Submitted:
25 November 2025
Posted:
27 November 2025
You are already at the latest version
Abstract
Background and Objectives: Dichrostachys glomerata and Cissus quadrangularis, two species traditionally used in Cameroon, are recognized for their weight-reducing potential. This study examined the effects of standardized extracts of these botanicals on glucagon-like peptide-1 (GLP-1), dipeptidyl peptidase-4 (DPP-4), and key metabolic outcomes in individuals with excess body weight. Materials and Methods: In this 16-week, randomized, double-blind, placebo-controlled trial, 248 adults (126 women and 122 men; mean age 41.3 ± 0.3 years; BMI 25–34.9 kg/m²) were assigned to receive 400 mg D. glomerata extract (DGE), 300 mg C. quadrangularis extract (CQE), semaglutide (dose-escalated from 3 mg to 14 mg), or placebo, administered once daily. Primary assessments included changes in GLP-1 levels and DPP-4 activity. Secondary evaluations included body composition, caloric intake, satiety response, fasting glucose levels, and lipid profiles. Results: Participants receiving DGE or CQE displayed notable elevations in circulating GLP-1 (+38.6 pg/mL and +42.2 pg/mL, respectively; p < 0.01) and significant reductions in DPP-4 activity (−15.3% and −17.8%; p < 0.01) compared with placebo. Both extracts produced substantial improvements in body weight (−5.2% and −5.8%), body fat (−10.3% and −10.9%), energy intake (−16.2% and −17.5%), and satiety (+25.6% and +27.4%) (p < 0.01). Significant changes in fasting glucose and serum lipid levels were also observed (p < 0.05). These responses are similar to those of semaglutide. Moreover, GLP-1 increments showed strong negative correlations with body fat percentage (r = −0.91 to −0.92; p < 0.001) and DPP-4 activity (r = −0.97 to −0.98; p < 0.001). Conclusion: Supplementation with D. glomerata and C. quadrangularis extracts enhanced GLP-1 secretion and reduced DPP-4 activity, yielding significant benefits for body composition and metabolic parameters. These findings indicate that both botanicals are promising natural agents for managing obesity through incretin-based mechanisms.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Test Materials
- DGE: standardized to contain ≥ 25% total polyphenols, expressed as gallic acid equivalents (GAE).
- CQE: standardized to contain ≥ 2.5% ketosteroids and ≥ 15% total flavonoids, expressed as quercetin equivalents (QE).
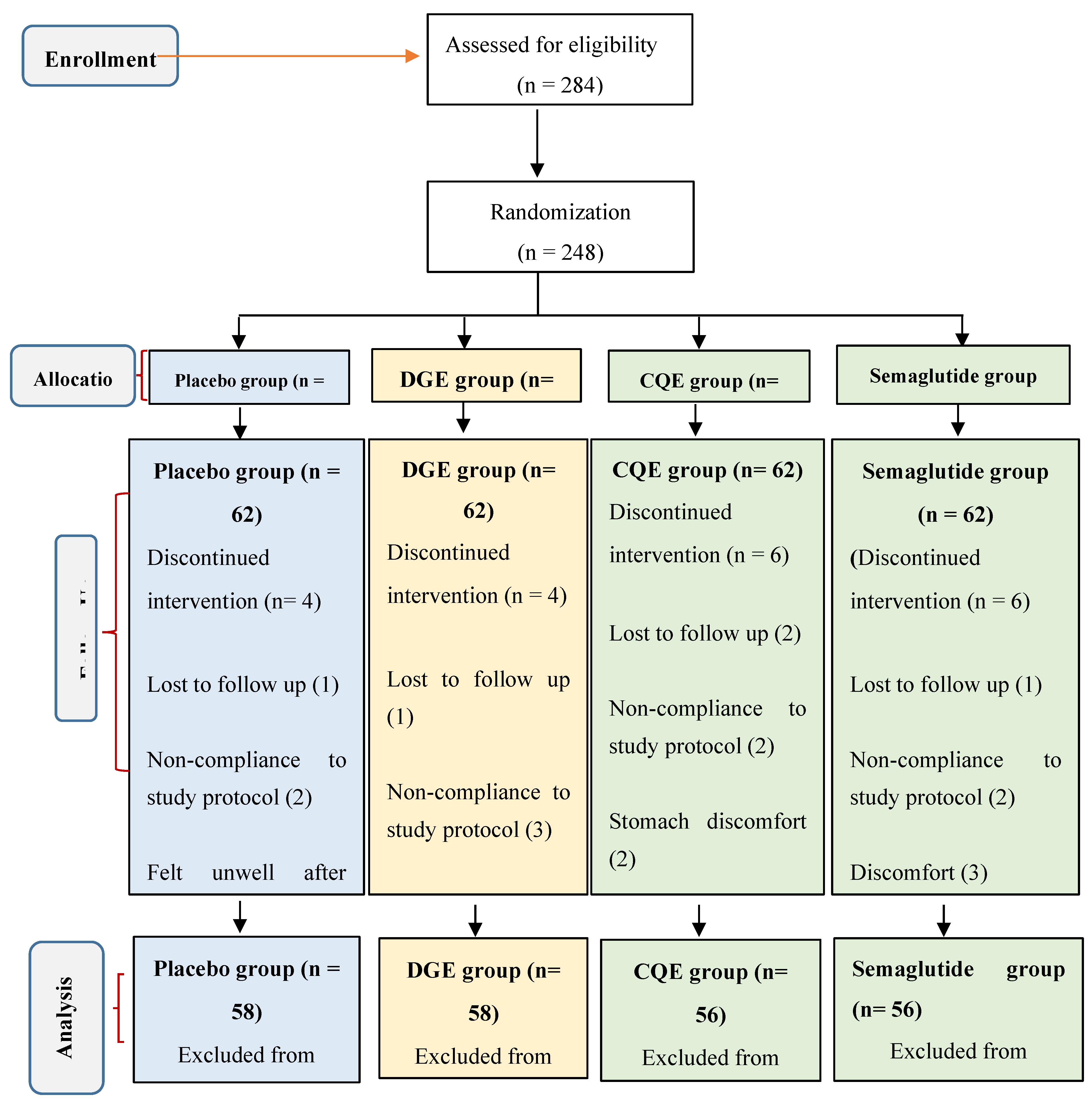
2.2. Participants and Study Design
2.3. Inclusion and Exclusion Criteria
- ➣
-
Inclusion criteria
- BMI between 25–34.9 kg/m²;
- Stable body weight (±2 kg) over the previous 3 months; and
- Willingness to maintain usual dietary and physical activity patterns
- ➣
-
Exclusion criteria
- Diagnosed and uncontrolled diabetes mellitus;
- Recently suffered from a stroke or heart attack;
- Impaired kidney or liver function;
- Pregnancy or lactation;
- Use of weight-loss medications or herbal supplements within the previous 3 months; and
- Known allergies to study materials.
2.4. Randomization
- DGE 400 mg/day;
- CQE 300 mg/day;
- Oral semaglutide (dose-escalation: 3 → 7 → 14 mg/day); and
- Placebo (400 mg dextrin/day).
2.5. Intervention Protocol
2.6. Dietary and Exercise Restrictions
2.7. Blood Sample Collection
2.8. Outcome Measures
2.8.1. Primary Outcomes
- GLP-1 levels: PW was used to measure active GLP-1 in fasting as well as 2 and 3-hour postprandial plasma. Concentrations were measured using the RayBio® Human GLP-1 (Active) ELISA Kit (Catalog #ELH-GLP1-1, RayBiotech, Peachtree Corners, GA, USA).
- DPP-4 activity: PWO was assayed using a Human DPP-4/CD26 Immunoassay Kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions.
2.8.2. Secondary Outcomes
- Anthropometric parameters: height (m) was measured using a stadiometer, weight (kg) using a digital scale (Omron HBF-511), BMI calculated as kg/m², and body fat (%) estimated through bioelectric impedance (HD Touch Body composition scale).
- Metabolic parameters: Fasting glucose was measured with a Accu-Chek® glucometer; total cholesterol, triglycerides, and HDL-c was measured using Roche Diagnostics kits; and LDL cholesterol levels were calculated using the Friedewald formula [10].
- Energy intake: Estimated from 7-day food diaries using the FAO Food Composition Tables for Cameroon. The energy intake (kcal/day) was calculated as follows:
- Satiety: Evaluated using the Visual Analogue Scale (VAS) questionnaire validated by Cazzo et al [11].
2.9. Statistical Analysis
3. Results
3.1. Participant Baseline Characteristics
3.2. Effects on Circulating GLP-1 and DPP-4 Activity
3.3. Anthropometric and Metabolic Parameters
3.3.1. Anthropometric Parameters
3.3.2. Metabolic Parameters
3.4. Energy Intake and Satiety Scores
3.5. Safety and Tolerability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statements
Informed Consent Statement
Data availability
Acknowledgements
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| DPP4 | Dipeptidyl Peptidase 4 |
| GLP-1 | Glucagon-Like Peptide-1 |
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 February 2024).
- Van Bloemendaal, L.; Ten Kulve, J.S.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, M.; Kumar, S.; Sood, A.K. Acute pancreatitis with gliptins: Is it a clinical reality? Indian J. Endocrinol. Metab. 2013, 17 (Suppl. 1), S323–S325. [Google Scholar] [CrossRef] [PubMed]
- Youovop, J.; Takuissu, G.; Mbopda, C.; Nwang, F.; Ntentié, R.; Mbong, M.; Azantsa, B.; Singh, H.; Oben, J. The effects of DGE (Dichrostachys glomerata extract) on body fat percentage and body weight: A randomized, double-blind, placebo-controlled clinical trial. Funct. Foods Health Dis. 2023, 13, 334–346. [Google Scholar] [CrossRef]
- Youovop, J.; et al. In Vitro Inhibition of Dipeptidyl Peptidase-4 (DPP-4) by Standardized Extracts of Dichrostachys glomerata and Cissus quadrangularis: A Mechanistic Insight into Their Anti-Diabetic Potential. [Journal Name] in press.
- Kim, H.L.; Lee, S.K.; Min, D.E.; Choi, B.K.; Lee, D.R. Anti-obesity effect of Dyglomera® is associated with activation of the AMPK signaling pathway in 3T3-L1 adipocytes and mice with high-fat diet-induced obesity. Molecules 2022, 27, 3288. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Le, B.; Lee, D.R.; Choi, B.K.; Yang, S.H. Cissus quadrangularis extract (CQR-300) inhibits lipid accumulation by downregulating adipogenesis and lipogenesis in 3T3-L1 cells. Toxicol. Rep. 2018, 5, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Conde, K.; Fang, S.; Xu, Y. Unraveling the serotonin saga: From discovery to weight regulation and beyond—A comprehensive scientific review. Cell Biosci. 2023, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Blundell, J.; Tetens Hoff, S.; Dahl, K.; Bauer, R.; Baekdal, T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Cazzo, E.; Pareja, J.; Chaim, E.; Coy, C.; Magro, D. Glucagon-like peptides 1 and 2 are involved in satiety modulation after modified biliopancreatic diversion: Results of a pilot study. Obes. Surg. 2017, 27, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Alkhalidy, H.; Liu, D. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef] [PubMed]
- Kuate, D.; Etoundi, B.; Ngondi, J.; Wan Muda, W.; Oben, J. Anti-inflammatory, anthropometric, and lipomodulatory effects of Dyglomera® (aqueous extract of Dichrostachys glomerata) in obese participants with metabolic syndrome. Funct. Foods Health Dis. 2013, 3, 416–427. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Lynch, T.A.; Befus, S.M.; Braverman, T.L.; Hooper, S.L. Efficacy of Dichrostachys glomerata supplementation on overweight and mildly obese adults’ weight, mood, and health-related quality of life: A randomized double-blind placebo-controlled trial. J. Diet. Suppl. 2024, 21, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Das, P.K.; Saha, S.; Dhiwar, P.S.; Khanra, R.; Chatterjee, A.; Zanchi, F.B. Exploration of antidiabetic and anti-apoptotic activity of Cissus quadrangularis stem through mechanistic pathway: An in vitro, in silico and in vivo approach. Appl. Biochem. Biotechnol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Lakthan, T.; Limpachayaporn, P.; Rayanil, K.O.; Charoenpanich, P.; Phuangbubpha, P.; Charoenpanich, A. Lupenone-rich fraction derived from Cissus quadrangularis L. suppresses lipid accumulation in 3T3-L1 adipocytes. Life 2023, 13, 1724. [Google Scholar] [CrossRef] [PubMed]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Cissus quadrangularis enhances UCP1 mRNA, indicative of white adipocyte browning, and decreases central obesity in humans in a randomized trial. Sci. Rep. 2021, 11, 2008. [Google Scholar] [CrossRef] [PubMed]
- Mondal, I.; Zilani, M.N.H.; Lisany, N.F.; Yasmin, F.; Bibi, S.; Biswas, P.; Tauhida, S.J.; Rahman, M.S.; Albadrani, G.M.; Al-Ghadi, M.Q.; et al. Cissus quadrangularis revealed as a potential source of anti-inflammatory and antidiabetic pharmacophore in experimental and computational studies. Chem. Biodivers. 2025, e00903. [Google Scholar] [CrossRef] [PubMed]
- Sowinski, R.J.; Grubic, T.J.; Dalton, R.L.; Schlaffer, J.; Reyes-Elrod, A.G.; Jenkins, V.M.; Williamson, S.; Rasmussen, C.; Murano, P.S.; Earnest, C.P.; et al. An examination of a novel weight loss supplement on anthropometry and indices of cardiovascular disease risk. J. Diet. Suppl. 2021, 18, 478–506. [Google Scholar] [CrossRef] [PubMed]
- Oben, J.; Kuate, D.; Agbor, G.; Momo, C.; Talla, X. The use of a Cissus quadrangularis formulation in the management of weight loss and metabolic syndrome. Lipids Health Dis. 2006, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Kuate, D.; Nash, R.J.; Bartholomew, B.; Penkova, Y. The use of Cissus quadrangularis (CQR-300) in the management of components of metabolic syndrome in overweight and obese participants. Nat. Prod. Commun. 2015, 10, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Sawangjit, R.; Puttarak, P.; Saokaew, S.; Chaiyakunapruk, N. Efficacy and safety of Cissus quadrangularis L. in clinical use: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2017, 31, 555–567. [Google Scholar] [CrossRef] [PubMed]
| Extract | Batch/Lot No | Standardization marker | Certificate of Analysis | Formulation | Supplier |
| DGE | GHA-DGE-0423 | Polyphenols (25% GAE) | COA #DGE0423-24 | Capsule, 400 mg | Gateway Health Alliances |
| CQE | GHA-CQE-0323 | Ketosterols (2.5%) Flavonoids (15% QE) |
COA #CQE0323-24 | Capsule, 300 mg | Gateway Health Alliances |
| Placebo (n=58) | DGE (n=58) | CQE (n=56) | Semaglutide (n=56) | ||
| Demographic characteristics | |||||
| Age (years) | 43.04 ± 1.05 | 41.37 ± 1.14 | 40.70 ± 1.12 | 40.09 ± 1.04 | |
| Gender (n) | |||||
| Males (%) | 50.00 (29) | 51.72 (30) | 44.64 (25) | 50.00 (28) | |
| Females (%) | 50.00 (29) | 48.28 (28) | 55.36 (31) | 50.00 (28) | |
| Primary outcomes | |||||
| GLP-1 level (pg/mL) | 13.00 ± 0.73 | 13.10 ± 0.65 | 13.13 ± 0.56 | 13.04± 0.34 | |
| DPP-4 activity (U/L) | 31.68 ± 0.96 | 31.82 ± 1.14 | 31.42 ± 1.36 | 31.60 ± 0.96 | |
| Secondary outcomes | |||||
| Anthropometric characteristics | |||||
| Body Weight (Kg) | 81.97 ± 10.09 | 81.96 ± 9.48 | 81.55 ± 9.49 | 81.80 ± 1.22 | |
| Body Mass Index (Kg/m2) | 30.62 ± 1.34 | 30.68 ± 1.10 | 30.93 ± 1.58 | 30.56 ± 1.44 | |
| Body Fat Percentage (%) | 30.94 ± 1.79 | 30.93 ± 1.86 | 31.26 ± 2.09 | 31.57 ± 2.56 | |
| Energy Intake & Satiety | |||||
| Energy intake (Kcal/day) | 2943.93 ± 859.18 |
2898.60 ± 839.75 |
2941.64 ± 918.33 |
2940.60 ± 885.99 |
|
| Satiety Score (VAS score 0-10) | 8.20 ± 0.60 | 8.00 ± 0.40 | 8.10 ± 0.50 | 8.00 ± 0.32 | |
| Metabolic characteristics | |||||
| Total Cholesterol (mg/dL) | 191.72 ± 3.09 | 191.52 ± 2.39 | 190.88 ± 2.78 | 190.80 ± 2.23 | |
| Triglyceride (mg/dL) | 58.78 ± 2.31 | 58.21 ± 2.46 | 58.71 ± 1.53 | 58.20 ± 2.07 | |
| LDL-c (mg/dL) | 166.38 ± 3.16 | 167.65 ± 2.54 | 166.93 ± 2.86 | 165.90 ± 2.76 | |
| HDL-c (mg/dL) | 66.89 ± 1.11 | 67.07 ± 1.74 | 66.71 ± 1.91 | 66.80 ± 1.60 | |
| Fasting blood glucose (mg/dL) | 106.41 ± 3.93 | 106.33 ± 3.65 | 106.51 ± 3.33 | 106.40 ± 3.65 | |
| Groups | Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Change over 16 weeks | |
| GLP-1 (pg/mL) | |||||||
| Placebo | 13.00 ± 0.73 | 13.58 ± 0.65 | 16.43 ± 0.64 | 17.48 ± 0.58 | 17.70 ± 0.67 | 4.70 (36.12%) | |
| DGE | 13.10 ± 0.65 | 21.92 ± 5.53ᵟ# | 31.57 ± 0.63ᵟ# | 44.90 ± 1.19ᵟ | 51.7 ± 1.02ᵟ | 38.6 (294.7%)ᵟ | |
| CQE | 13.13 ± 0.56 | 22.56 ± 0.88ᵟ | 34.15 ± 1.12ᵟ | 45.56 ± 1.11ᵟ | 55.33 ± 1.20ᵟ | 42.2 (321.4%)ᵟ | |
| Semaglutide | 13.04 ± 0.34 | 27.52 ± 3.12 | 45.28 ± 0.82 | 53.65 ± 1.24ᵟ | 59.84 ± 1.28ᵟ | 46.80 (358.9%)ᵟ | |
| DPP4 (U/L) | |||||||
| Placebo | 31.68 ± 0.96 | 31.40 ± 2.15 | 31.10 ± 8.93 | 31.20 ± 8.05 | 30.90 ± 8.37 | -0.78 (-2.9%) | |
| DGE | 31.82 ± 1.14 | 31.54 ± 2.77ᵟ# | 28.91 ± 3.16ᵟ | 28.32 ± 3.12ᵟ | 27.71 ± 2.93ᵟ | -4.11 (-15.3%)ᵟ | |
| CQE | 31.42 ± 1.36 | 30.01 ± 1.90ᵟ | 28.27 ± 3.17ᵟ | 27.50 ± 2.84ᵟ | 26.72 ± 3.80ᵟ | -4.70 (-17.8%)ᵟ | |
| Semaglutide | 31.60 ± 0.96 | 28.95 ± 1.74ᵟ | 27.34 ± 1.60ᵟ | 26.38 ± 2.68ᵟ | 25.34 ± 3.18ᵟ | -6.26 (-23.5%)ᵟ | |
| Parameters | Groups | Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Change over 16 weeks |
| Body weight (Kg) | Placebo | 81.97 ± 10.09 | 81.74 ± 9.80 | 81.52 ± 9.86 | 81.42 ± 10.87 | 81.31 ± 9.78 | - 0.60 (-0.7%) |
| DGE | 81.96 ± 9.48 | 81.22 ± 12.00 | 80.45 ± 13.12 | 79.86 ± 14.67ᵟ | 77.66 ± 12.52ᵟ | -4.3 (-5.2%)ᵟ | |
| CQE | 81.55 ± 9.49 | 80.92 ± 10.62 | 80.30 ±11.75 | 79.15 ± 12.84ᵟ | 76.85 ± 10.48ᵟ | -4.7 (-5.8%)ᵟ | |
| Semaglutide | 81.80 ± 1.22 | 80.18 ± 9.42 | 78.55 ± 9.92 | 77.58 ± 12.77ᵟ | 77.00 ± 10.78ᵟ | -4.8 (-5.9%)ᵟ | |
| Body Mass Index (Kg/m2) | Placebo | 30.62 ± 1.34 | 30.58 ± 1.36 | 30.50 ± 1.38 | 30.45 ± 1.24 | 30.40 ± 1.42 | -0.22 (-0.7%) |
| DGE | 30.68 ± 1.10 | 30.44 ± 1.83# | 30.20 ± 1.68ᵟ | 29.93 ± 2.68ᵟ | 29.10 ± 1.82ᵟ | -1.58 (-5.1%)ᵟ | |
| CQE | 30.93 ± 1.58 | 30.11 ± 1.52* | 29.86 ± 1.83ᵟ | 29.62 ± 1.52ᵟ | 29.02 ± 2.10ᵟ | -1.91 (-6.2%)ᵟ | |
| Semaglutide | 30.56 ± 1.44 | 30.00 ± 1.28 | 29.41 ± 2.70ᵟ | 28.65 ± 0.96ᵟ | 28.44 ± 1.02ᵟ | -2.12 (-6.9%)ᵟ | |
| Body fat percentage (%) | Placebo | 30.94 ± 1.79 | 30.97 ± 1.14 | 30.46 ± 0.71 | 30.48 ± 1.37 | 30.49 ± 1.73 | -0.45 (-1.5%) |
| DGE | 30.93 ± 1.86 | 30.42 ± 1.47 | 29.19 ± 4.49* | 29.35 ± 6.86* | 27.73 ± 4.42ᵟ | -3.2 (-10.3%)ᵟ | |
| CQE | 31.26 ± 2.09 | 30.86 ± 3.23 | 30.46 ± 4.18 | 29.46 ± 3.31ᵟ | 27.86 ± 3.14ᵟ | -3.4 (-10.9%)ᵟ | |
| Semaglutide | 31.57 ± 2.56 | 30.37 ± 4.73* | 29.17 ± 4.89* | 28.58 ± 4.74ᵟ | 27.97 ± 3.47ᵟ | -3.60 (-11.4%)ᵟ | |
| Parameters | Groups | Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Change over 16 weeks |
| Total cholesterol (TC) |
Placebo | 191.72 ± 3.09 | 191.45 ± 3.00 | 191.20 ± 3.21 | 191.09 ± 3.50 | 190.98 ± 3.78 | -0.74 (-0.4%) |
| DGE | 191.52 ± 2.39 | 189.85 ± 2.60ᵟ# | 187.45 ± 2.84ᵟ# | 185.12 ± 4.02ᵟ# | 181.40 ± 3.50ᵟ | -10.12 (-5.28%)ᵟ | |
| CQE | 190.88 ± 2.78 | 187.12 ± 5.05ᵟ | 183.56 ± 4.73ᵟ | 179.45 ± 4.99ᵟ | 176.90 ± 4.20ᵟ | -13.98 (-7.32%)ᵟ | |
| Semaglutide | 190.80 ± 2.23 | 185.25 ± 4.82ᵟ | 179.90 ± 2.37ᵟ | 177.23 ± 2.84ᵟ | 174.55 ± 3.30ᵟ | -16.25 (-8.5%)ᵟ | |
| Triglycerides (TG) | Placebo | 58.78 ± 2.31 | 58.65 ± 2.60 | 58.50 ± 2.05 | 58.46 ± 2.38 | 58.40 ± 2.68 | -0.38 (-0.6%) |
| DGE | 58.21 ± 2.46 | 57.35 ± 2.68ᵟ | 56.10 ± 0.94ᵟ# | 55.05 ± 2.76ᵟ# | 53.30 ± 2.12ᵟ | -4.9 (-8.42%)ᵟ | |
| CQE | 58.71 ± 1.53 | 57.10 ± 1.66ᵟ | 55.65 ± 1.37ᵟ | 54.25 ± 1.81ᵟ | 52.50 ± 1.40ᵟ | -6.21 (- 10.58%)ᵟ | |
| Semaglutide | 58.20 ± 2.07 | 55.95 ± 1.58ᵟ | 53.85 ± 1.10ᵟ | 52.65 ± 1.24ᵟ | 51.45 ± 1.78ᵟ | -6.75 (-11.60%)ᵟ | |
| LDL-c | Placebo | 166.38 ± 3.16 | 166.20 ± 3.12 | 166.05 ± 3.07 | 165.95 ± 3.46 | 165.85 ± 3.86 | -0.53 (-0.3%) |
| DGE | 167.65 ± 2.54 | 165.90 ± 2.76ᵟ# | 163.85 ± 2.92ᵟ# | 161.75 ± 4.18ᵟ# | 159.80 ± 3.08ᵟ | -7.85 (-4.68%)ᵟ | |
| CQE | 166.93 ± 2.86 | 163.55 ± 4.97ᵟ | 160.35 ± 4.65ᵟ | 157.10 ± 5.99ᵟ | 155.15 ± 5.02ᵟ | -11.78 (-7.06%)ᵟ | |
| Semaglutide | 165.90 ± 2.76 | 161.45 ± 3.15ᵟ | 157.10 ± 2.92ᵟ | 154.74 ± 2.84ᵟ | 152.35 ± 4.73ᵟ | -13.55 (-8.2%)ᵟ | |
| HDL-c | Placebo | 66.89 ± 1.11 | 66.95 ± 0.94 | 67.00 ± 0.55 | 67.03 ± 0.72ᵟ | 67.05 ± 0.87 | +0.16 (+0.2%) |
| DGE | 67.07 ± 1.74 | 67.45 ± 1.02ᵟ# | 67.80 ± 0.71ᵟ# | 68.15 ± 1.18ᵟ# | 68.50 ± 1.02ᵟ | +1.43 (+2.13%)ᵟ | |
| CQE | 66.71 ± 1.91 | 67.25 ± 3.00ᵟ | 67.75 ± 2.21ᵟ | 68.30 ± 2.76ᵟ | 68.75 ± 2.01ᵟ | +2.04 (+3.06%)ᵟ | |
| Semaglutide | 66.80 ± 1.60 | 67.50 ± 1.73ᵟ | 68.25 ± 0.94ᵟ | 68.68 ± 0.86ᵟ | 69.05 ± 0.79ᵟ | +2.25 (+3.4%)ᵟ | |
| Fasting Blood Glucose | Placebo | 106.41 ± 3.93 | 106.35 ± 3.94 | 106.25 ± 3.39 | 106.20 ± 3.47 | 106.15 ± 3.55 | -0.26 (-0.2%) |
| DGE | 106.33 ± 3.95 | 104.85 ± 4.86ᵟ# | 102.95 ± 5.76ᵟ# | 101.25 ± 6.24ᵟ# | 97.02 ± 5.55ᵟ | -9.31 (-8.8%)ᵟ | |
| CQE | 106.51 ± 3.33 | 104.10 ± 4.10ᵟ | 101.85 ± 3.16ᵟ | 99.45 ± 2.25ᵟ | 96.21 ± 2.70ᵟ | -10.30 (-9.7%)ᵟ | |
| Semaglutide | 106.40 ± 3.65 | 102.95 ± 3.94ᵟ | 99.25 ± 3.32ᵟ | 97.30 ± 3.16ᵟ | 95.35 ± 2.18ᵟ | -11.05 (-10.4%)ᵟ | |
| Parameters | Groups | Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Change over 16 weeks |
| Energy Intake (Kcal/day) | Placebo | 2943.93 ± 859.18 |
2921.39 ± 858.67 |
2818.39 ± 832.75 |
2835.16 ± 845.71 |
2851.93 ± 794.39 |
-92.0 (-3.1%) |
| DGE | 2898.60 ± 839.75 |
2891.06 ± 839.73 |
2704.06 ± 839.40* |
2660.60 ± 847.92* |
2428.40 ± 843.83* |
-470.20 (-16.2%)ᵟ | |
| CQE | 2941.64 ± 918.33 |
2856.46 ± 927.41 |
2856.46 ± 933.48* |
2665.64 ± 942.98* |
2427.84 ± 902.42* |
-513.8 (-17.5%)ᵟ | |
| Semaglutide | 2940.60 ± 885.99 |
2757.72 ± 852.78 |
2537.00 ± 931.12* |
2463.50 ± 891.95* |
2390.00 ± 866.76* |
-550.0 (18.7%)ᵟ | |
| Satiety Score (VAS score 0-10) | Placebo | 8.20 ± 0.60 | 8.24 ± 0.80 | 8.36± 0.60 | 8.61 ± 0.75 | 8.85 ± 0.70 | 0.65 (5.3%) |
| DGE | 8.00 ± 0.40 | 8.14 ± 0.70ᵟ | 8.38 ± 0.60ᵟ | 9.24 ± 0.40ᵟ | 10.05 ± 0.80ᵟ | 2.05 (25.6%)ᵟ | |
| CQE | 8.10 ± 0.50 | 8.05 ± 0.60ᵟ | 8.90 ± 0.70ᵟ | 9.51 ± 0.60ᵟ | 10.32 ± 0.52ᵟ | 2.22 (27.4%)ᵟ | |
| Semaglutide | 8.00 ± 0.32 | 9.72 ± 0.52ᵟ | 10. 45 ± 0.62ᵟ | 10.63 ± 0.80 | 10.77 ± 0.42ᵟ | 2.77 (34.6%)ᵟ | |
| Parameters | GLP-1 | DPP-4 | Body fat% | Energy intake | VAS score | |
| DGE | ||||||
| GLP-1 | 1 | -0.82* | -0.74* | -0.75* | 0.84* | |
| DPP-4 | -0.82* | 1 | 0.70* | 0.70* | -0.79* | |
| Body fat | -0.74* | 0.70* | 1 | 0.63* | -0.67* | |
| Energy intake | -0.75* | 0.70* | 0.63* | 1 | -0.82* | |
| VAS score | 0.84* | -0.79* | -0.67* | -0.82* | 1 | |
| CQE | ||||||
| GLP-1 | 1 | -0.86* | -0.78* | -0.80* | 0.88* | |
| DPP-4 | -0.86* | 1 | 0.74* | 0.76* | -0.84* | |
| Body fat | -0.78* | 0.74* | 1 | 0.70* | -0.73* | |
| Energy intake | -0.80* | 0.76* | 0.70* | 1 | -0.86* | |
| VAS score | 0.88* | -0.84* | -0.73* | -0.86* | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




