1. Introduction
Neonatal morbidity and mortality remain unacceptably high worldwide, particularly among preterm infants, in whom immature organ systems and a fragile immune response predispose to life-threatening complications. Early-onset neonatal sepsis (EONS) and necrotizing enterocolitis (NEC) are among the most severe and socially significant disorders in this population, contributing substantially to both acute mortality and long-term neurodevelopmental and somatic sequelae [
1,
2,
3,
4,
5,
6]. Both conditions arise at the interface between an immature host and a rapidly changing microbial environment, and there is growing interest in how gut-derived metabolites, particularly short-chain fatty acids (SCFAs), may modulate susceptibility and clinical trajectories [
7,
8].
EONS, typically defined as sepsis occurring within the first 72 hours of life [
6], remains a major cause of death and severe morbidity in preterm and preterm neonates. Its pathogenesis reflects a complex interplay between vertical and early postnatal microbial exposures, inadequate innate immune responses, and impaired epithelial and endothelial barrier function [
9]. Recent work increasingly points to the gut as an important reservoir and signalling hub in neonatal sepsis: dysbiosis, delayed colonisation by beneficial taxa, enrichment of opportunistic pathogens, and altered microbial metabolic output are all associated with sepsis risk [
7,
10]. Among microbial metabolites, SCFAs and related fatty acids have attracted particular attention in the context of systemic inflammation and septic shock [
11,
12,
13]. However, most available data come from cross-sectional measurements or from non-intestinal compartments, and, to date, the temporal dynamics of fecal SCFAs during EONS have not been comprehensively characterised.
NEC, in turn, remains one of the most feared gastrointestinal emergencies of the neonatal period, with multifactorial pathogenesis involving prematurity, enteral feeding, microbial imbalances, and exaggerated inflammatory responses [
1,
2,
3]. Disturbances in host–microbiota interactions and barrier integrity are central to NEC development, and SCFAs have been proposed as key mediators in this triad [
14,
15,
16]. Early clinical stages of NEC suspicion (Bell stage I) are now recognised as a distinct and clinically relevant phenotype rather than mere false alarms [
17,
18,
19]. Metabolomic and microbiome studies indicate that infants in this “NEC-risk” state already exhibit altered microbial composition and metabolic signatures, even when the disease does not progress to overt intestinal necrosis [
8,
17]. Importantly, some of these early intestinal disturbances may reflect broader systemic inflammatory vulnerability, conceptually linking NEC-risk states and sepsis-prone phenotypes without equating the two conditions [
20].
SCFAs—including acetic, propionic, and butyric acids—and related branched-chain fatty acids (BCFAs) exert multiple homeostatic functions at the mucosal interface. They provide energy substrates for colonocytes, regulate epithelial proliferation and differentiation, influence tight-junction protein expression, and modulate cytokine production and immune-cell activation [
14,
15,
21,
22,
23,
24]. BCFAs such as isobutyric and isovaleric acids, which arise from proteolytic fermentation, offer additional insight into microbial metabolic activity in the immature gut [
25,
26]. Imbalances in these metabolites have been implicated in inflammatory bowel diseases and in microbiota disturbances in preterm infants [
27,
28,
29,
30], suggesting that SCFA patterns may serve as sensitive indicators of host–microbe dysregulation.
Despite this growing body of evidence, a critical gap remains in our understanding of how fecal SCFA profiles evolve over time in preterm infants who develop EONS. Existing studies in neonatal sepsis have largely focused on single timepoints, heterogeneous septic phenotypes, or systemic (plasma/serum) markers [
11,
12,
13,
31,
32], providing limited insight into the intestinal metabolic trajectories that accompany early systemic infection. It is therefore unclear whether EONS is associated with reproducible, time-dependent shifts in the intestinal SCFA and BCFA pools, and whether such patterns might reflect either vulnerability, compensatory adaptation, or metabolic exhaustion of the gut ecosystem.
In this context, we designed a longitudinal cohort study originally motivated by the pathophysiology of NEC, but implemented in a clinical setting where NEC incidence in the targeted gestational window was unexpectedly low. This circumstance, together with the shared axes of gut–immune interaction in NEC and EONS, provided an opportunity to focus on preterm infants with and without EONS while preserving the high-resolution, time-structured sampling framework. The present study therefore aimed to characterise absolute and relative fecal SCFA concentrations across the first month of life in preterm neonates initially enrolled as at high risk for NEC but subsequently stratified by EONS status. By performing dynamic, timepoint-specific analyses rather than relying on single measurements, we sought to determine whether EONS is accompanied by distinct intestinal SCFA signatures. To our knowledge, this is the first longitudinal assessment of a comprehensive fecal SCFA and BCFA panel in the context of early-onset neonatal sepsis, laying groundwork for future studies that will extend this approach to dedicated NEC cohorts and refined risk phenotypes.
2. Materials and Methods
2.1. Study Design
This prospective longitudinal cohort pilot study was conducted at the Neonatal Intensive Care Unit of the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology from June 2024 to February 2025. Preterm neonates (gestational age ⩽ 32 weeks) were enrolled; the study was approved by the local ethics committee (Protocol 04, April 18, 2024), and written informed consent was obtained from parents or legal guardians.
Eligible infants were admitted to the NICU with gestational age ⩽ 32 weeks; exclusion criteria included severe congenital malformations, chromosomal abnormalities, inherited metabolic disorders, hydrops fetalis. In addition, we collected stool samples in more mature neonates (GA>32 weeks) if they developed signs suspicious for NEC (one or more signs: pronounced abdominal distension, pain on abdominal palpation, frequent regurgitation, blood in the stool, lack of intestinal peristalsis). The samples were taken on the day these signs appeared . NEC diagnosis was based on the modified Bell’s criteria [
33] and assessed by two independent clinical experts (I.N., O.K.J.) before obtaining the results of SCFA testing.
Given real–world enrollment, the original NEC–centered plan was adapted to focus on
early–onset neonatal sepsis (EONS) versus absence of sepsis while retaining an exploratory NEC–risk subgroup. Early–onset neonatal sepsis was defined as the presence of at least two clinical symptoms and at least two laboratory signs in the presence of, or as a result of, suspected or proven infection (positive culture, microscopy, or polymerase chain reaction) within the first 72 h of life, according to the criteria listed in the expert report on neonatal and paediatric sepsis by the European Medicines Agency [
34]. Infants fulfilling these criteria comprised the EONS group; infants without clinical or laboratory evidence of sepsis during the observation windows comprised the non–EONS group. All cases of EONS were evaluated independently by two experts (I.N. and O.K.J.) before the results of SCFA testing became available. All neonates were followed up until discharge from the NICU. The small NEC–risk subgroup (Bell stage I during hospitalization) was analyzed descriptively for hypothesis generation and was not included in the between–group comparisons in
Table 1.
Fecal sampling followed predefined postnatal windows centered at approximately day of life (DoL) 3, 7, 14, 21, and 28 (hereafter TP1–TP5); when multiple samples occurred within a window, the specimen closest to the window’s median timing was selected for primary analyses.
The comparative analysis set comprised preterm infants: EONS () and non–sepsis (). Continuous variables are reported as median (Q1–Q3) and were compared using the Mann–Whitney U test; categorical variables are shown as n (%) and were compared using two–sided Fisher’s exact test, with considered significant.
Clinical and demographic characteristics are summarized in
Table 1. Groups did not differ in gestational age at birth (31 [28.5–32] vs. 31.2 [29.3–32] weeks;
) or sex distribution (
). Birth weight was lower in EONS (1121 [840–1329] vs. 1440 [1092–1679] g;
). Apgar scores were lower in EONS at 1 min (
) and 5 min (
). Surfactant treatment and invasive mechanical ventilation were more frequent in EONS (
and
, respectively). Length of NICU stay was longer in EONS (24 [15–35] vs. 11 [7–25] days;
). Lethal cases were observed only in the EONS group (4 cases, 22% vs. 0%;
). Other perinatal and feeding variables were comparable between groups (all
).
Notwithstanding this design, we additionally characterized, for descriptive and contextual purposes, a small ancillary cohort of infants who developed NEC (Bell stage II) with gestational age
weeks. These cases were reviewed under the same ethics approval and are presented here solely as a post hoc, hypothesis–generating extension; they were not included in the primary EONS vs. non–sepsis longitudinal analyses or in
Table 1.
2.2. Chemicals and Reagents
For the quantitative GC–MS analysis of SCFAs in feces, analytical-grade standards and reagents were obtained from reliable suppliers (Sigma-Aldrich, Saint Louis, MO, USA). The reference standards included acetic (AA, ≥99%), propionic (PA, ≥99%), butyric (BA, ≥99%), valeric (VA, ≥99%), isobutyric (iBA, ≥99%), isovaleric (iVA, ≥99%), 2-methylbutyric (2mBA, ≥99%), 2-methylvaleric (2mVA, ≥99%), 3-methylvaleric (3mVA, ≥99%), hexanoic (HA, ≥99%), and octanoic (OA, ≥99%) acids.
To improve quantification accuracy and correct for matrix effects, internal standards (IS) were used. AA-d4 (≥99.5%) served as the internal standard for acetic acid, while BA-1,2-13C2 (≥98%) was applied for other SCFAs, including PA, BA, VA, iBA, iVA, 2mBA, 2mVA, 3mVA, HA, and OA.
Milli-Q water was used for the preparation and dilution of calibration and working solutions, while 2.0 M hydrochloric acid (HCl) was employed for acidification prior to extraction. Methyl tert-butyl ether (MTBE) served as the solvent for liquid–liquid extraction (LLE) of SCFAs, ensuring efficient recovery and phase separation.
All reagents and consumables were handled in accordance with standard laboratory protocols and quality-control procedures to ensure the reliability and reproducibility of analytical results [
35,
36].
2.3. Sample Collection
Naturally-excreted stool samples were collected on days 3, 7, 14, 21, and 28 of life, preferably before antibiotic therapy or the morning feeding. Samples were placed in sterile containers with inert plastic inserts to prevent contact with the diaper surface and minimize volatile loss. All samples were immediately frozen at -80 °C until analysis.
In infants who developed acute intestinal symptoms during hospitalization (e.g., cases later evaluated for suspected or confirmed NEC), stool samples were additionally collected within 24 h after symptom onset according to unit protocol. Enteral feeding was discontinued at the time of symptom appearance; therefore, the feeding characteristics reported in the Results correspond to the last 24 h of enteral nutrition prior to clinical deterioration. These cases were not part of the longitudinal comparison and are presented descriptively.
2.4. Sample Preparation
A 50–100 mg aliquot of native stool was weighed into 1.6–2.0 mL Eppendorf tubes. Milli-Q water (1000 µL per 100 mg stool) was added, and the exact dilution factor was determined gravimetrically. After GC–MS analysis, SCFA concentrations (µM) were recalculated to µmol per g of stool. Samples were vortexed, sonicated for 20 min at room temperature, vortexed again for 10 min, and centrifuged (5000 rpm, RT). Then 100 µL of supernatant was transferred to a 0.5 mL tube, 5 µL of internal standard solution (d4-acetic acid 103 µM, 13C2-butyric acid 102 µM) and 5 µL of 2 M HCl were added, followed by 200 µL of methyl tert-butyl ether (MTBE). After vortexing (10 min) and centrifugation (15000 rpm, 5 min), the upper organic phase was transferred to an autosampler vial for GC–MS analysis.
The protocol was adapted from previously published methods, demonstrating the applicability of water as a surrogate matrix and MTBE extraction after acidification. Kim et al. (2022) [
36] established a methodological basis validated across several bio-matrices, whereas our work [
35] applied and tested it on clinical stool and plasma samples. In this study, the protocol was further modified for neonatal stool, to reduce matrix effects and improve reproducibility.
2.5. Calibration Standards and Quality Controls
Calibration standards were prepared in Milli-Q water, with sample preparation identical to that of the stool extracts, beginning with a 100 µL aqueous aliquot. Stock solutions of the analytes were prepared in acetonitrile at the following concentrations: AA at 106 µM; PA, BA, and iBA acids at 105 µM; and VA, iVA, 2mBA, 2mVA, 3mVA, HA, and OA at 104 µM. These stock standards were stored at and serially diluted with water to create working solutions. The internal standards (IS) consisted of d4-AA (104 µM, stored at ) and 13C2-BA (10 mg/mL, stored at ). A working internal standard (ISTD) solution containing 103 µM d4-AA and 102 µM 13C2-BA was prepared, with 5 µL added to each sample.
Seven-point calibration curves and four levels of quality-control (QC) standards were prepared in Milli-Q water, which was selected as a surrogate matrix based on previous validation data [
35,
36] and confirmed by standard-addition tests in this study. Calibration and QC samples underwent identical preparation steps to fecal extracts, starting from the stage of 100 µL aliquot acidification and MTBE extraction. For AA, calibration levels were 2, 4, 10, 20, 40, 100, and 160 µM, with an additional high-level point at 2000 µM when needed to account for elevated concentrations. For PA, BA and iBA, calibration levels were 0.2, 0.4, 1, 2, 4, 10, and 16 µM, with an optional extended level of 200 µM for high-concentration samples. For other acids (2mBA, iVA, VA, 2mVA, 3mVA, HA, OA), levels were 0.02, 0.04, 0.1, 0.2, 0.4, 1, and 1.6 µM, optionally extended to 20 µM.
Calibration curves were constructed by plotting the peak area ratio of each SCFA to its corresponding internal standard against the analyte concentration, followed by linear regression. The linearity for each SCFA was confirmed by a coefficient of determination (R2) exceeding 0.995. The limit of detection (LOD) was calculated as 3.3×SD/b, where SD is the standard deviation of the Y-intercept and b is the slope of the regression curve. The limit of quantification (LOQ) was defined as 3×LOD. For several samples, the quantification was additionally verified using the standard addition method with four spiked concentration levels.
Calibration curves were constructed by plotting the peak area ratio of each SCFA to its corresponding internal standard against the analyte concentration, followed by linear regression (
Table 2). The linearity for each SCFA was confirmed by a coefficient of determination (R
2) exceeding 0.995. The limit of detection (LOD) was calculated as 3.3×SD/b, where SD is the standard deviation of the Y-intercept and b is the slope of the regression curve. The limit of quantification (LOQ) was defined as 3×LOD. For several samples, the quantification was additionally verified using the standard addition method with four spiked concentration levels.
2.6. GC–MS Analysis
Analyses were performed using an Agilent 7890B/5977B GC–MS system equipped with an HP-FFAP capillary column (30 m × 0.25 mm × 0.25 µm). Helium was used as the carrier gas at a constant flow of 1.7 mL/min. The oven temperature was programmed as follows: initial temperature 60 ∘C (held for 1.5 min), ramped at 20 ∘C/min to 240 ∘C, and held for 7.5 min. Electron ionization (EI) at 70 eV was used with a solvent delay of 4 min. Injector, ion source, and quadrupole temperatures were maintained at 240 ∘C, 230 ∘C, and 150 ∘C, respectively.
A preliminary series of full-scan measurements was performed to determine retention times and identify characteristic fragment ions by spectral matching with the NIST17 library [
37]. Subsequently, ion registration was carried out in SIM (Single Ion Monitoring) mode for quantitative determination of individual analytes (
Table 2). Quantification was performed in Selected Ion Monitoring (SIM) mode by targeting characteristic fragment ions for each analyte. The ion at m/z 43 was monitored for isobutyric acid (iBA), while the ion at m/z 60 was used for the simultaneous detection of acetic, butyric, valeric, isovaleric, and 3-methylvaleric acids. Propionic, 2-methylbutyric, and 2-methylvaleric acids were quantified using the ion at m/z 74. For the IS, the ions m/z 46 and 63 were selected for the deuterated standard d
4-acetic acid, and the ion m/z 62 was selected for
13C
2-butyric acid. Compound identity was confirmed by retention time and spectral comparison with authentic standards (NIST17). Method performance (R
2 > 0.995, LOD 0.5–1 µM, accuracy ±10 %, CV < 15 %) and extract stability at
up to 14 days were consistent with published data [
35,
36].
2.7. Statistical Analysis
All statistical analyses were performed in Python 3.13 using the packages pandas (data handling), NumPy (numerical operations), SciPy (non-parametric tests), scikit-learn (multivariate modelling), and matplotlib/seaborn (visualisation).
Continuous variables are summarised as median (Q1; Q3), and categorical variables as n (%). Between-group comparisons for clinical characteristics were carried out using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Statistical significance was defined as ; values with were interpreted as trends.
For fecal short-chain fatty acid (SCFA) analysis, both absolute concentrations and derived metrics were evaluated. Derived variables included:
relative fractions, (percentage of total SCFAs);
composite branched-chain indices, and ;
pairwise ratios of metabolites, .
For each analyte, timepoint-specific differences between the EONS and non-sepsis groups were assessed at TP1–TP5 (approximately 3, 7, 14, 21, and 28 days of life) using the Mann–Whitney U test. Infants evaluated for suspected or confirmed NEC formed a small exploratory subgroup; their SCFA profiles were summarised descriptively and were not included in inferential testing.
Multivariate analysis was performed using partial least squares–discriminant analysis (PLS–DA) implemented in scikit-learn. Variable Importance in Projection (VIP) scores were calculated for each metabolite and ratio; variables with VIP were considered informative contributors to group separation. Temporal patterns were visualised using boxplots, PLS–DA score plots, and median trend curves across days of life, with p-values annotated on timepoint-specific plots where appropriate.
2.8. Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee (2024, April 18, # 04). Informed consent was obtained from parents or legal guardians of all participants.
3. Results
3.1. Fecal SCFA Levels Across Postnatal Windows (All Patients)
Table 3 summarizes absolute fecal SCFA concentrations (µmol/g) for all enrolled preterm infants across predefined postnatal windows (TP1–TP5; ∼3, 7, 14, 21, and 28 days of life).
Acetic acid (AA) predominated at all timepoints, showing a steady increase from TP1 (median 360, IQR 222–1530) and TP2 (428, 310–971) toward substantially higher levels by TP3 (4286, 633–4989) and a marked peak at TP4 (21205, 8670–26372), followed by a moderate decline at TP5 (9843, 5543–24117).
Propionic acid (PA) demonstrated a more variable course, with modest levels at TP1–TP2 (17.3 and 10.8 µmol/g) and a pronounced rise by TP3 (34.4, 9.9–67.8), remaining elevated at TP4 (22.2, 20.0–77.5) and stabilizing thereafter (25.2, 14.5–27.1 at TP5).
Butyric acid (BA) exhibited a gradual and comparatively moderate increase (2.4, 2.8, 4.9, 6.2, and 4.3 µmol/g for TP1–TP5, respectively), with the highest interindividual variability observed after the third week of life.
Among minor SCFAs, isobutyric acid (iBA) remained relatively stable during the first two weeks (3.7–5.3 µmol/g at TP1–TP3) and tended to increase thereafter (4.8 at TP4–TP5). Valeric acid (VA) concentrations were low throughout the neonatal period, without a clear monotonic pattern (median range 1.6–3.3 µmol/g).
Isovaleric acid (iVA) and 2-methylbutyric acid (2mBA) were detected at submicromolar to low micromolar levels and displayed slight increases by the end of the first month (TP4–TP5).
In contrast, medium-chain acids hexanoic acid (HA) and octanoic acid (OA) showed higher absolute values and dynamic fluctuations. HA rose from 21.2 µmol/g at TP1 to transient peaks at TP3–TP4 (9.9–13.4), while OA peaked earlier at TP1 (15.7, 6.5–28.1) and gradually declined toward TP5 (6.6, 5.3–9.2).
Overall, total fecal SCFA output increased across the neonatal period, driven primarily by acetic acid and, to a lesser extent, propionic and butyric acids, whereas branched- and medium-chain acids contributed smaller, yet dynamically variable, fractions of the total pool. These aggregate patterns provide a developmental context for group-wise contrasts; comparisons between EONS and non-sepsis infants across the same time windows are presented in the subsequent sections.
Branched-chain acids and valeric acid exhibited low early values with late increases in both medians and dispersion: isobutyrate (iBA) rose from 3.63 (2.66–-4.72) at TP1 to 8.59 (3.06–-16.02) and 11.02 (3.28-–34.58) at TP4–TP5; isovalerate (iVA) from 0.44 (0.14–-1.39) at TP1 to 1.85 (0.68–3.80) and 2.91 (0.78–-8.65) at TP4–TP5; valerate (VA) from 1.88 (1.43–3.75) to 5.40 (2.26–-12.20) and 8.64 (2.62–-25.18). Medium-chain acids showed a similar late pattern: hexanoate (HA) and octanoate (OA) remained low through TP1–TP3 and rose at TP4–TP5 (HA to 83.28 and 136.64; OA to 24.69 and 37.73, medians).
Across several analytes, the interquartile ranges and observed ranges widened markedly after TP3 (e.g., AA and PA), indicating increasing between-infant heterogeneity as feeding advances and postnatal adaptation progresses. These distributions provide the reference context for subsequent groupwise and trajectory analyses in this cohort.
3.2. Between-Group Differences: EONS vs. Non-Sepsis (Day-Specific Analyses)
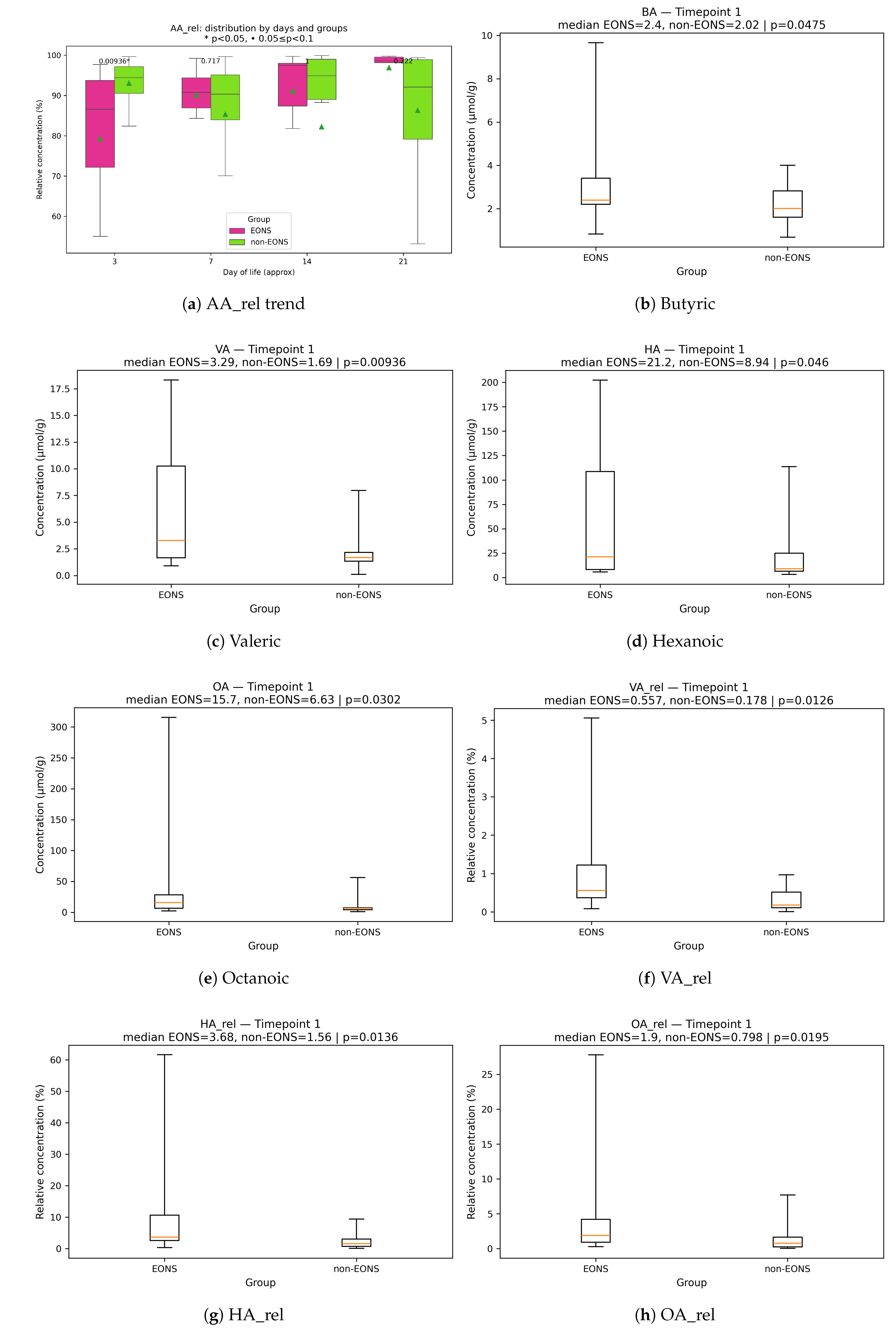
At TP1 (∼3 DoL), among infants originally enrolled as high risk for NEC but analyzed here for EONS, we observed a pronounced redistribution of the fecal SCFA pool (
Table 4). The median relative fraction of acetate (AA_rel) was significantly lower in EONS (86.6 % vs 94.5 %,
), consistent with the temporal increase of acetate in EONS versus near-stable levels in non-sepsis infants (trend,
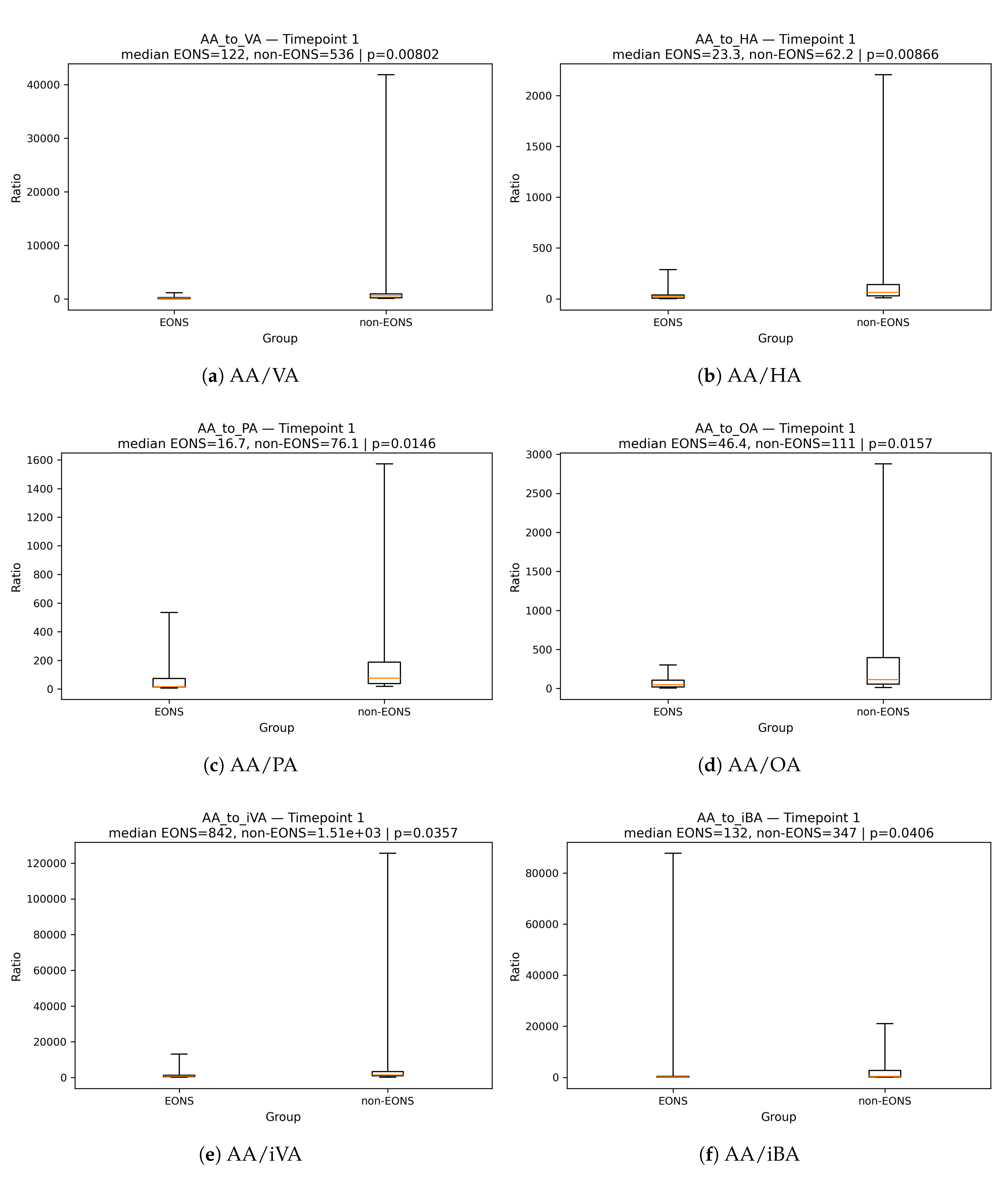
Figure 1). Concordantly, acetate-to-non-acetate ratios were consistently reduced in EONS, indicating a redistribution of the SCFA pool toward non-acetate and branched-chain acids. The strongest differences were observed for AA/VA (121.7 vs 535.6,
), AA/HA (23.26 vs 62.15,
), AA/PA (16.72 vs 76.11,
), AA/OA (46.35 vs 111.27,
), AA/iVA (842.45 vs 1511.37,
), and AA/iBA (131.64 vs 347.45,
), with a similar tendency for AA/BA (
) (
Figure A1–
Figure A1).
Non-acetate components predominated in EONS at TP1: butyric (BA,
vs
M,
), valeric (VA,
vs
M,
), hexanoic (HA,
vs
M,
), and octanoic acids (OA,
vs
M,
) were elevated, with concordant increases in their relative fractions (VA_rel,
; HA_rel,
; OA_rel,
;
Figure 1Figure 1).
The combined relative contribution of branched SCFAs showed an early elevation in EONS and a decline in statistical significance after week 2 (trend, Figure ); at TP1, individual branched fractions demonstrated only tendencies (PA_rel,
; iBA_rel,
; iVA_rel,
) and are therefore not illustrated here (see
Table 4).
At TP2 (∼7 DoL), between-group contrasts attenuated: no comparisons reached statistical significance (all
). A single borderline trend was observed for the PA/BA ratio, which was lower in EONS (
;
Table 4). The temporal patterns persisted—AA_rel continued to increase in EONS relative to non-sepsis (
Figure 1), and the combined branched fraction remained qualitatively higher without statistical support at this timepoint (Figure ). By TP3 (∼14 DoL), selective differences were confined to a single branched-chain ratio: BA/2mBA was higher in EONS (8.90 vs 4.80;
), consistent with a transient reweighting among BCFAs rather than a global pool shift; other comparisons, including iBA/2mBA, were not significant (
Table 4). By TP4 (∼21 DoL), early-case differences largely attenuated, with a single residual contrast: valeric acid (VA) was lower in EONS (1.88 vs 3.34
M;
). Weak tendencies were also observed for OA/VA (higher in EONS;
) and for PA-based ratios (PA/iVA and PA/2mBA; both
). Overall, EONS-associated alterations appear predominantly
transient: a broad acetate-to-non-acetate redistribution at TP1, narrowing to a selective BCFA-related ratio by TP3, and minimal residual differences by TP4 (see
Table 4).
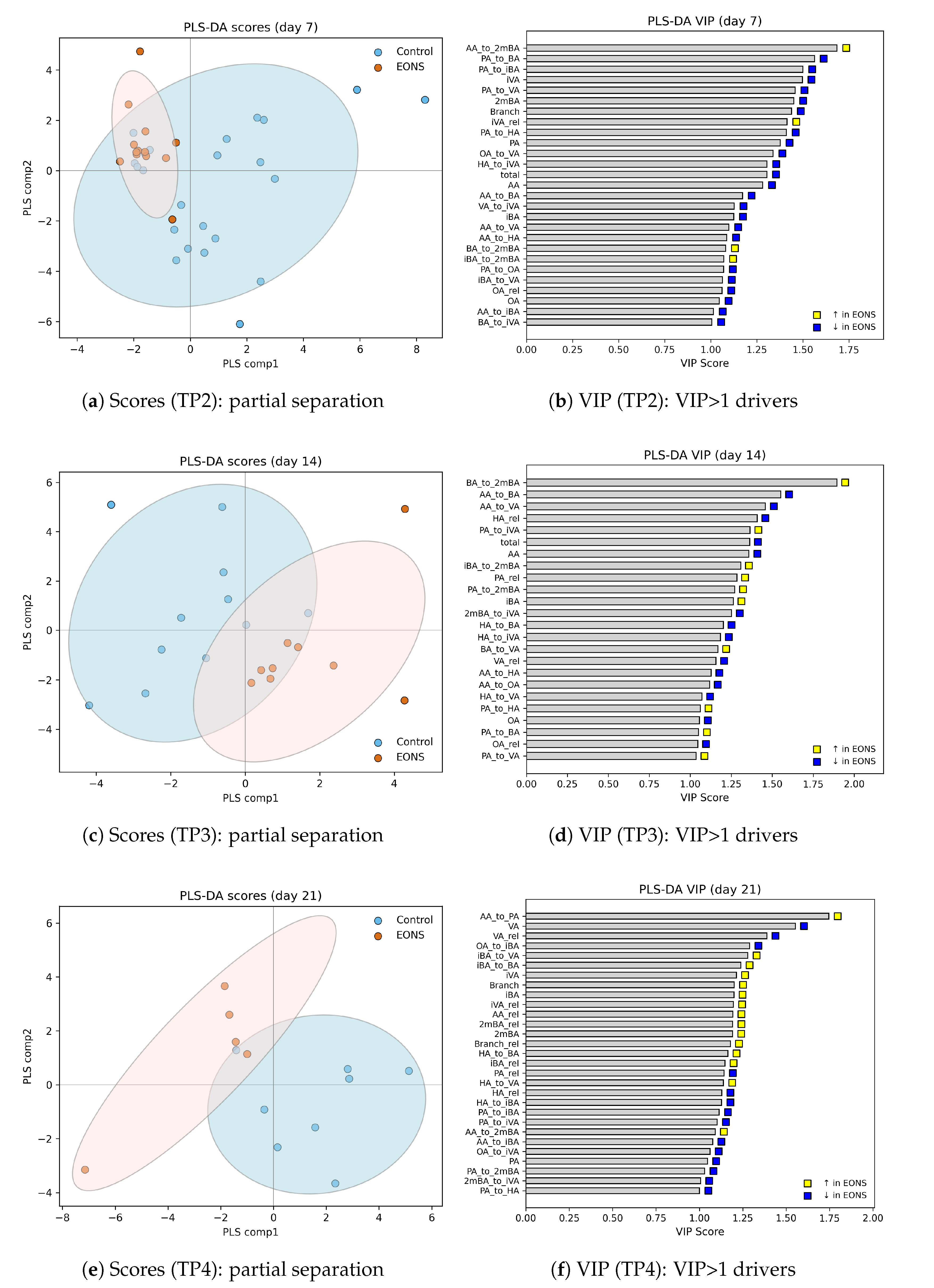
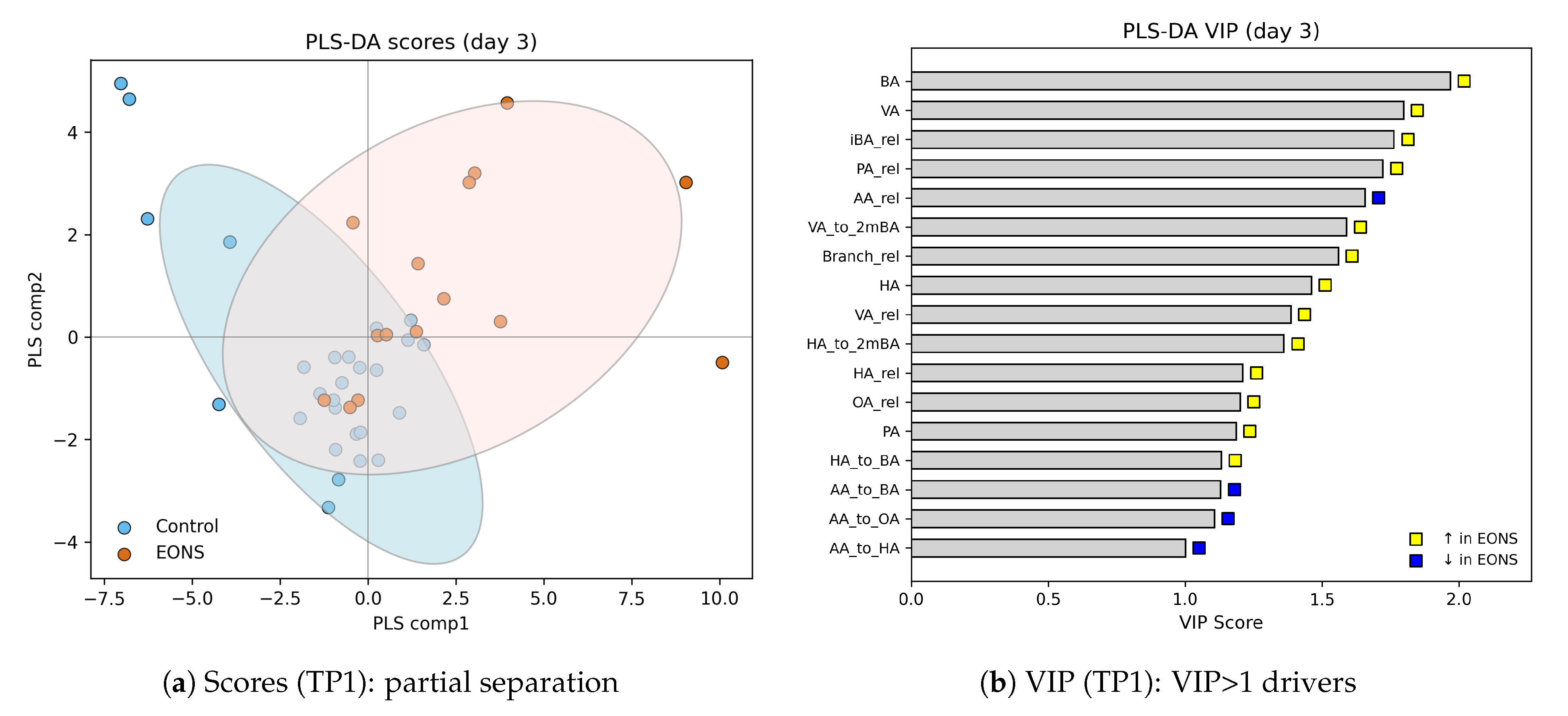
3.3. Multivariate Discrimination at the Earliest Timepoint (PLS–DA at TP1)
To complement the day-specific tests, we performed timepoint-wise PLS–DA (EONS vs. non-EONS). Because the strongest redistribution of the SCFA pool occurred at the earliest window (TP1, ≈3 DoL), we report TP1 in the main text, whereas PLS–DA panels for TP2–TP4 (scores and VIP) are provided in Supplementary Materials (
Figure A2).
At TP1, the PLS–DA scores showed a clear partial separation between groups (
Figure 2), in agreement with the univariate contrasts. The VIP ranking (
Figure 2) highlighted multiple non-acetate features among the strongest contributors to discrimination, including butyric and valeric acids (BA, VA), the relative abundance of branched-chain acids (
,
), propionic and valeric fractions (
,
), and several BCFA-related ratios (e.g.,
). Acetic acid contributed in the opposite direction, with
decreased in EONS. Overall, the multivariate pattern at TP1 reflects a coordinated shift away from acetic acid toward propionate-, valerate-, and BCFA-related components of the fecal SCFA pool.
3.4. Fecal SCFA Profiles in Older Infants with Confirmed and Suspected NEC
A small auxiliary subgroup of five infants was assessed due to clinical suspicion of necrotizing enterocolitis (NEC). Three of these neonates were the only confirmed NEC cases among all infants admitted to the NICU during the study period (n = 540). One infant demonstrated intestinal bleeding associated with infection during the perinatal period, and one infant had suspected NEC (Bell stage Ib). The clinical characteristics of these five infants are summarised in
Table 5. Clinical signs of intestinal pathology in this subgroup appeared between day 4 and day 17 of life.
Fecal samples for SCFA analysis in this subgroup were collected within 24 h after the onset of intestinal symptoms in all five infants. Thus, the SCFA measurements reflect the early phase of NEC suspicion or perinatal intestinal infection rather than a baseline, asymptomatic state. At the time of sampling, these infants were more mature than the main cohort in terms of gestational age (median 36.6 weeks [36.0; 37.0]). Enteral feeding was discontinued at the onset of symptoms; therefore, the feeding type and volume indicated in
Table 5 refer to the last 24 h of enteral nutrition prior to clinical deterioration (i.e., the day preceding stool collection). Their fecal SCFA concentrations (
Table 6) demonstrated substantial heterogeneity across all measured acids. Acetic acid (AA) reached high values (median 7369 µmol/g), while propionic acid (PA) showed wide interindividual variation (19.7–4111.2 µmol/g). Branched-chain fatty acids—including isobutyric (iBA), isovaleric (iVA), and 2-methylbutyric acid (2mBA)—were consistently detectable, and medium-chain acids such as hexanoic (HA) and octanoic acid (OA) exhibited the highest variability, particularly in infants with perinatal intestinal infection or suspected NEC. In descriptive terms, infants with confirmed NEC (Bell IIa–IIb) tended to have lower PA and branched-chain SCFA levels, as well as lower HA and OA concentrations, whereas the highest PA, branched-chain SCFA, and MCFA values were observed in the subgroup with perinatal infection-related intestinal symptoms and suspected NEC.
These descriptive findings illustrate the diversity of fecal SCFA profiles in infants evaluated for suspected NEC and highlight the potential metabolic signatures that may characterize this clinical setting. Although the sample size is insufficient for formal statistical comparisons, the observed differences between confirmed NEC, perinatal intestinal infection, and suspected NEC suggest that targeted SCFA profiling may help refine early metabolic phenotyping in future NEC-focused cohorts. The results support the methodological framework used in the present study and emphasize the importance of integrating SCFA measurements into prospective investigations of NEC risk, early diagnosis, and pathophysiology.
3.5. Discussion
In this longitudinal cohort study, we identified distinct temporal shifts in fecal short-chain fatty acids in preterm infants who developed early-onset neonatal sepsis (EONS). Although SCFAs are increasingly recognised as key microbial metabolites involved in epithelial maturation, immune modulation, and inflammatory signalling [
14,
15,
16,
21,
22,
23,
24], their dynamic behaviour during systemic infection has remained largely unexplored. Recent reviews highlight the potential importance of SCFAs in neonatal sepsis [
11,
12,
13], but existing evidence relies mostly on single-timepoint measurements or on systemic rather than intestinal readouts. To our knowledge, this is the first study to characterise time-resolved fecal SCFA and BCFA trajectories during EONS across the first month of life.
A consistent finding was the early reduction in the relative dominance of acetic acid, accompanied by higher proportions of non-acetate acids—including propionic, valeric, and branched-chain fatty acids—at the initial timepoint. Such deviations suggest that EONS may disrupt microbial metabolic output from the earliest days of life. Reduced acetate dominance may reflect delayed establishment of key saccharolytic commensals or impaired epithelial utilisation of acetate during systemic inflammation, both of which have been reported in preterm infants with inflammatory morbidities [
7,
27,
28,
29,
30]. Conversely, the relative enrichment of non-acetate SCFAs and BCFAs may reflect early perturbations in fermentation pathways or disruption of normal ecological succession in the immature gut [
8,
10].
The pronounced differences observed in acetate-to-non-acetate ratios provide additional evidence of metabolic imbalance. Ratio-based measures offer an internally normalised view of fermentation activity and are robust against the large inter-individual variation in total SCFA output typical in preterm infants, where feeding regimens, transit time, and microbial maturity vary considerably [
38,
39]. Previous studies on SCFAs in NEC and sepsis [
20,
32,
40,
41] have reported inconsistent absolute concentrations, underscoring the limitations of single-point measurements and supporting the value of longitudinal, ratio-informed approaches.
An additional consideration is that we did not adjust for the absolute volume of enteral substrate at each timepoint, which may influence the availability of medium-chain triglycerides and, consequently, the fecal levels of medium-chain fatty acids (MCFAs). Prior neonatal nutrition studies demonstrate that dietary fat quantity and formulation—particularly the proportion of medium-chain triglycerides—can substantially affect MCFA absorption and downstream fecal levels [
31,
42,
43,
44]. Although our time-structured design provides internally consistent trajectories, future work should explore feeding-volume normalisation to refine interpretation of MCFA-related dynamics.
These findings align with emerging evidence linking intestinal immaturity, dysbiosis, and systemic inflammation in preterm infants. Neonatal sepsis has been repeatedly associated with delayed colonisation by beneficial anaerobes, instability of microbial communities, and enrichment of facultative or opportunistic taxa [
7,
8,
45]. Because SCFAs modulate epithelial energy supply, tight-junction expression, mucus production, and innate immune responses [
21,
22,
23,
24], the alterations documented here likely represent both a marker and a mediator of early gut–systemic inflammatory crosstalk.
Although NEC and EONS are distinct clinical entities, early-stage NEC-risk phenotypes (Bell stage I) also demonstrate metabolic and microbial disturbances before overt intestinal injury [
17,
19]. Some of these early deviations—such as altered acetate dominance or increased variability in non-acetate fractions—may reflect a broader inflammatory or maturational vulnerability in the preterm gut [
20]. Our findings extend this concept to the setting of systemic infection, indicating that EONS is accompanied by reproducible SCFA shifts that may represent a condition-specific metabolic signature rather than a generic consequence of illness.
The principal strengths of this study include its longitudinal design and high-resolution sampling across predefined postnatal windows. Although a handful of preliminary studies have explored gut-microbial metabolites in preterm infants during the first month of life [
9,
46,
47], time-resolved, fecal SCFA trajectories in the context of systemic neonatal infection remain almost entirely uncharted. This serial approach captures within-infant evolution and reveals structured metabolic patterns that would be missed by single-timepoint sampling.
Limitations include the modest cohort size, which reduces statistical power, and the lack of microbiome or immune profiling, which precludes direct mechanistic mapping. Clinical factors such as feeding modality, antibiotic exposure, and respiratory support may have contributed to the observed variability. Larger, multicentre cohorts integrating SCFA measurements with metagenomic and immunologic readouts will be essential to determine whether these metabolic trajectories represent causal contributors to EONS vulnerability, compensatory responses, or epiphenomena of severe illness [
7,
8].
Overall, this study provides the first longitudinal evidence that EONS in preterm infants is accompanied by structured changes in the fecal SCFA landscape. The early reduction in acetic-acid dominance and the enrichment of non-acetate components highlight the gut’s metabolic response as a potential early indicator of systemic inflammatory stress. These findings lay the groundwork for future research on gut–systemic communication in neonatal sepsis and support the development of metabolically informed approaches to early risk assessment in high-risk neonatal populations.
4. Conclusions
This study provides the first longitudinal characterisation of fecal short-chain fatty acid dynamics in preterm infants with early-onset neonatal sepsis (EONS). We demonstrate that EONS is accompanied by reproducible, time-structured alterations in intestinal fermentation, including an early reduction in acetic-acid dominance and a relative enrichment of non-acetate components. These shifts are evident from the initial days of life and are most clearly captured through ratio-based metrics, which offer an internally normalised view of microbial metabolic function and are more robust than absolute concentrations in the highly variable preterm setting.
The metabolic signature identified here suggests that the intestinal microbiome of infants with EONS undergoes early functional reorganisation during systemic infection, supporting growing evidence that gut–systemic inflammatory crosstalk is highly active in the neonatal period. Although NEC and EONS represent distinct clinical entities, some early deviations observed in NEC-risk phenotypes parallel the initial SCFA imbalance seen in EONS, indicating potential points of convergence in inflammatory vulnerability.
Our findings highlight fecal SCFAs as a promising non-invasive marker of early systemic infection in preterm infants. The serial sampling approach used in this study captures within-infant trajectories that are not accessible through single-timepoint measurements and may provide a basis for developing metabolically informed risk stratification tools. Future work should validate these observations in larger, multicentre cohorts and integrate SCFA profiling with microbiome and immune analyses to elucidate mechanistic pathways and identify actionable targets for early intervention.
Author Contributions
Conceptualization, E.N., N.S., A.L., and G.S.; methodology, E.K., O.K.J., I.N., N.S., and V.F.; software, A.T., and E.K.; validation, O.K.J., I.N., A.L., and V.F.; formal analysis, O.K.J., I.N., A.L., and V.F.; investigation, E.K., A.T., N.S, and V.F.; resources, V.F. and G.S.; data curation, O.K.J., I.N., A.L., N.S. and A.T.; writing—original draft preparation, E.K., N.S., O.K.J., I.N., and A.T.; writing—review and editing, A.L., V.F., and G.S.; visualization, E.K., N.S., and A.T.; supervision, E.K., V.F., N.S. and G.S.; project administration, E.K., V.F., N.S. and G.S.; funding acquisition, E.K., V.F., and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation (RSF), grant number 24-25-00068.
Institutional Review Board Statement
The study was approved by the Ethical Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology, named after Academician V.I. Kulakov (Moscow, Russia, protocol code 04 of 18 April 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to ethical and privacy restrictions related to clinical data from preterm neonates. De-identified data may be made available from the corresponding author upon reasonable request and subject to institutional approval.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
|
EONS |
Early-onset neonatal sepsis |
| NEC |
Necrotizing enterocolitis |
| NICU |
Neonatal intensive care unit |
| GA |
Gestational age |
| DoL |
Day of life |
| TP |
Timepoint |
| SCFA |
Short-chain fatty acid |
| BCFA |
Branched-chain fatty acid |
| MCFA |
Medium-chain fatty acid |
| MCT |
Medium-chain triglyceride |
| GC–MS |
Gas chromatography–mass spectrometry |
| PLS–DA |
Partial least squares–discriminant analysis |
| LOD |
Limit of detection |
| LOQ |
Limit of quantification |
| SIM |
Selected ion monitoring |
| IQR |
Interquartile range |
| AA |
Acetic acid |
| PA |
Propionic acid |
| BA |
Butyric acid |
| VA |
Valeric acid |
| iBA |
Isobutyric acid |
| iVA |
Isovaleric acid |
| 2mBA |
2-methylbutyric acid |
|
Combined relative fraction of branched-chain fatty acids (iBA, iVA, 2mBA, etc.) |
| HA |
Hexanoic acid |
| OA |
Octanoic acid |
Appendix A
Appendix A.1
Figure A1.
Acetate-to-non-acetate ratios at TP1 (∼3 days of life) in infants with EONS and non-sepsis controls. All ratios (AA/VA, AA/HA, AA/PA, AA/OA, AA/iVA, AA/iBA, and AA/BA) were lower in EONS, indicating a redistribution of the fecal SCFA pool away from acetate and toward branched- and medium-chain acids. Boxplots represent median and interquartile range;
p-values from Mann–Whitney
U tests are reported in
Table 4.
Figure A1.
Acetate-to-non-acetate ratios at TP1 (∼3 days of life) in infants with EONS and non-sepsis controls. All ratios (AA/VA, AA/HA, AA/PA, AA/OA, AA/iVA, AA/iBA, and AA/BA) were lower in EONS, indicating a redistribution of the fecal SCFA pool away from acetate and toward branched- and medium-chain acids. Boxplots represent median and interquartile range;
p-values from Mann–Whitney
U tests are reported in
Table 4.
Figure A2.
PLS–DA at TP2–TP4 (≈7, 14, 21 DoL). Compared with TP1, group separation is weaker or unstable; VIP patterns are attenuated. Yellow = ↑EONS, blue = ↓EONS.
Figure A2.
PLS–DA at TP2–TP4 (≈7, 14, 21 DoL). Compared with TP1, group separation is weaker or unstable; VIP patterns are attenuated. Yellow = ↑EONS, blue = ↓EONS.
References
- Neu, J.; Mshvildadze, M.; Mai, V. A roadmap for understanding and preventing necrotizing enterocolitis. Current gastroenterology reports 2008, 10, 450–457. [Google Scholar] [CrossRef]
- Ahearn-Ford, S.; Berrington, J.E.; Stewart, C.J. Development of the gut microbiome in early life. Experimental Physiology 2022, 107, 415–421. [Google Scholar] [CrossRef]
- Thänert, R.; Sawhney, S.S.; Schwartz, D.J.; Dantas, G. The resistance within: antibiotic disruption of the gut microbiome and resistome dynamics in infancy. Cell host & microbe 2022, 30, 675–683. [Google Scholar]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nature medicine 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. The ISME journal 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Davies, H.D. Early-Onset Neonatal Sepsis. Clinical Microbiology Reviews 2014, 27, 21–47. [Google Scholar] [CrossRef]
- Lai, M.Y.; Chang, Y.H.; Lee, C.C.; (NEMO), N.M.O.S.G. The impact of gut microbiota on morbidities in preterm infants. Journal of Perinatology and Neonatology, 1 July. [CrossRef]
- Erickson, I.; Tung, J.; Schwartz, D.J. Consequences of host–microbiome interactions in preterm infants. Infection and Immunity 2025, 93, e00501–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Li, N.; Guan, K.; Yin, D.; Zhang, H.; Ding, G.; Hu, Y. Evolution of Intestinal Gases and Fecal Short-Chain Fatty Acids Produced in vitro by Preterm Infant Gut Microbiota During the First 4 Weeks of Life. Frontiers in Pediatrics 2021, 9, 726193. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Marrs, E.C.L.; Nelson, A.; Lanyon, C.; Perry, J.D.; Embleton, N.D.; Cummings, S.P.; Berrington, J.E. Development of the Preterm Gut Microbiome in Twins at Risk of Necrotising Enterocolitis and Sepsis. PLoS ONE 2013, 8, e73465. [Google Scholar] [CrossRef]
- Iqbal, F.; Lewis, L.E.S.; Siva, N.; K E, V.; Purkayastha, J.; Shenoy, P.A. Modulation of gut microbiota: An emerging consequence in neonatal sepsis. Clinical Epidemiology and Global Health 2023, 20, 101245. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Khachatryan, L.G.; Younis, N.K.; Mustafa, M.A.; Ahmad, N.; Athab, Z.H.; Polyanskaya, A.V.; Kasanava, E.V.; Mirzaei, R.; Karampoor, S. Microbiota-derived short chain fatty acids in pediatric health and diseases: from gut development to neuroprotection. Frontiers in Microbiology 2024. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, Y.Q.; Kim, D.; Sung, D.K.; Kim, K.S.; Lee, J.S.; Cho, J.Y.; Lee, W.; Sung, S.I.; Lee, D.Y. Deciphering dynamic antibiotics-microbiome-metabolome interactions in preterm infants using systems biology. iScience, 1130. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Xue, G.; Han, L.; Jia, H.; Wang, Z.; Zhang, J.; Liu, P.; Yang, C.; Zhou, Y. Role of short-chain fatty acids in host physiology. Animal Models and Experimental Medicine 2024, 7, 641–652. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nakanishi, Y.; Ohno, H. Microbial metabolites and gut immunology. Annual review of immunology 2024, 42, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: mechanisms and functional importance in the gut. Proceedings of the Nutrition Society 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Brehin, C.; Dubois, D.; Dicky, O.; Breinig, S.; Oswald, E.; Serino, M. Evolution of Gut Microbiome and Metabolome in Suspected Necrotizing Enterocolitis: A Case-Control Study. Journal of Clinical Medicine 2020, 9, 2278. [Google Scholar] [CrossRef] [PubMed]
- Cuna, A.; Chan, S.; Jones, J.; Sien, M.; Robinson, A.; Rao, K.; Opfer, E. Feasibility and acceptability of a diagnostic randomized clinical trial of bowel ultrasound in infants with suspected necrotizing enterocolitis. European Journal of Pediatrics 2022, 181, 3211–3215. [Google Scholar] [CrossRef]
- Guo, X.; Feng, J.; Zhao, X.; Ying, E.; Liu, D.; Tu, H.; Yan, Y.; Huang, H.; Li, X.; Chen, X.; et al. Online registry of neonatal necrotising enterocolitis in Shenzhen: protocol for a multicentre, prospective, open, observational cohort study. BMJ Open 2024, 14, e091290. [Google Scholar] [CrossRef]
- Liu, X.C.; Du, T.T.; Gao, X.; Zhao, W.J.; Wang, Z.L.; He, Y.; Bao, L.; Li, L.Q. Gut microbiota and short-chain fatty acids may be new biomarkers for predicting neonatal necrotizing enterocolitis: A pilot study. Frontiers in Microbiology 2022, 13, 969656. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of lipid research 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Advances in immunology 2014, 121, 91–119. [Google Scholar]
- Ma, J.; Liu, Z.; Gao, X.; Bao, Y.; Hong, Y.; He, X.; Zhu, W.; Li, Y.; Huang, W.; Zheng, N.; et al. Gut microbiota remodeling improves natural aging-related disorders through Akkermansia muciniphila and its derived acetic acid. Pharmacological Research 2023, 189, 106687. [Google Scholar] [CrossRef]
- Saha, S.; Day-Walsh, P.; Shehata, E.; Kroon, P.A. Development and validation of a LC-MS/MS technique for the analysis of short chain fatty acids in tissues and biological fluids without derivatisation using isotope labelled internal standards. Molecules 2021, 26, 6444. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Olmo, B.; Lopez-Gonzalvez, A.; Cornejo, L.; Rupérez, F.; Barbas, C. Capillary electrophoresis for short chain organic acids in faeces: reference values in a Mediterranean elderly population. Journal of pharmaceutical and biomedical analysis 2008, 46, 356–361. [Google Scholar] [CrossRef]
- Luu, M.; Visekruna, A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. European Journal of Immunology 2019, 49, 842–848. [Google Scholar] [CrossRef]
- Thomas, S.P.; Denu, J.M. Short-chain fatty acids activate acetyltransferase p300. Elife 2021, 10, e72171. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nature communications 2018, 9, 3555. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Wang, L.; Jin, J.; Mi, L.; Pang, J.; Liu, Z.; Gong, J.; Sun, C.; Li, J.; Wei, W.; et al. Role Medium-Chain Fatty Acids in the Lipid Metabolism of Infants. Frontiers in Nutrition 2022, 9, 804880. [Google Scholar] [CrossRef]
- Chun, J.; Toldi, G. The Impact of Short-Chain Fatty Acids on Neonatal Regulatory T Cells. Nutrients 2022, 14, 3670. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Kliegman, R.M. Necrotizing Enterocolitis: Treatment Based on Staging Criteria. Pediatric Clinics of North America 1986, 33, 179–201. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Report on the Expert Meeting on Neonatal and Paediatric Sepsis. Technical report, European Medicines Agency (EMA), London, UK, 2010. Available online: https://www.ema.europa.eu/en/documents/report/report-expert-meeting-neonatal-paediatric-sepsis_en.pdf (accessed on 15 October 2025).
- Kukaev, E.; Kirillova, E.; Tokareva, A.; Rimskaya, E.; Starodubtseva, N.; Chernukha, G.; Priputnevich, T.; Frankevich, V.; Sukhikh, G. Impact of Gut Microbiota and SCFAs in the Pathogenesis of PCOS and the Effect of Metformin Therapy. International Journal of Molecular Sciences 2024, 25, 10636. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, Y.; Chae, W.; Cho, J.Y. An improved method to quantify short-chain fatty acids in biological samples using gas chromatography–mass spectrometry. Metabolites 2022, 12, 525. [Google Scholar] [CrossRef]
- NIST. NIST Standard Reference Database 17: NIST Mass Spectral Library (NIST17). National Institute of Standards and Technology, Gaithersburg, MD, USA, 2017. Available online: https://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:nist17 (accessed on 20 October 2025).
- Pourcyrous, M.; Nolan, V.; Goodwin, A.; Davis, S.; Buddington, R. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. Journal of pediatric gastroenterology and nutrition 2014, 59, 725–731. [Google Scholar] [CrossRef]
- Cifuentes, M.P.; Chapman, J.A.; Stewart, C.J. Gut microbiome derived short chain fatty acids: Promising strategies in necrotising enterocolitis. Current Research in Microbial Sciences 2024, 6, 100219. [Google Scholar] [CrossRef]
- He, Y.; Du, W.; Xiao, S.; Zeng, B.; She, X.; Liu, D.; Du, H.; Li, L.; Li, F.; Ai, Q.; et al. Colonization of fecal microbiota from patients with neonatal necrotizing enterocolitis exacerbates intestinal injury in germfree mice subjected to necrotizing enterocolitis-induction protocol via alterations in butyrate and regulatory T cells. Journal of Translational Medicine 2021, 19, 510. [Google Scholar] [CrossRef]
- Casaburi, G.; Wei, J.; Kazi, S.; Liu, J.; Wang, K.; Tao, G.Z.; Lin, P.Y.; Dunn, J.C.; Henrick, B.M.; Frese, S.A.; et al. Metabolic model of necrotizing enterocolitis in the premature newborn gut resulting from enteric dysbiosis. Frontiers in Pediatrics 2022, 10, 893059. [Google Scholar] [CrossRef]
- Carnielli, V.P.; Sulkers, E.J.; Moretti, C.; Wattimena, J.L.; van Goudoever, J.B.; Degenhart, H.J.; Zacchello, F.; Sauer, P.J. Conversion of octanoic acid into long-chain saturated fatty acids in premature infants fed a formula containing medium-chain triglycerides. Metabolism 1994, 43, 1287–1292. [Google Scholar] [CrossRef]
- Rodriguez, M.; Funke, S.; Fink, M.; Demmelmair, H.; Turini, M.; Crozier, G.; Koletzko, B. Plasma fatty acids and [13C]linoleic acid metabolism in preterm infants fed a formula with medium-chain triglycerides. Journal of Lipid Research 2003, 44, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhu, X.; Sun, S.; Yu, C.; Huang, X.; Ness, R.; Dugan, L.L.; Shu, L.; Seidner, D.L.; Murff, H.J.; et al. Ca:Mg ratio, medium-chain fatty acids, and the gut microbiome. Clinical Nutrition 2022, 41, 2490–2499. [Google Scholar] [CrossRef]
- Bresesti, I.; Salvatore, S.; Valetti, G.; Baj, A.; Giaroni, C.; Agosti, M. The Microbiota-Gut Axis in Premature Infants: Physio-Pathological Implications. Cells 2022, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Wandro, S.; Osborne, S.; Enriquez, C.; Bixby, C.; Arrieta, A.; Whiteson, K. The Microbiome and Metabolome of Preterm Infant Stool Are Personalized and Not Driven by Health Outcomes, Including Necrotizing Enterocolitis and Late-Onset Sepsis. mSphere 2018, 3, e00104–18. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Xu, W.; Janton, S.; Henderson, W.A.; Matson, A.; McGrath, J.M.; Maas, K.; Graf, J. Gut Microbiome Developmental Patterns in Early Life of Preterm Infants: Impacts of Feeding and Gender. PLoS ONE 2016, 11, e0152751. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).