPING-Pmax-Catch-all-05-Dec-23&gross_price=true |
| Sensor CO2, temperature and relative humidity |
1 |
61,14 |
61,14 |
https://es.farnell.com/seeed-studio/101020952/m-dulo-sensor-arduino-raspberry/dp/4007751?st=modulo%20sensor%20%20co2 |
Voltage regulator 5V and 9V
|
2 |
0,28 |
0,56 |
https://www.amazon.com/valores-Paquete-regulador-positivo-corriente/dp/B07T5ZHY63/ref=sr_1_1_sspa?__mk_es_US=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=1651XJCJIY90X&dib=eyJ2IjoiMSJ9.z7VZ01yDMzS7FNoFVZYjfIIDIqJf7MWuKacqC0FVcexkbaaq2K3koWWNRLSjCUnNocgOZhSxSL_K2NLBBZrMZcpmaEX61JZhaHNEK6GLg-pEYKA2nXRwSKnqndWUS3hKDuTBenbCbF9ouzxqIJhS3vUE_hhOFrJp8Tlc9ngNTITlqc6fLt1BAs_ijswPUxupFI1jSGK1lUobc-yQ_mRvKIzIJFYo4byvBh0iwoiQtwk.e5isTzn0mwmTiPh46biH3ZfvGoMPDzyBZDeiYAUNsKQ&dib_tag=se&keywords=voltage+regulator+7809&qid=1749637707&sprefix=voltage+regulator+780%2Caps%2C158&sr=8-1-spons&sp_csd=d2lkZ2V0TmFtZT1zcF9hdGY&psc=1 |
| Diode |
1 |
0,04 |
0,04 |
https://www.amazon.com/conmutaci%C3%B3n-miliamp-voltios-silicio-electr%C3%B3nicos/dp/B07Q4F3Y5W/ref=sr_1_1_sspa?crid=19S08TO69SSPJ&dib=eyJ2IjoiMSJ9.G4ZHuurkn3IVHsyuKzuoxxIKGXN42qvZAc9mcrW5r69d231gJdkC9262W6Y9Ge7VLNqRy653RUZFWEvndlAY5xd59nQGcFeStKJK1_vYzKzwS6160cq9R0J4r7gITSLqXn6BMuL67mi8kVQTMqTT2NHGUpL0ptQOIsJUrpLD2EnGLzXHuZAQ9FXGqVdy_-a-TAHyH_NyhFEjnBrFpiu8voqXx_8EnP7By8SCqog0cLuR0ymxSKV6cAbdcwyaRp9ebK8oHe41a7807tuTpKDn0sVd7oFUAog0Dy4GwfcQmjw.AWgWGJvW0Wjxyzk9oo0Ga_ChksybQGJP_y22Lfu2cvI&dib_tag=se&keywords=in4148&qid=1749637823&sprefix=%2Caps%2C115&sr=8-1-spons&sp_csd=d2lkZ2V0TmFtZT1zcF9hdGY&psc=1 |
| Transistor |
1 |
0,28 |
0,28 |
https://www.amazon.com/valores-transistor-potencia-epitaxial-Darlington/dp/B08BFYVK6C/ref=sr_1_2_sspa?__mk_es_US=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=F7L9BPIUJJ1J&dib=eyJ2IjoiMSJ9.h3c-xIy3MyTNjNTX3uCVtQcwfWZK_sKfZ5uAxLmZxsqRtPa0gU6bxTYAyV5DnWuyAXByYs1n1nX0i6Q_l5MTe88pNAxBZxrPfEN8voxaQCtiZdJVnQwGgVRG-ODWzeXTkUsDoi6EbqcQMcgAnbQ2VqfPOWZgFRMchaxT0K9n-Gp6LGWk1V-iedqbZlP-o_bcyz1OLHvcxVl1vjiv74yYgciAfzE5yGDDIyRHC4qiHCw.mcSg2LgzvVTPouRlbtI4MDXSFdDEva1NHrZGLCBTpZU&dib_tag=se&keywords=tip122&qid=1749637875&sprefix=tip12%2Caps%2C198&sr=8-2-spons&sp_csd=d2lkZ2V0TmFtZT1zcF9hdGY&psc=1 |
| Solid state Relay |
1 |
2,20 |
2,20 |
https://www.amazon.com/-/es/HiLetgo-m%C3%B3dulo-estado-Control-fusible/dp/B00WSN9CJC/ref=sr_1_6?__mk_es_US=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=1DCIOST74VCPI&dib=eyJ2IjoiMSJ9.q91PYm6OKAKVxQl4ebBf0AcV_wFfLUw6yFpImY74y9NNuAwuMA_hpIqRP2tM66p4flq29UABM9fA-Vt8GxLx1_6p2ie44_AJ84mtp5V95vCcb2_E7tkDR2bruMwsij8DGnkhkGYR3yGb3K202Yot5Uw3FOzQ5Em4ew4ZXLH8U5mn9tf4yMoJdQZ_yI1NPchNJZzmKsWA_Omo89YqgdAJFDpTr6nQ7RvzFNO0pdqxyZg.B-yP_CUBkbA0UKhlF3XE1GJNXnzZo4iaBUsoYxd5gl0&dib_tag=se&keywords=rele+solid+state+5V&qid=1749637984&sprefix=rele+solid+state+5v%2Caps%2C131&sr=8-6 |
| Connectors |
6 |
0,11 |
0,66 |
https://www.amazon.com/s?k=conectores+arduino&__mk_es_US=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=ZOJJGKBHPROE&sprefix=conectores+arduino%2Caps%2C133&ref=nb_sb_noss |
| Capacitors |
4 |
0,04 |
0,16 |
https://www.amazon.com/ALLECIN-surtido-condensadores-electrol%C3%ADticos-aluminio/dp/B0C1VBXCQM/ref=sr_1_1_sspa?__mk_es_US=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=O5JYUB0HNKY2&dib=eyJ2IjoiMSJ9.ekYH7jx2EeGYfnpDMkTUbA5_8nAZM5NXRF7ISTCDYRdiGRRate4MlrQd9ldvZs-A_pzD-zjApyYWkhjoUOqNALpVcJsc6DD3JHlReRoBcxMOy5HnZgoPPDGKGQ7eKIroLYErlCkXUuGwYEHo_DbuP4dZ3WHupx5R5NgErb4ax20-0bVA56GPbf6AHFW0x2BVjzMCRqJcM72pPARxXIipVzthtF2eEvVnVx2oaxKJv7E.rax_WABdj5Kg3iZg0-DCwqXoqhX58FBhrXxgFDcFrrk&dib_tag=se&keywords=condensadores&qid=1749638632&sprefix=condensadore%2Caps%2C169&sr=8-1-spons&sp_csd=d2lkZ2V0TmFtZT1zcF9hdGY&psc=1 |
| Arduino Mega |
1 |
23,25 |
23,25 |
https://www.amazon.es/ELEGOO-Microcontrolador-ATmega2560-ATmega16U2-Compatible/dp/B06Y3ZHPWC/ref=sr_1_2_sspa?__mk_es_ES=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=16IXP8IYVEZIP&dib=eyJ2IjoiMSJ9.VNlP3CQ0NzQDPWLu2uC7KkHA__lwuDLf45_9fQSmtbWX2NOfw9qL2kbiUGF2MnDCMVOVg4_-bUWaN20Oo-pyASW_fsBa_0BKxKMcKOIPvQIcAQr6LkANQDjtDT_nyyHx2ukyr-PD484nwxI2IANpNtI_E_-62_pEWMiufkU8QHI7q1S8JpvgGJMgdeHwqG-1ufXxIfMmGLtVCMEVGeAfOb1MSvMF3AFAV1wK7aIIIRbpWm8kf6G-NnivmR22WzIv0Qw8t_W8qhw9jDU8I6Lsm2-z2Id8eIaxHEbcPxL0E-4.c8KUwVEiJ7T0gRfOboB311pEUyYhsvdQTwCAj5wkmN4&dib_tag=se&keywords=arduino+mega+con+pantalla&qid=1749642004&sprefix=arduino+mega+con+pantalla%2Caps%2C90&sr=8-2-spons&sp_csd=d2lkZ2V0TmFtZT1zcF9hdGY&psc=1 |
| Screen 3,5’’ |
1 |
18,99 |
18,99 |
https://www.amazon.es/Binghe-Bol%C3%ADgrafo-Contacto-Resoluci%C3%B3n-Compatible/dp/B0D6B9M4ZH/ref=sr_1_4?__mk_es_ES=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=16IXP8IYVEZIP&dib=eyJ2IjoiMSJ9.VNlP3CQ0NzQDPWLu2uC7KkHA__lwuDLf45_9fQSmtbWX2NOfw9qL2kbiUGF2MnDCMVOVg4_-bUWaN20Oo-pyASW_fsBa_0BKxKMcKOIPvQIcAQr6LkANQDjtDT_nyyHx2ukyr-PD484nwxI2IANpNtI_E_-62_pEWMiufkU8QHI7q1S8JpvgGJMgdeHwqG-1ufXxIfMmGLtVCMEVGeAfOb1MSvMF3AFAV1wK7aIIIRbpWm8kf6G-NnivmR22WzIv0Qw8t_W8qhw9jDU8I6Lsm2-z2Id8eIaxHEbcPxL0E-4.c8KUwVEiJ7T0gRfOboB311pEUyYhsvdQTwCAj5wkmN4&dib_tag=se&keywords=arduino+mega+con+pantalla&qid=1749642106&sprefix=arduino+mega+con+pantalla%2Caps%2C90&sr=8-4 |
| Chamber polymethyl methacrylate |
2,5m2 |
65,72(m2) |
164,30 |
https://planchasdeplastico.es/producto/policarbonato-transparente-3-mm/?gad_source=1&gad_campaignid=16996510449&gbraid=0AAAAAoVwwA-2JSUGanHN_CR0-AZc1Ivjj&gclid=Cj0KCQjw0qTCBhCmARIsAAj8C4Y5dcppkkNshgAS0GoqejuNiEiCO95D5VttoEFx4nHkqewEDAKf7SoaAnJJEALw_wcB |
| Rack |
3 |
11,00 |
33,00 |
https://www.amazon.es/Mallard-Ferri%C3%A8re-cromado-rejilla-60/dp/B00BMKWP5U/ref=sr_1_7?__mk_es_ES=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=2V6WL13VKL0ZY&dib=eyJ2IjoiMSJ9.D1aLnzF4G36EJHClT1wbHuzBq6CHue7ZO_5WTr09IPf_FcX3ORDatQU4OY5709laVOA0idmW5NpqLC6RQz7T87oYfxxrDxjSR5eTrB0yLd1bjZbrDPnmBd7xqRa9mAxg3pcdKfTQlQGtVIfaI4jVlV9tNLabpyse-1ln13VbL5By94Qt2BkDkJo4coagITwtE3pNG-Drcpuch8YyzA4YgWY2huacB4vj4O4oZJ7au3IjG-Y1rjsIMG4AVEJtxHMyniUypJ0bWs_USfhGiPeVA_JXeFG7aufLo6DtSsklo-c.9nLvIyT4-DDJM9Y6EGfGY1Slq_SyAkWfgoQLRpgNxXE&dib_tag=se&keywords=rejilla+60x50&qid=1749639549&sprefix=rejilla+60x50%2Caps%2C83&sr=8-7 |
| Pump 30l/min |
1 |
61,18 |
61,18 |
https://www.amazon.es/Hailea-bomba-minuto-incluye-hidrop%C3%B3nico/dp/B00NSOQZO0/ref=sr_1_6?__mk_es_ES=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=2QV3ZCZZQW2EP&dib=eyJ2IjoiMSJ9.8EvrXsj1Rve6ppqRPADz0-VaZ0Vpe3Ad9jM2YO1LJHfGVrsnI4xbu3vmb0839HdqjEGE8U7JU9FCOhZENWH55rtt5RVu7eWzqa-n7OEE70Ot9CG2JJupy_W4fQCEEgj1ojhxiMCgqONskinhanDqchDC4PTigzll2OZqpo_y1HUKPAySq8f8n4WtXCsYTqWiHs4TX7fCgu7U6xTNvHZS8mB-U7dgI-JvHv9Z9ODIOE6h5fac7dldxlGhVDIBLOF2mTGdx8rXOzodLoEyiE1r_xtG-xHwmzzBHrQsUgGERRc.QgNQ_J8WkHaYqVuGzuyWAp5TwTee0tubDkAzO8SgjEs&dib_tag=se&keywords=bomba+60l%2Fmin&qid=1749639830&sprefix=bomba+60l%2Fmin%2Caps%2C94&sr=8-6 |
| Pump 60l/min |
1 |
110,00 |
110,00 |
https://www.amazon.es/AquaForte-aluminio-Silenciosa-Capacidad-regulable/dp/B006SYHCI0/ref=sr_1_5?__mk_es_ES=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=1Z9OHLAOKFU5L&dib=eyJ2IjoiMSJ9.NRkweEtlZU8--IlxdeJnBVnA57aO3bZv5hAEsbZEYcl9xBXcXFNMVAeKlU_86n4GJzWpJ4OIB5EVtu1BudyX06OpzDGO0m-QAcaWxxlnw97CIZDvWj0Q6BCLHxdqOjyrizC-5bgqUfTjGLc7AgJx9N7xc5UT6tWYHqJTycqoAMjZkBHAgosIT_-LUFZez5Z_CAMOPlR3IbjEVCJU9N_bvnvFdB68xiLwvSfY2hTUrhiUBE75D232bex3DA3G01LUZhVONH23IfRAz2U_0am5jKcT0EFB2WKabKOpAJOVNUM.SmnSqG7K3XQG0_bTTbTVbFH7hzXOCEkY8uRXlZar1lc&dib_tag=se&keywords=Bomba%2Bv30&qid=1749640500&sprefix=bomba%2Bv30%2Caps%2C130&sr=8-5&th=1 |
| 3D printing |
- |
31,00 |
31,00 |
https://es.farnell.com/ultimaker/1609/filament-pla-black-750g/dp/2992628?gross_price=true&CMP=KNC-GES-GEN-SHOPPING-Pmax-High_ROAS&gad_source=1&gad_campaignid=18071281895&gbraid=0AAAAAD8yeHlKxiGMy79WCfJwNBT_BsqP9&gclid=Cj0KCQjw0qTCBhCmARIsAAj8C4b-IsF7T5oIuiyewkZV-nNr8tDFVX0Vy5St76HWuHN5j-02Z0rQICEaAhJBEALw_wcB |
| Thermoelectric cooler |
1 |
47,41 |
47,41 |
https://www.amazon.es/Refrigerador-Refrigeraci%C3%B3n-Termoel%C3%A9ctrico-Enfriamiento-Semiconductores/dp/B0F6NWNWJM/ref=sr_1_16?__mk_es_ES=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=1XPI9W7G6FJMY&dib=eyJ2IjoiMSJ9.9IY2M3EO0bKYlISgJaOjbFR0CO1MNnuPKF7FQmBmf24793Shg3oHhO-N_JNhPTuMwtWic163ggGxBsgEaRs6K16S-PeHerDs4niKZfdYLnI9DrVWBIBR1iV60ukXPu3hhjJTvsIpVfQ1XD-gxILKkw2GAAQosMOAUKuB3SloCD-4lxuFh2BLEdseAl5S91JsiijAaGn6LtfQBiIioHr3EaD2cnSsogdszxBCcni6g-tGlKIw5CnGXptQwrq8BEX4UCFTLGcdRjignZ0kLzgelIU7xlUn5pjFYNRXYykJEW0.kDaw92Wqmkk6qb-3Jdq5KTzDpfY6PgVISbfYPW0V8aU&dib_tag=se&keywords=peltier+12v+120W+liquido&qid=1749724430&sprefix=peltier+12v+120w+liquido%2Caps%2C89&sr=8-16 |
| Water cooling radiator |
1 |
15,99 |
15,99 |
https://www.amazon.es/CENPEK-Radiador-refrigeraci%C3%B3n-intercambiador-computadora/dp/B09FXL787S/ref=sr_1_12?__mk_es_ES=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=PQGXM2YHEEV3&dib=eyJ2IjoiMSJ9.yjbbkS_YR53lsFDVB9rz0eYd5sZpphUjuTHx0GHaUkYI5UlNnAXcYGrK5NPn7pJfDK7bzqOrTpZhn_NPIxlIyE2goUsz_bcNBKzDvYGkaIZlo9KPsHoiczEU67CN8_tpfdQOO35OF_DK9ZAaofcYhHLr3qrTsUEJqPhkwz7V0Bg8SO2REwV15OTBLg_uwcM5tloSxGjuGmAhrcS_X7Qrxw18W9LjGTun564uKLiel05dAQiT5TZ6nAGbaw8igOMGZmzpsWvEZrDNOEXwrxasGjp5itvNilLNFstgyNaFOJc.G1-K8oQzQAXi-uTpVrxW7Fyd0eNC0x3PsQHYMwMlq-4&dib_tag=se&keywords=radiador+liquido&qid=1749724514&sprefix=radiador+liquido%2Caps%2C77&sr=8-12 |
| Water cooling pump |
1 |
19,72 |
19,72 |
https://www.amazon.es/Diyeeni-refrigeraci%C3%B3n-expansi%C3%B3n-m%C3%A1x-Bomba-enfriamiento/dp/B07ZRQRV7J/ref=sr_1_4?__mk_es_ES=%C3%85M%C3%85%C5%BD%C3%95%C3%91&crid=2PGY6ZH64SWIS&dib=eyJ2IjoiMSJ9.fLA9IrZ-4xIiJiCZDNLIo0iOr0srTdzEtk26K6yuy7gC27QZgzKO3yCZpTJPTes98E8sfvWLDY58QdMIxf7WFjIln4dTfSQemugONaQwo8cqYc70UEWTb35qg50KeciM-G5VzBf4mZiaAk483FHoIRd8rsp9fGP9PY_fHRqzVkEE9MAFjzffeJJapV2YuOewTKz0QHwzmhtHavNzgWzUONq1C4nojCNiIV_by89kzD9YEq5_OL82FUaG-W6l5QxrdNt7rga4DEEUKZW26d28iO6IUbgxF22iBQ_jiEh9h6o.T2DoqO2ThfgHihFKC9f4I2e8n3kWlOl2-tvC-n82Zew&dib_tag=se&keywords=bomba+liquido+refrigeracion&qid=1749724631&sprefix=bomba+liquido+refrigeracion%2Caps%2C77&sr=8-4 |
5. Build Instructions
5.1. 3D Design and Printing
The chamber was built using polymethylmethacrylate sheets, forming a sealed enclosure with three hinged doors that allow the insertion of mice cages. To ensure proper closure, a metal rod is used to apply pressure on each door against the chamber frame. Custom 3D-printed holders were designed and fabricated to hold the metal rods securely in place. On the side of the chamber, additional 3D-printed holders are used as storage for the metal rods when they are not in use. Beyond structural mounting, 3D printing was also leveraged to develop custom protective enclosures for the gas sensors affixed to the chamber. These cases were specifically designed with dedicated slots and openings for efficient gas exchange. A custom 3D-printed case was also created to house the electronic circuit incorporating the Arduino board. This enclosure includes ventilation slots to prevent overheating, a front opening for the display module that visualizes real-time data and system graphics, and a rear connector interface for power supply integration. 3D printing was also employed to create accessory components for the soda lime containers. These components ensure optimal airflow for efficient CO₂ removal. Furthermore, a funnel specifically designed for refilling the soda lime containers was also produced using a 3D printer.
Figure 3 shows the 3D-printed components.
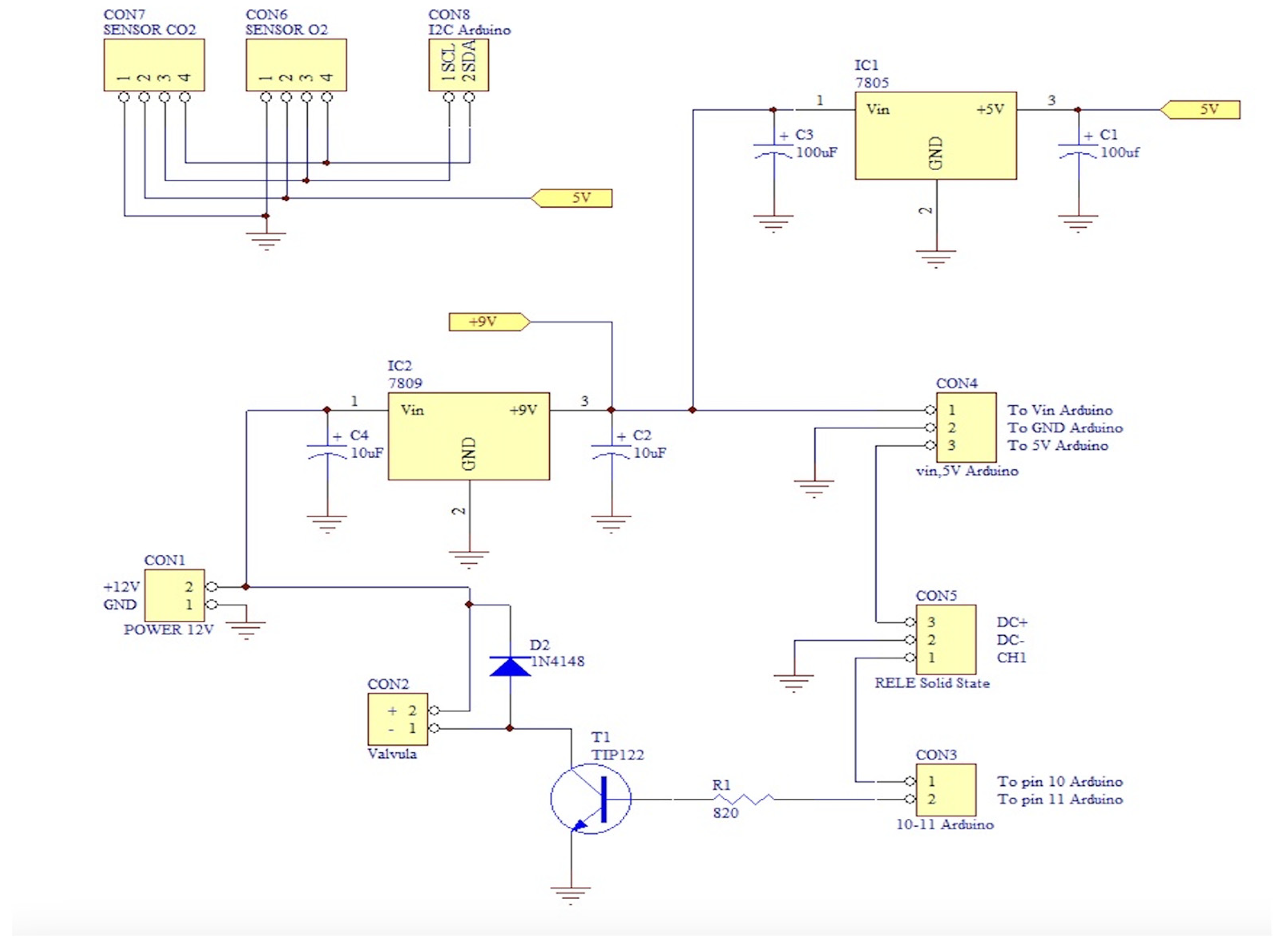
5.2. Electronics
The electronic system, shown in
Figure 4, has been designed to interface the gas sensors with a microcontroller unit (MCU), specifically an Arduino Mega, while also enabling control of electromechanical actuators such as a solenoid valve and pumps via a transistor switch and a solid-state relay, respectively. An I²C-based Sensirion SCD4x sensor is employed for measuring CO₂ concentration, temperature, and humidity, while oxygen levels are monitored using a DFRobot I²C oxygen sensor module. The schematic is organized into distinct functional blocks: sensor interfacing, power regulation, and actuator control. The system operates from a single 12V DC power source. Two linear voltage (7805 for 5V and 7809 for 9V) regulators are used to derive the required supply voltages for the other components.
The injection of N2 into the chamber is allowed by a solenoid valve driven by TIP122 NPN Darlington transistor that acts as a switch. A flyback diode (1N4148) is placed in parallel with the solenoid coil to protect the transistor from voltage spikes. This valve remains closed except when activated. The introduction of room air through the pump is controlled via a digitally-activated solid-state relay, which provides electrical isolation and long-term durability in switching operations.
To regulate humidity inside the chamber, a cooling system based on a Peltier cell is used. This system consists of a thermoelectric cooler, a radiator, and a fan. The Peltier cell cools the air circulating through the chamber. As the air cools, it condenses on the cold surfaces inside the dryer. This process effectively removes the water vapor produced by the mice, which helps maintain a stable relative humidity. The cooled and dehumidified air is then returned to the chamber.
5.5. Arduino Control
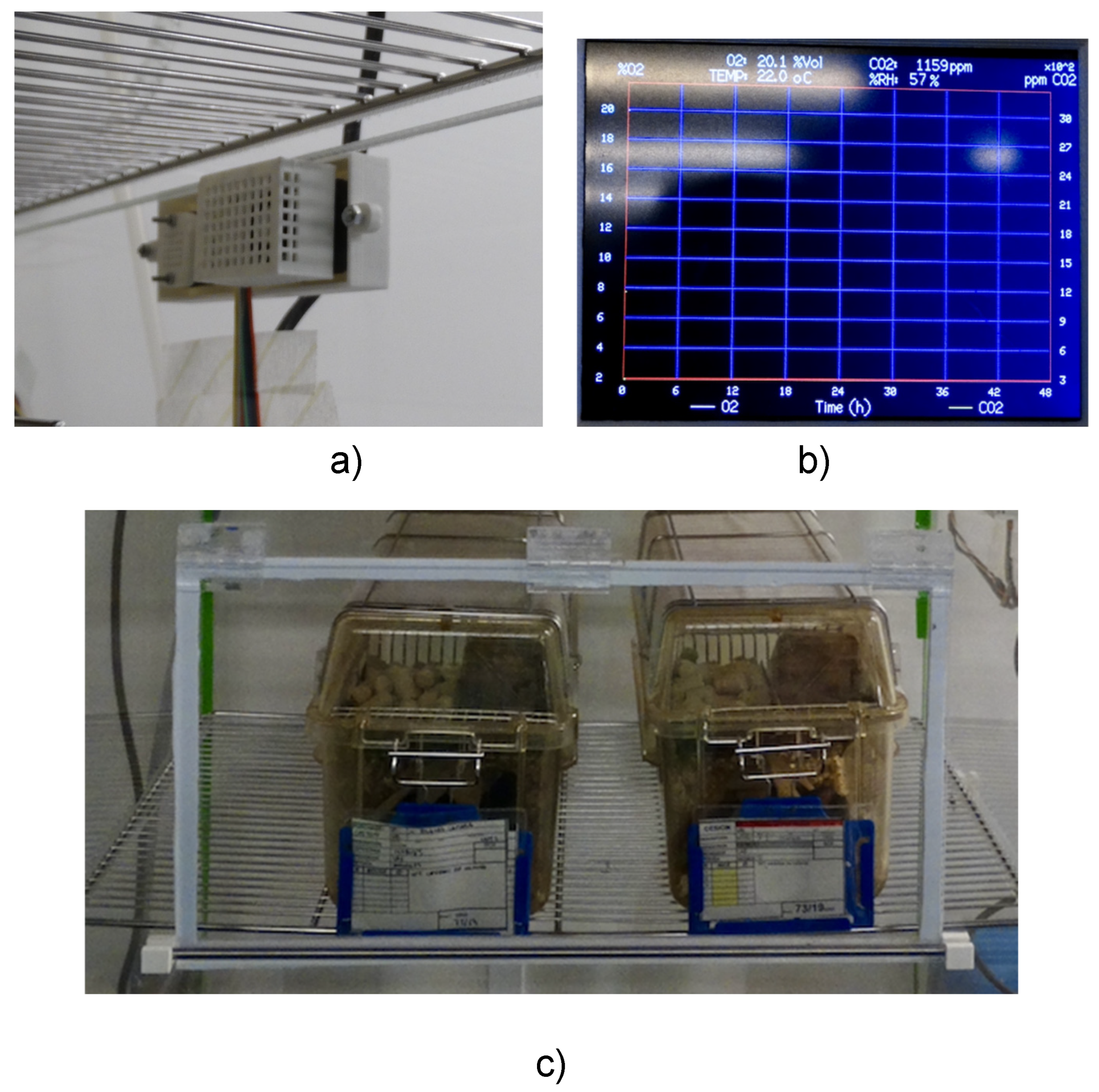
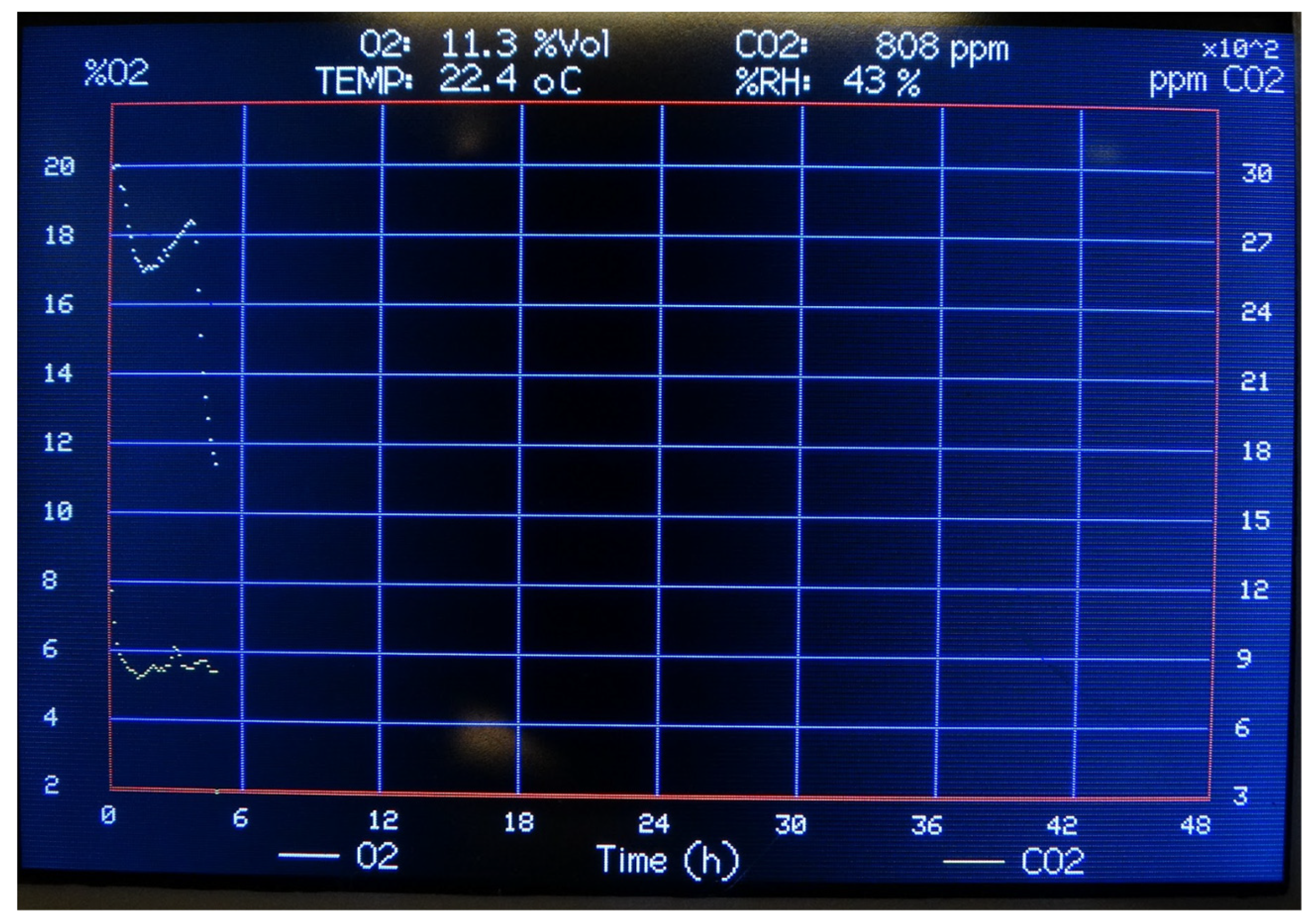
The Arduino firmware is structured to manage real-time acquisition, display, control, and recording of oxygen (O₂) and carbon dioxide (CO₂) concentrations, along with temperature and relative humidity. These values are periodically sampled and visualized on a 3.5” TFT LCD driven by the TFT_HX8357 library. Upon initialization, communication with all peripherals is established via I²C and SPI interfaces. A graphical interface is then rendered on the display, including axes for time and gas concentration, grid lines, and fixed legends for the visual interpretation of data. A rectangular plotting area is drawn for graphing real-time measurements. Initial error checks are performed to verify sensor integrity. The main function of the code contains the core operational logic and it is executed iteratively. At each iteration, the system checks whether new data is available from the CO₂ sensor. In the event of a sensor error (basically checked by the I2C bus), the nitrogen solenoid valve is closed and the air pump is activated, so animals are subjected to room air until the error is fixed. Once valid data are retrieved, the CO₂ concentration, temperature, and humidity values are updated. Simultaneously, the O₂ sensor performs an averaged measurement based on ten samples, providing a smoothed value of the ambient oxygen concentration. The numerical values of humidity, temperature, CO₂, and O₂ are updated on the display interface every 3–5 seconds, in accordance with the conversion time of the CO₂ and O₂ sensors. Approximately every 7 minutes, the system plots instantaneous values of O₂ and CO₂ on a dynamic scrolling graph. The X-axis represents time (scaled to cover 48 hours with 412 data points), while the Y1 and Y2 axes are scaled to appropriately display O₂ (%) and CO₂ (ppm) concentrations, respectively. A circular buffer mechanism ensures efficient redrawing of the graph without overloading memory.
Figure 5 shows the graphical interface on the display. Simultaneously, the system logs environmental data to an SD card every 60 seconds, including timestamp, temperature, humidity, O₂ concentration, and CO₂ concentration. This allows for offline analysis and long-term monitoring.
A bang-bang controller is used to maintain the gas mixture at the desired values during the experiments. On the one hand, if the oxygen concentration exceeds the setpoint (11% in the examples), the system activates the N₂ valve until the level reaches the setpoint. Once this setpoint value is reached, the valve is deactivated, cutting off the N₂ supply. On the other hand, if the O₂ level drops below a critical value (setpoint-0.5% in the examples), or the CO2 concentration exceeds a critical value (2000 ppm), the N₂ valve is automatically closed, and a room air (i.e., oxygenation) pump is activated until the O₂ / CO2 levels are restored to the defined setpoints (11% and 1500 ppm respectively). Depending on the specific experiment aim, if the 2000 ppm threshold is considered too high, it can be lowered by simply modifying its value in the code.
The choice for bang-bang control is made, apart from being simple to implement, because this type of controller offers faster spin up times. This is especially important in applications where the system needs to be frequently manipulated (long periods with the door open or the equipment switched off). Nevertheless, it is well-known that bang-bang controllers present two main drawbacks, which could lead to experimental problems if very precise FiO2 is required: ripple in the signal and risk of overdamping. As is shown in the next section, we have not observed overdamping, and the ripple is within acceptable range for most applications. If more precision is required to mimic certain pathologies, we advise implementing a more complex control scheme, such as proportional-integral-derivative (PID). It should be noted that, however, the PID parameters are more difficult to tune, so the intervention of technical personnel will be needed whenever any of the experimental parameters are changed (number of animals, gas pressures, etc.)
6. Operation Instructions
Starting application of chronic hypoxia to mice requires the following initial actions: connecting the device to an electrical power line (preferably with backup), placing a recipient at the outlet tube of the Peltier-based air conditioning unit to collect the condensed water, filling the soda lime containers, connecting the device N2 inlet to a pressured source (e.g, a cylinder), setting its low pressure/flow regulator to provide a N2 flow able to reduce the chamber O2 concentration from room air (21%) to the 11% set point in 10-20 min, place the animal cages into the chamber, carefully closing the windows and pressing the power on button.
During application of continuous hypoxia, the real time values of O2 and CO2 concentrations, temperature and relative humidity within the chamber can be seen in the front panel of the control unit. The screen in the front panel shows an updated time course of O2 and CO2 concentrations for the last 48 h (e.g., allowing checking that the system has worked correctly along an unattended weekend).The most important maintenance task is to replace the soda lime when required (either because it starts changing color form white to blue or because CO2 concentration starts to increase above desired values). When a mice cage is extracted from the chamber (either for cleaning, feeding replacement or for animal examination), the window must be closed immediately to minimize gas concentration changes into the chamber. Users should be aware of the information provided by the sensors technical data sheets regarding periodic calibration checking and sensors lifelong.
For simplicity and taking into account that the process of reducing O2 from room air (21%) to the chronic hypoxic target (11%) only occurs once at the beginning of the experiment, we did not include an automatically controlled time-course for the O2 reduction. This initial process of animal adaptation should be carried out under the direct supervision of the investigator, and he/she can manually modulate the rate of hypoxia application by partially opening one of the box door’s. However, it would be easy to include a very few sentences in the code to limit the rate of O2 concentration reduction.
In case the device is used at conditions involving high air humidity and halogenated anesthetics, it is advisable to include a CO sensor [
16,
17]. In this work we have quantified the O
2 concentration by its percentage in air, as is it more usual by assuming working at sea level. However, in case the chamber is used in high altitude places where atmospheric pressure is below that of see level, it should be taken into consideration that the physiologically relevant variable for O
2 concentration is its partial pressure.
7. Validation and Characterization
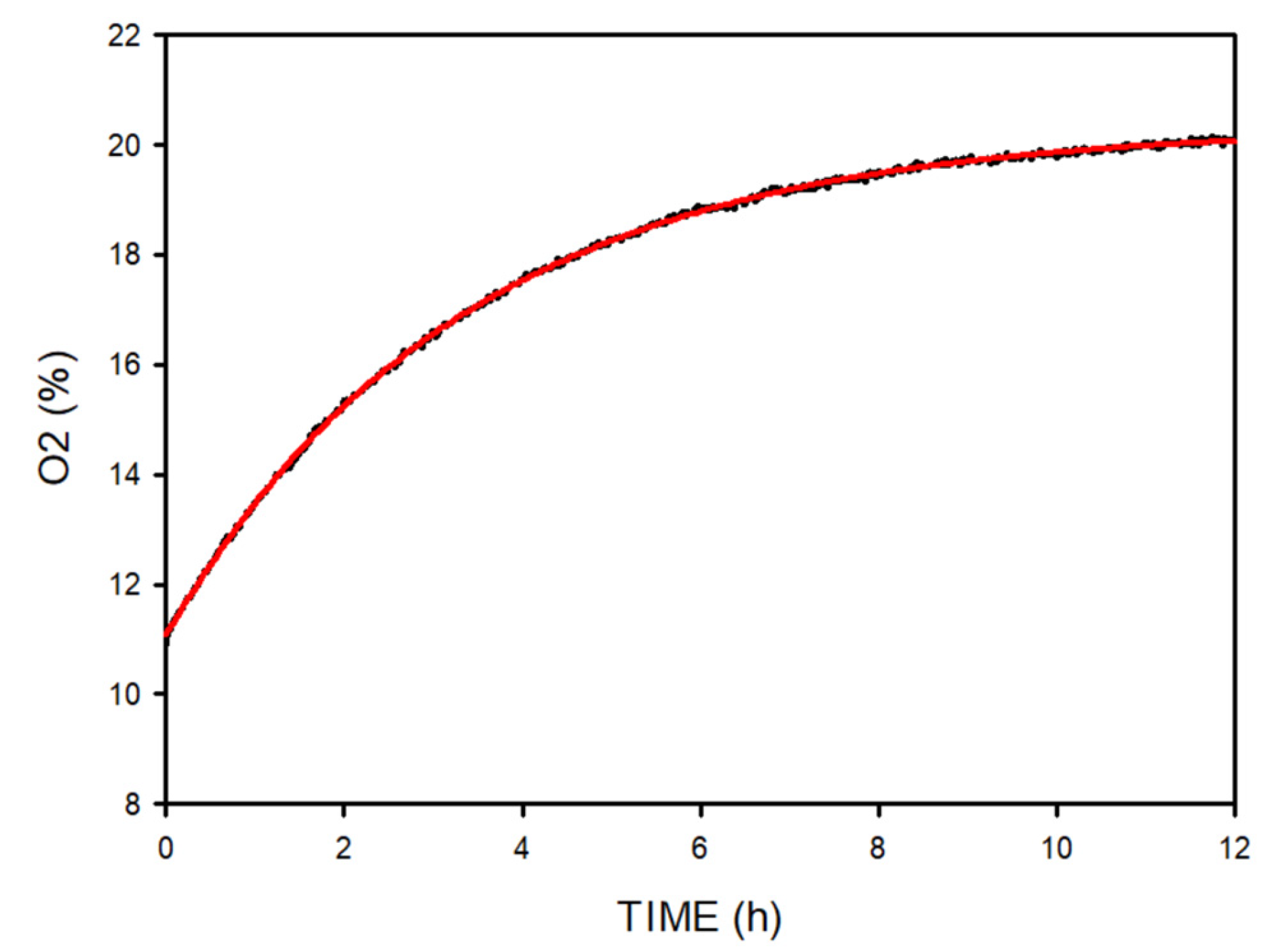
The passive dynamic change in gas concentration depends on both the chamber volume and the flow of gas leakage between the chamber and the room. We first characterized the passive time constant of gas concentration change in the chamber by measuring the O
2 concentration when the provision of N
2 was purposely interrupted when the chamber was at a stable 11% O
2 concentration.
Figure 6 shows an exponential variation corresponding to a time constant (τ) of 3.34 h.
The in vivo performance of the device was validated when applying it in research studies where mice were experiencing chronic hypoxia.
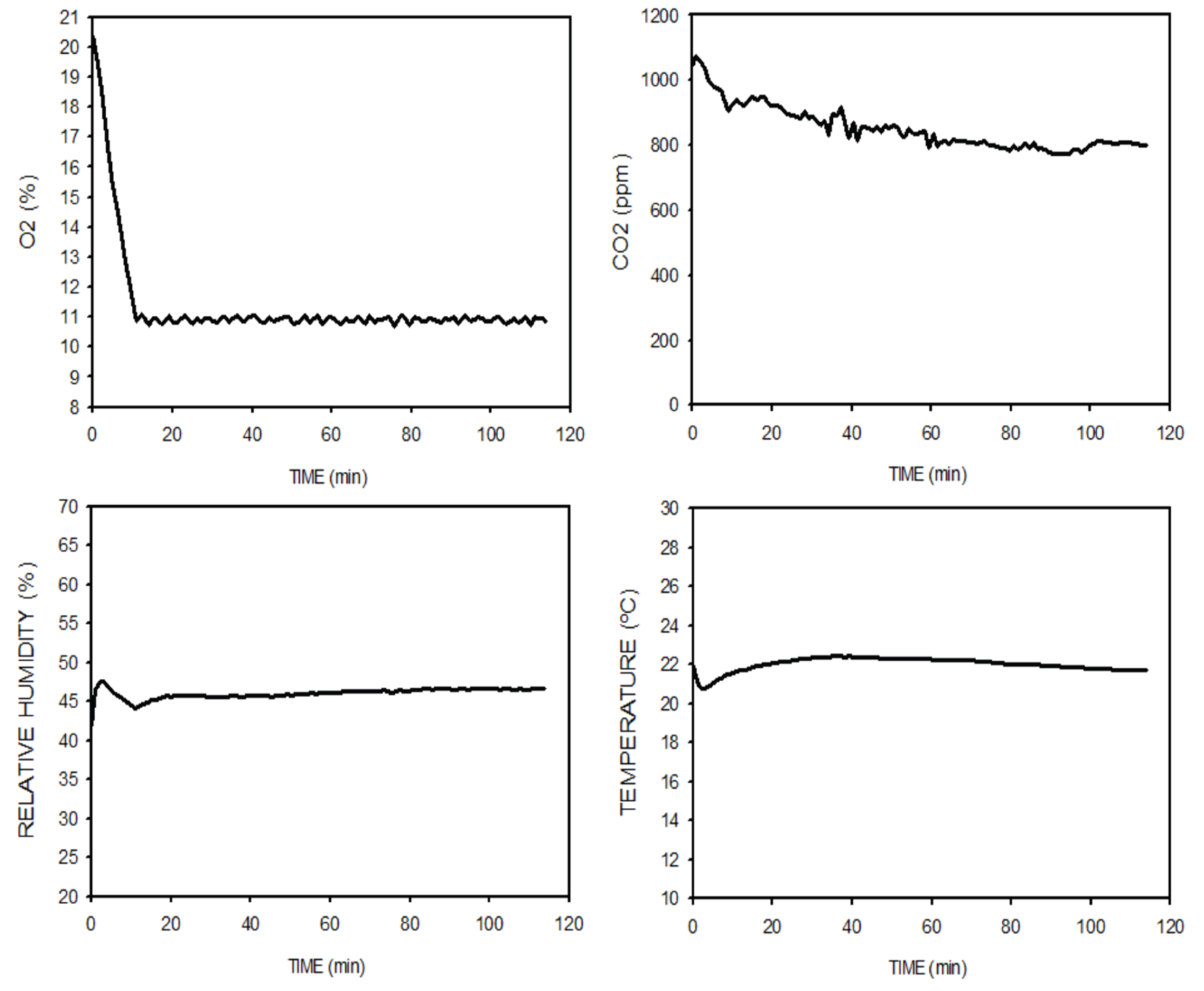
Figure 7 shows an example of the O
2 and CO
2 concentrations, temperature and relative humidity signals recorded when 17 wild-type mice were subjected to 11% O
2. Data in the figure starts when the mice were initially introduced into the hypoxia chamber at conventional lab ambient conditions and hypoxia application was initiated. As expected, O
2 concentration decreased from lab conditions (21%) to the 11% set point and subsequently remained stable at this value with negligible oscillations (10.8–11.2%). CO
2 absorption was very effective since its concentration was reduced from the initial room lab value ≈1000 ppm; i.e., 0.1%) to ≈800 ppm, showing a considerable reserve capacity of the device to keep safe levels of CO
2 concentration. The figure also shows that, after a very minor initial fluctuation resulting from the sudden N
2 injection to lower O
2 concentration until the set point, ambient temperature and relative humidity in the hypoxia chamber were maintained at values very close to the external lab room air ≈45% and ≈22 ºC, respectively. N
2 consumption to keep the 11%-O
2 state steady was only ≈2 l/min.
Uniform distribution of CO2 concentration within the chamber was confirmed by measuring it when the 19 mice were maintained under steady state condition. Indeed, by placing a CO2 sensor at the 9 different chamber sites potentially occupied by the mice cages, we observed that the variability (coefficient of variation) in the CO2 concentration within the chamber was only 6%.
These validation data and the suitability of the corresponding setting parameters correspond to the specific device built and animals employed. Users should adapt the device parameters, and validate them, in case of different applications, mainly depending on the number of animals and their metabolic rate (i.e., O2 consumption).
The maneuver consisting of opening a box door, taking out a mice cage and closing the door again (e.g., for cleaning and checking the animals) minimally disturbs the O2 concentration within the box. Indeed, data from repeated measurements when the box was at a stable 11% O2 concentration with the 17 mice inside resulted in a maximum O2 concentration increased to 11.8%±0.1% (mean±SD) 65±9 s after ending the maneuver, and O2 concentration automatically recovered the 11% steady state value 67±12 s after the transient maximum increase. Thus, O2 concentration was altered by less than 1% for just 2 min.
Ethics statements
The mice experimental procedure was approved by the Ethical Committee for Animal Research of the Vall d’Hebrón Research Institute of Barcelona (approval number 51/24).
CRediT Author Statement
Jorge Otero: Technical conceptualization, Methodology, Writing-Reviewing; Daniel Mbanze: Methodology; Miguel A. Rodríguez-Lázaro: Methodology, Validation; Raffaella Salama: Methodology, Writing; Gorka Solana: Methodology; Vicent Muñoz-Vaño: Animal model testing; Yolanda Cámara: Animal model testing; Isaac Almendros: Animal model testing; Ramon Farré: General conceptualization, Methodology, Validation, Writing-Reviewing, Editing, and Supervision.
Acknowledgments
This work was partially funded by the Spanish Ministry of Science, Innovation and Universities (project reference: PID2023-147668OB-I00).
References
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020, 8(6), 585–596. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G; Fuster, V; Murray, C.; et al. Global Burden of Cardiovascular Diseases and Risks, 1990-2022. JACC 2023, 82(25), 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, TM; Poirier, P; Burke, LE; Després, JP; Gordon-Larsen, P; Lavie, CJ; Lear, SA; Ndumele, CE; Neeland, IJ; Sanders, P; St-Onge, MP. American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143(21), e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Shah, NM; Kaltsakas, G. Respiratory complications of obesity: from early changes to respiratory failure. Breathe (Sheff) 2023, 19(1), 220263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D; Wang, Y; Wong, ND; Wang, J. Impact of Aging on Cardiovascular Diseases: From Chronological Observation to Biological Insights: JACC Family Series. JACC Asia 2024, 4(5), 345–358. [Google Scholar] [CrossRef] [PubMed]
- Cho, SJ; Stout-Delgado, HW. Aging and Lung Disease. Annu Rev Physiol. 2020, 82, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, PS.; Chiu, WT.; Hsu, PL.; et al. Pathophysiological implications of hypoxia in human diseases. J Biomed Sci 2020, 27, 63. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, BM; Taylor, CT; Rocha, S. Hypoxia research, where to now? Trends Biochem Sci 2024, 49(7), 573–582. [Google Scholar] [CrossRef] [PubMed]
- Hillman, TC; Idnani, R; Wilson, CG. An Inexpensive Open-Source Chamber for Controlled Hypoxia/Hyperoxia Exposure. Front Physiol 2022, 13, 891005. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M; Jain, IH; Goldberger, O; Rezoagli, E; Thoonen, R; Cheng, KH; Sosnovik, DE; Scherrer-Crosbie, M; Mootha, VK; Zapol, WM. Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome. Proc Natl Acad Sci U S A 2017, 114(21), E4241–E4250. [Google Scholar] [CrossRef] [PubMed]
- Kim, E. R.; Tong, Q. Oxygen consumption rate and energy expenditure in mice: indirect calorimetry. Methods Mol. Biol. 2017, 1566, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Olesen, B. W.; Bogatu, D.-I.; Kazanci, O.; Coakley, D. The use of CO2 as an indicator for indoor air quality and control of ventilation according to EN16798-1 and TR16798-2. Paper presented at 15th ROOMVENT Conference Available at, 2021; Available online: https://orbit.dtu.dk/en/publications/the-use-of-cosub2sub-as-an-indicator-for-indoor-airquality-and-c.
- Hirabayashi, G; Uchino, H; Sagara, T; Kakinuma, T; Ogihara, Y; Ishii, N. Effects of temperature gradient correction of carbon dioxide absorbent on carbon dioxide absorption. Br J Anaesth 2006, 97(4), 571–5. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, GA. Measurement of respiratory volume for virus retention studies in mice. Appl Microbiol 1972, 24(5), 812–8. [Google Scholar] [CrossRef] [PubMed]
- Haines, H; Shield, CF; Twitchell, C. Reduced evaporation from rodents during prolonged water restriction. Life Sciences 1969, 8(Issue 19, Part 1), 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Fang, ZX; Eger, EI, 2nd; Laster, MJ; Chortkoff, BS; Kandel, L; Ionescu, P. Carbon monoxide production from degradation of desflurane, enflurane, isoflurane, halothane, and sevoflurane by soda lime and Baralyme. Anesth Analg. 1995, 80(6), 1187–93. [Google Scholar] [CrossRef] [PubMed]
- Keijzer, C.; Perez, R.S.; De Lange, J.J. Carbon monoxide production from five volatile anesthetics in dry sodalime in a patient model: halothane and sevoflurane do produce carbon monoxide; temperature is a poor predictor of carbon monoxide production. BMC Anesthesiol 2005, 5, 6. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).