Submitted:
22 November 2025
Posted:
27 November 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Material & Methods
Search Strategy
Data Selection/Eligibility and Exclusion Criteria
Analysis Process
Results
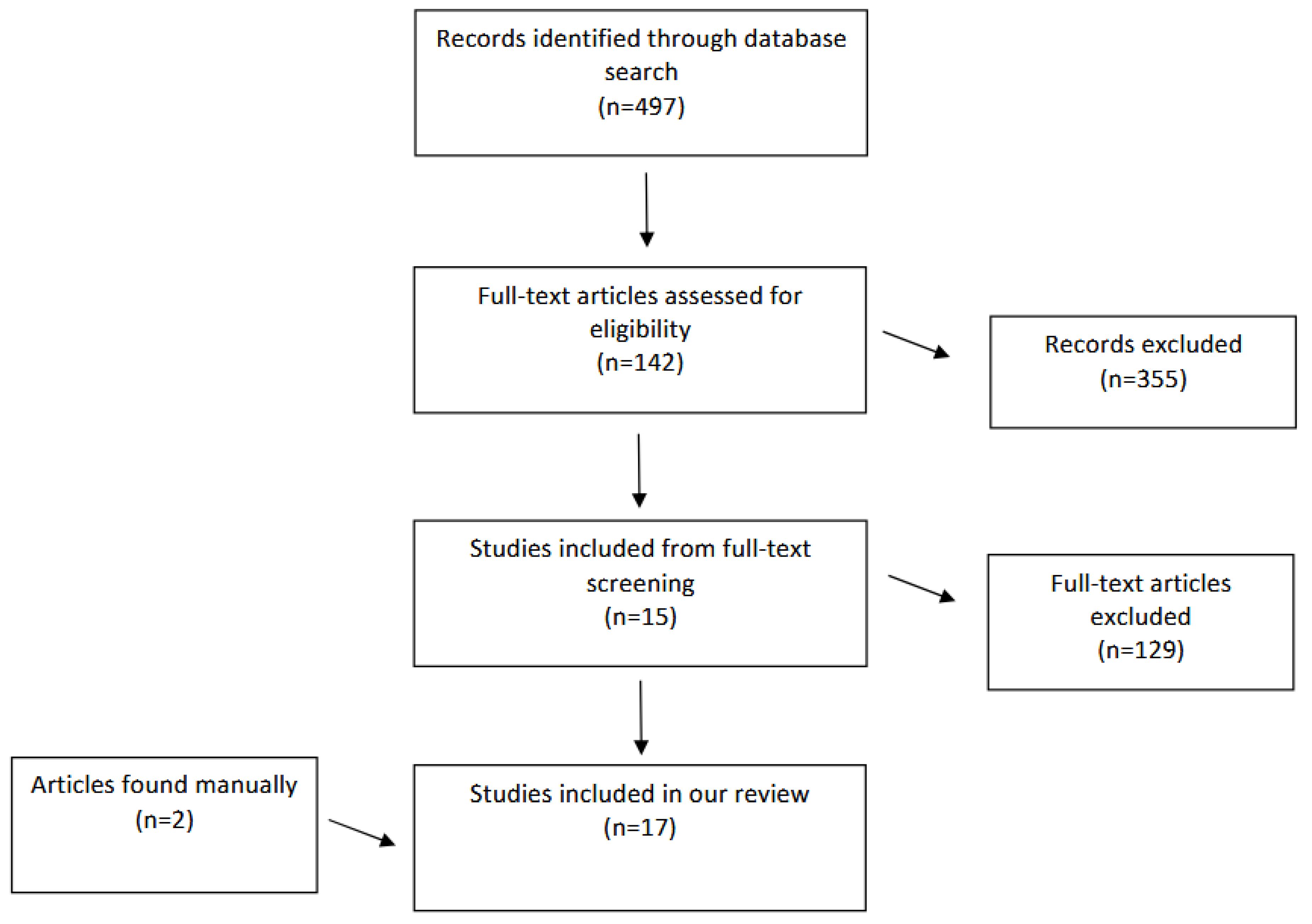
Literature Search
Characteristics of Included Studies
| Studies (year) | Study type | Intervention | Study participants | Results | Adverse effects | Notes | ||
|---|---|---|---|---|---|---|---|---|
| NVA Improvement | % showing improvement | Mean pupil size | ||||||
| Kaufman[9] (2012) | Non-randomized prospective study | Pilocarpine 1% Carbachol 3% Brimonidine 0.2% Placebo (artificial tear) |
12 patients with presbyopia |
Pilocarpine alone: Improvement by 2.3J lines Pilocarpine with brimonidine: Improvement by 3J lines Carbachol: Improvement by 6.3J lines. Carbachol with brimonidine: Improvement by 6.3J lines. |
Minor ocular discomfort by 10-30% of patients including the placebo group | |||
| Benozzi et al[10] (2012) | Non-randomized prospective study | Pilocarpine 1% Diclofenac 0.1% |
100 patients with presbyopia aged 45-50 years | Improvement to J1 | 1% of patients complained of ocular burning and discomfort | The improvement in NVA was maintained for a period of 5 years | ||
| Allergan pharmaceutical[11] (2015) | Phase 2 randomized clinical trial | Pilocarpine (AGN-190584) Oxymetazoline (AGN-199201) |
65 patients with presbyopia with a mean age of 49.2 years | At least 2 lines improvement | In 70.6% of patients using AGN-190584, and 46.7% in patients using AGN-199201. | Eyelid retraction was seen in 26% of patients using oxymetazoline alone. | ||
| Krader and Feinbaum[12] (2015) | Case series | PresbiDrops (pilocarpine (0.247%) | 81 patients with presbyopia in the age group of 42-74 years | Improvement from 0.3J to 0.6J lines. | After treatment with 2 drops, the diameter decreased significantly from 3.77 mm to 2.63 mm. | No serious adverse reactions. 4 patients experience nausea, headache each which spontaneously resolved | ||
| Abdelkader[13] (2015) |
A prospective, double-masked, randomized, placebo-controlled clinical trial | Carbachol 2.25% Brimonidine 0.2% |
48 patients with presbyopia aged between 43 and 56 years | Improvement in all subjects who received carbachol plus brimonidine drops (p < 0.0001) In the >= 50-year-old group, improved significantly from 7.68J to 4.75J at 8 hr (p < 0.0001) In the < 50-year-old group, improved significantly from 6.29J to 4.64J at 8 hr (p < 0.0001) |
In the >=50-year-old group, decreased significantly from 4.77±0.47 mm to 3.48±0.36 mm at 8 hr (P<0.0001) In the < 50-year-old group, decreased significantly from 4.72±0.51 mm to 3.42±0.48 mm at 8 hr (P<0.0001), |
Mild burning (3.3%) in 1 subject Headache (10%) Low luminosity (3.3%) in 1 subject |
||
| Abdelkader and Kaufman[14] (2016) |
A prospective, double-masked, randomized, controlled clinical trial | Carbachol 3% Brimonidine 0.2% |
10 patients with presbyopia aged between 42 and 58 years | Statistically significant improvement was achieved in all subjects who received combined 3% carbachol and 0.2% brimonidine compared with those who received separate forms of carbachol alone or brimonidine alone (P < 0.0001) Improved from 8.6J ± 1.5 before treatment to 2.3J ± 0.5 at 8 h post-treatment (P<0.01) |
Decreased significantly from 4.3 ± 0.5 mm to 2.1 ± 0.3 mm at 8 h post-treatment (P < 0.0001). | None reported | ||
| Abdelkader[15] (2018) |
A prospective, double-masked, randomized, placebo-controlled clinical trial | Carbachol 3% Brimonidine 0.2% |
40 pseudophakic patients with presbyopia aged between 30 and 80 years | In the treatment group, improved significantly from 7.5J ± 1 before treatment to 2.35J ± 0.49 at 8 hours posttreatment (p < 0.0001) | Dropped significantly from 4.1 ± 0.5 mm to 2.5 ± 0.4 mm at eight hours following drops (p < 0.0001). |
None reported | ||
| Vargas et al[16] (2019) | Consecutive, non-randomized, interventional clinical study | FOV Tears: pilocarpine (0.247%) phenylephrine (0.78%) polyethylene glycol (0.09%) nepafenac (0.023%) pheniramine (0.034%) naphazoline (0.003%) |
117 presbyopia patients in the age group of 41 to 60 years were recruited | Improved from 0.35 LogMAR to 0.16 LogMAR at 2 h after use of the eye drop (p = 0.000) | 92.3% of patients |
Decreased significantly under photopic conditions (p = 0.001), from 3.3 mm to 3.05 mm at 2 h after treatment. Also decrease significantly under scotopic conditions (p = 0.000), from 4.9 mm to 3.9 mm at 2 h after treatment |
14 patients (11.9%) complained of headache | |
| Benozzi et al[17] (2021) | Non-randomized case-series retrospective study | Patented formulation: pilocarpine and diclofenac preservative-free eye drops (Benozzi method; US 8.524.758 B2- EP1.938.839 B1) | 148 patients, aged 40 to 60. | At baseline, the NVA for the different groups were between 3J and 8J which was improved to 1J to 2J. | Decrease in light perception (24.6%) Headaches (11.3%) Ocular surface burning (6.6%) Side effects were spontaneously resolved. |
|||
| Price et al[18] (2021) | Two concurrent Phase 2, double-masked, randomized, vehicle-controlled studies, 1 short-term and 1 extended study |
Various concentrations and combinations of Pilocarpine (0%, 0.5% 1.0%, and 1.5%) and oxymetazoline (0%, 0.0125%, 0.05%, and 0.125%). | 163 presbyopia patients were recruited in the short-term study and 151 patients in the extended study. Mean age 48.6 years. | In the short and extended term studies, pilocarpine produced a significant dose response in the average increase of letters (P <0.001). |
All treatment groups had a reduction in diameter | The most common adverse event was headache (<5% when Pilo was dosed alone in the short-term study; up to 28.1% in the High OU group of the extended study) |
A dose response was seen as early as 15 minutes post administration, with peak effect at 1 hour. Peak improvement increased from day 1 to day 14 and was maintained up to day 28. | |
| Waring et al[19] (2022) | Vehicle-controlled, participant- and investigator-masked, randomized, Phase 3 clinical study, GEMINI 1 | AGN-190584, an optimized topical formulation of pilocarpine 1.25% (Vuity) or AGN-190584 formulation vehicle | Individuals with presbyopia, aged 40 to 55. 163 randomized to treatment. 160 randomized to vehicle | The proportion of participants with improvement of 3 or more lines was statistically significantly higher with AGN-190584 treatment compared with vehicle on day 30. | The most common adverse event was headache (23% in treatment group), followed by visual impairment (7% in treatment group). | |||
| Jackson et al[20] (2022) | In Vitro and Clinical Pilot Study | Pilocarpine 1.25% in the proprietary vehicle (Vuity) and a generic 1% Pilocarpine | 5 presbyopia patients aged 26 to 56. | 1 adverse event of brow ache was reported in the Optimized Formulation with 1.25% pilocarpine while 8 adverse events including eye pressure/pain, brow ache, vision blur, stinging, itching, and light sensitivity was reported in the Generic Formulation with 1% pilocarpine. | This study primarily assessed the side effects of the Optimized Formulation compared to Generic. | |||
| Eton et al[21] (2022) | Case Report | Pilocarpine 1.25% | 74 and 68 year old men with pre-existing retinal detachment risk factors | Unilateral retinal detachment occurring within 10 days of initiation of pilocarpine for treatment of presbyopia. | ||||
| Amarikwa et al[22] (2022) | Case Report | Pilocarpine 1.25% | 65 year old woman | Developed vitreomacular traction immediately following first administration of pilocarpine. Follow-up: The associated structural changes and scotoma persisted at follow-up, four weeks later. |
||||
| Al-Khersan et al[23] (2022) | Case Report | Pilocarpine 1.25% | 47 and 46 year old men | 47 year old: Bilateral Retinal detachment following 1 month of pilocarpine 1.25% drop use for presbyopia treatment. 46 year old: Unilateral Retinal detachment following 5 weeks of pilocarpine 1.25% drop use for presbyopia treatment. |
||||
| Vejarano et al[24] (2023) | Case series | FOV Tears: pilocarpine (0.247%) phenylephrine (0.78%) polyethylene glycol (0.09%) nepafenac (0.023%) pheniramine (0.034%) naphazoline (0.003%) |
363 participants with presbyopia aged 40-70 | Mean spherical equivalent (SE) changed significantly (− 0.17 Diopters) after instillation of the FOV Tears formulation (p < 0.001). logMAR NVA improved significantly by nearly two lines (p < 0.01). |
Diameter of the scotopic pupil decreased significantly by 0.97 ± 0.98 mm (p < 0.001) Diameter of the photopic pupil decreased by a non-significant amount (0.07 ± 0.69 mm). |
None mentioned | ||
| Kannarr et al[25] (2023) | Randomized (1:1), vehicle-controlled, double-masked, multicenter, phase 3 study, VIRGO |
Pilocarpine 1.25% (in AGN-190584 vehicle; Vuity) | Individuals with presbyopia, aged 40 to 55. 114 randomized to treatment. 116 randomized to vehicle | The proportion of participants who gained ≥3 lines in NVA on Day 14 was significantly greater with pilocarpine HCl 1.25% BID than vehicle. |
Pilocarpine HCl 1.25% twice daily significantly reduced pupil diameter in nondominant eyes under mesopic conditions (≥1.23 mm) compared to vehicle ( ≤0.08 mm) across all post-dose time points at all visits | Most common adverse reactions reported in >5% of participants were headache and eye irritation | ||
Discussion
Pilocarpine Formulations and Use of NSAIDs
Near Visual Acuity Improvements
Adverse Effects
Conclusion and Future Steps
Declaration of Conflicting Interests
Funding
References
- Grzybowski, A.; Markeviciute, A.; Zemaitiene, R. A Review of Pharmacological Presbyopia Treatment. Asia-Pacific J. Ophthalmol. 2020, 9, 226–233. [Google Scholar] [CrossRef]
- Glasser, A.; Kaufman, P.L. The mechanism of accommodation in primates. Ophthalmology 1999, 106, 863–872. [Google Scholar] [CrossRef]
- Holden, B.A.; Fricke, T.R.; Ho, S.M.; Wong, R.; Schlenther, G.; Cronjé, S.; Burnett, A.; Papas, E.; Naidoo, K.S.; Frick, K.D. Global Vision Impairment Due to Uncorrected Presbyopia. Arch. Ophthalmol. 2008, 126, 1731–1739. [Google Scholar] [CrossRef]
- Charman, W.N. Developments in the correction of presbyopia I: spectacle and contact lenses. Ophthalmic Physiol. Opt. 2014, 34, 8–29. [Google Scholar] [CrossRef]
- Alió, J.; Balgos, M.T.D.; Vargas, V. Correction of presbyopia: An integrated update for the practical surgeon. Taiwan J. Ophthalmol. 2018, 8, 121–140. [Google Scholar] [CrossRef]
- Wilkie, J.; Drance, S.M.; Schulzer, M. The Effects of Miotics On Anterior-Chamber Depth. Arch. Ophthalmol. 1969, 68, 78–83. [Google Scholar] [CrossRef]
- A Croft, M.; Kaufman, P.L.; Erickson-Lamy, K.; Polansky, J.R. Accommodation and ciliary muscle muscarinic receptors after echothiophate. Invest Ophthalmol Vis Sci. 1991, 32, 3288–97. [Google Scholar]
- Schalnus, R. Topical Nonsteroidal Anti-Inflammatory Therapy in Ophthalmology. Ophthalmologica 2003, 217, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S. Addressing presbyopia pharmacologically. Ophthalmology Times, https://www.ophthalmologytimes.com/article/addressing-presbyopia-pharmacologically (2012, accessed 23 February 2023).
- Benozzi, J.; Benozzi, G.; Orman, B. Presbyopia: a New Potential Pharmacological Treatment. Med Hypothesis Discov Innov Ophthalmol. 2012, 1, 3–5. [Google Scholar] [PubMed]
- Allergan. Safety and Efficacy of AGN-199201 and AGN-190584 in Patients with Presbyopia. ClinicalTrials.gov Identifier: NCT02197806, https://clinicaltrials.gov/ct2/show/NCT02197806 (2015, accessed 15 March 2023).
- Krader CG, Feinbaum C. Simple solution for presbyopia: topical agent acts by reducing pupil size to increase depth of focus. Ophthalmology Times, https://www.ophthalmologytimes.com/article/simple-solution-presbyopia (2012, accessed 25 February 2023).
- Abdelkader, A. Improved Presbyopic Vision With Miotics. Eye Contact Lens: Sci. Clin. Pr. 2015, 41, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, A.; Kaufman, H.E. Clinical outcomes of combined versus separate carbachol and brimonidine drops in correcting presbyopia. Eye Vis. 2016, 3, 31. [Google Scholar] [CrossRef]
- Abdelkader, A. A novel pharmacological treatment of pseudophakic presbyopia. Int J Ophthalm Res. 2018, 4. [Google Scholar]
- Vargas, V.; Vejarano, F.; Alió, J.L. Near Vision Improvement with the Use of a New Topical Compound for Presbyopia Correction: A Prospective, Consecutive Interventional Non-Comparative Clinical Study. Ophthalmol. Ther. 2019, 8, 31–39. [Google Scholar] [CrossRef]
- Benozzi, G.; Cortina, M.E.; Gimeno, E.; Vantesone, D.L.; Solas, A.E.; Lorda, G.M.; Facal, S.; Leiro, J.; Orman, B. A multicentric study of pharmacological treatment for presbyopia. Graefe's Arch. Clin. Exp. Ophthalmol. 2021, 259, 2441–2450. [Google Scholar] [CrossRef]
- Price, F.W.; Hom, M.; Moshirfar, M.; et al. Combinations of Pilocarpine and Oxymetazoline for the Pharmacological Treatment of Presbyopia: Two Randomized Phase 2 Studies. Ophthalmol Sci. 2021, 1, 100065. [Google Scholar] [CrossRef]
- Waring, G.O.; Price, F.W., Jr; Wirta, D.; et al. Safety and Efficacy of AGN-190584 in Individuals With Presbyopia: The GEMINI 1 Phase 3 Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Giyanani, J.; Shabaik, Y.; Penzner, J.; Gore, A.V.; Robinson, M.R.; Waring, G.O. In Vitro and In-Eye Comparison of Commercial Pilocarpine Ophthalmic Solution and an Optimized, Reformulated Pilocarpine for Presbyopia Treatment. Ophthalmol. Ther. 2022, 11, 869–879. [Google Scholar] [CrossRef]
- Eton, E.A.; Zhao, P.Y.; Johnson, M.W.; Rao, R.C.; Huvard, M.J. Rhegmatogenous retinal detachment after initiation of pilocarpine hydrochloride ophthalmic solution 1.25% for treatment of presbyopia. Retin. Cases Brief Rep. 2022, 18, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Amarikwa, L.; Michalak, S.M.; Caul, S.; Mruthyunjaya, P.; Rahimy, E. Vitreofoveal Traction Associated With Pilocarpine for Presbyopia. Ophthalmic Surgery, Lasers Imaging Retin. 2022, 53, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Al-Khersan, H.; Flynn, H.W.; Townsend, J.H. Retinal Detachments Associated With Topical Pilocarpine Use for Presbyopia. Arch. Ophthalmol. 2022, 242, 52–55. [Google Scholar] [CrossRef]
- Vejarano, F.; Alió, J.; Iribarren, R.; Lança, C. Non-Miotic Improvement in Binocular Near Vision with a Topical Compound Formula for Presbyopia Correction. Ophthalmol. Ther. 2023, 12, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Kannarr, S.; El-Harazi, S.M.; Moshirfar, M.; Lievens, C.; Kim, J.L.; Peace, J.H.; Safyan, E.; Liu, H.; Zheng, S.; Robinson, M.R. Safety and Efficacy of Twice-Daily Pilocarpine HCl in Presbyopia: The Virgo Phase 3, Randomized, Double-Masked, Controlled Study. Arch. Ophthalmol. 2023, 253, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Sheeladevi, S.; Seelam, B.; Nukella, P.B.; Borah, R.R.; Ali, R.; Keay, L. Prevalence of refractive errors, uncorrected refractive error, and presbyopia in adults in India: A systematic review. Indian J. Ophthalmol. 2019, 67, 583. [Google Scholar] [CrossRef]
- Fricke, T.R.; Tahhan, N.; Resnikoff, S.; Papas, E.; Burnett, A.; Ho, S.M.; Naduvilath, T.; Naidoo, K.S. Global Prevalence of Presbyopia and Vision Impairment from Uncorrected Presbyopia: Systematic Review, Meta-analysis, and Modelling. Ophthalmology 2018, 125, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Balal S, Gil-Cazorla R, Naroo SA, et al. Refractive surgery’s holy grail. Eyedrops for presbyopia. Myth or medicine? The Ophthalmologist, March 2017, p.18-29.
- Benozzi, G.; Perez, C.; Leiro, J.; Facal, S.; Orman, B. Presbyopia Treatment With Eye Drops: An Eight Year Retrospective Study. 2020, 9, 25.
- Alio, J.L.; Plaza-Puche, A.B.; Férnandez-Buenaga, R.; Pikkel, J.; Maldonado, M. Multifocal intraocular lenses: An overview. Surv. Ophthalmol. 2017, 62, 611–634. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





