Introduction
Pars plana vitrectomy (PPV) combined with internal limiting membrane (ILM) peeling and gas tamponade remains the standard surgical approach for full-thickness macular holes (FTMHs), with reported closure rates generally ranging between 80% and 100%.[
1,
2,
3,
4,
5,
6] Despite these favourable outcomes, anatomical failure can still occur, and in some cases a macular hole that initially closes may later reopen.[
6,
7] In the literature, FTMHs that do not achieve closure following the first surgical attempt are referred to as
persistent or
refractory, whereas those that reappear after having been successfully sealed for at least four weeks are classified as
recurrent.[
6,
8]
When a second intervention is required, surgeons must account for several challenges typical of recurrent or refractory FTMHs. These include the scarcity or complete absence of ILM available for further peeling, the impracticality of recreating an inverted ILM flap, and the generally lower anatomical success rates reported for re-operations.[
9] Even so, undertaking an additional procedure is often considered worthwhile, given the meaningful anatomical and functional improvements that can still be obtained.[
6,
10,
11]
Moreover, the natural progression of an open FTMH tends to involve enlargement, leading to progressive atrophy and visual loss [
12], while repeated surgeries are less likely to result in worsening vision.[
6,
13]
A range of surgical strategies is available for addressing recurrent or refractory FTMHs; however, for several of these approaches the published evidence remains relatively scarce, largely because they have only recently been incorporated into clinical practice.[
6,
7] Consequently, there is no consensus regarding the best surgical technique for secondary repair [
14] of FTMHs, unlike the primary surgery. Numerous approaches have been suggested to treat macular holes that remain open after the first surgery [
6,
7], such as autologous platelet-rich plasma (aPRP) [
15], revisional PPV with or without ILM peeling enlargement [
16], subretinal fluid (SRF) injection [
17], relaxing retinotomies[
18,
19,
20], outpatient fluid/gas exchange[
21] (with or without laser photocoagulation), retinal massage[
22], microdrain[
12,
23], macular buckling[
24], autologous neurosensory retinal free flap[
25], human amniotic membrane (hAM) graft[
7,
26,
27], ILM free flap with or without autologous blood[
28] and lens capsular flap (LCF)[
29,
30].
With respect to relaxing retinotomies [
18,
19,
20,
31,
32,
33], a recent review[
6] summarised the evidence currently available for this approach in the secondary treatment of recurrent and refractory FTMHs, and also outlined the historical development of the technique together with the different methods by which relaxing retinotomies can be carried out. In 2013, Charles and coworkers described the use of a full-thickness arcuate relaxing retinotomy temporal to a macular hole in eyes with large FTMHs that had failed to close following primary repair[
6,
19].
The rationale for performing a temporal arcuate retinotomy is to increase retinal elasticity beyond what can be achieved through ILM peeling alone, thereby enabling the temporal retinal bridge to relax and shift into a position that promotes macular hole closure.[
6,
19] Charles et al. reported that combining this arcuate incision with ILM peeling enhances retinal compliance and supports the closure of large FTMHs that may not respond adequately to standard techniques.[
6]
It is well established that, in the region temporal to the fovea and near the horizontal raphe, the retinal ganglion cell axons course both above and below the macula and the papillomacular bundle before entering the optic nerve.[
19] As a result, their trajectory as they diverge from the raphe becomes oblique and almost vertical.[
19] Understanding this orientation allows surgeons to design an arcuate (longitudinal) retinotomy aligned with the natural path of these axons, minimising the risk of cutting nerve fibres and reducing the likelihood of inducing a nasal visual field defect.[
19]
In this retrospective case series, we aimed to assess both anatomical closure rates and functional outcomes following the use of temporal arcuate relaxing retinotomy for the management of persistent FTMHs.
Material and Methods
This retrospective, single-centre interventional study assessed the effectiveness of temporal arcuate relaxing retinotomy in eyes with persistent full-thickness macular holes (FTMHs). Eligible patients were recruited at Beauregard Hospital (Aosta, Italy) between November 2022 and April 2024.
Inclusion criteria included patients with persistent FTMHs despite having undergone one or more standard repair procedures involving pars plana vitrectomy (PPV) and internal limiting membrane (ILM) peeling.
Exclusion criteria included significant macular pathologies (e.g., advanced diabetic retinopathy, retinal vein occlusions, or late-stage age-related macular degeneration) and inability to adhere to postoperative face-down positioning.
Before surgery, the risks and benefits of the procedure were discussed with patients deemed eligible by the investigators.
Preoperative data collected included patient demographics (age, sex), concurrent ocular conditions, laterality of the FTMH, hole diameter and duration, and previous FTMH repair interventions. The size of the FTMH was measured pre- and postoperatively using spectral-domain optical coherence tomography (OCT Spectralis, Heidelberg Engineering), with aperture diameter defined as the minimum width at any macular level and basal diameter as the maximum width at the retinal pigment epithelium (RPE) level. Additional assessments included best-corrected visual acuity (BCVA), lens status, initial hole diameter, subfoveal ellipsoid zone restoration, and the interval between primary and secondary PPV. From two months post-PPV onwards, patients were questioned at each follow-up regarding any new scotomas or visual field defects in the operated eye. After the secondary PPV, all patients were allowed to undergo a formal Humphrey visual field test using the HFA3 (Carl Zeiss Meditec, Dublin, CA).
All surgeries were performed by a single surgeon (LV) using either 23-gauge or 25-gauge transconjunctival PPV (Constellation, Alcon, Texas). Residual ILM at the retinotomy site was stained with intravitreal Blulife dye (view ILM, Alchimia, Italy), and, if present, an expanded circumferential ILM peel was performed. Procedures were conducted using a 3D visualization system (NGENUITY, Alcon, Texas). A full-thickness arcuate (semicircular) incision was made one hole diameter temporal to the macular hole edge using vertical or horizontal scissors, as per Charles' technique.[
19] The retinotomy arc was centred 90° on the temporal horizontal meridian (
Figure 1), followed by fluid-air exchange and intravitreal gas tamponade with either sulphur hexafluoride (SF6) or perfluoropropane (C3F8). Patients were instructed to maintain a face-down position for four days after surgery. A surgical video is available in the Supplementary Materials.
Postoperative assessment was conducted after complete absorption of the gas tamponade. The main outcome measure was anatomical closure of the FTMH, confirmed by OCT imaging. In eyes that achieved closure, the integrity of the subfoveal ellipsoid zone was also examined. The secondary endpoint consisted of evaluating the variation in BCVA from the preoperative examination to the postoperative follow-up visit.
Baseline characteristics and postoperative outcomes are presented using descriptive statistics, including mean, standard deviation (SD), median, and range. Decimals of BCVA were converted to the logarithm of the minimal angle of resolution (logMAR) units for quantitative analysis, and a paired t test was used to detect a significant difference between preoperative and postoperative mean BCVA. Statistical significance was defined as P less than 0.05.
Results
This retrospective case series comprised 9 consecutive eyes from 9 individuals with persistent FTMHs. The sample included 7 males and 2 females, with a median age of 70 years (range: 58–76 years).
FTMH minimum diameters varied between 412 and 1037 µm, with a median of 613 µm. Six cases were idiopathic, two were long-standing traumatic macular holes (patients 5 and 9), and one macular hole developed after retinal detachment repair (patient 4). Patients 5 and 9 had chronic macular holes lasting at least six months; notably, patient 9 had a macular hole documented for over four years.All eyes had previously undergone one or more PPV procedures with ILM peeling, with or without the use of an ILM flap, yet the macular holes remained unclosed and were therefore classified as refractory. All 9 eyes underwent PPV combined with a temporal relaxing retinotomy. In 4 cases, a residual ILM remnant was present at the site of the planned retinotomy; in these eyes, an extended circumferential ILM peel was performed. After surgery, every patient adhered to a face-down positioning regimen for a minimum of 4 days. A summary of baseline characteristics, macular hole features, surgical details, and postoperative outcomes is provided in
Table 1.
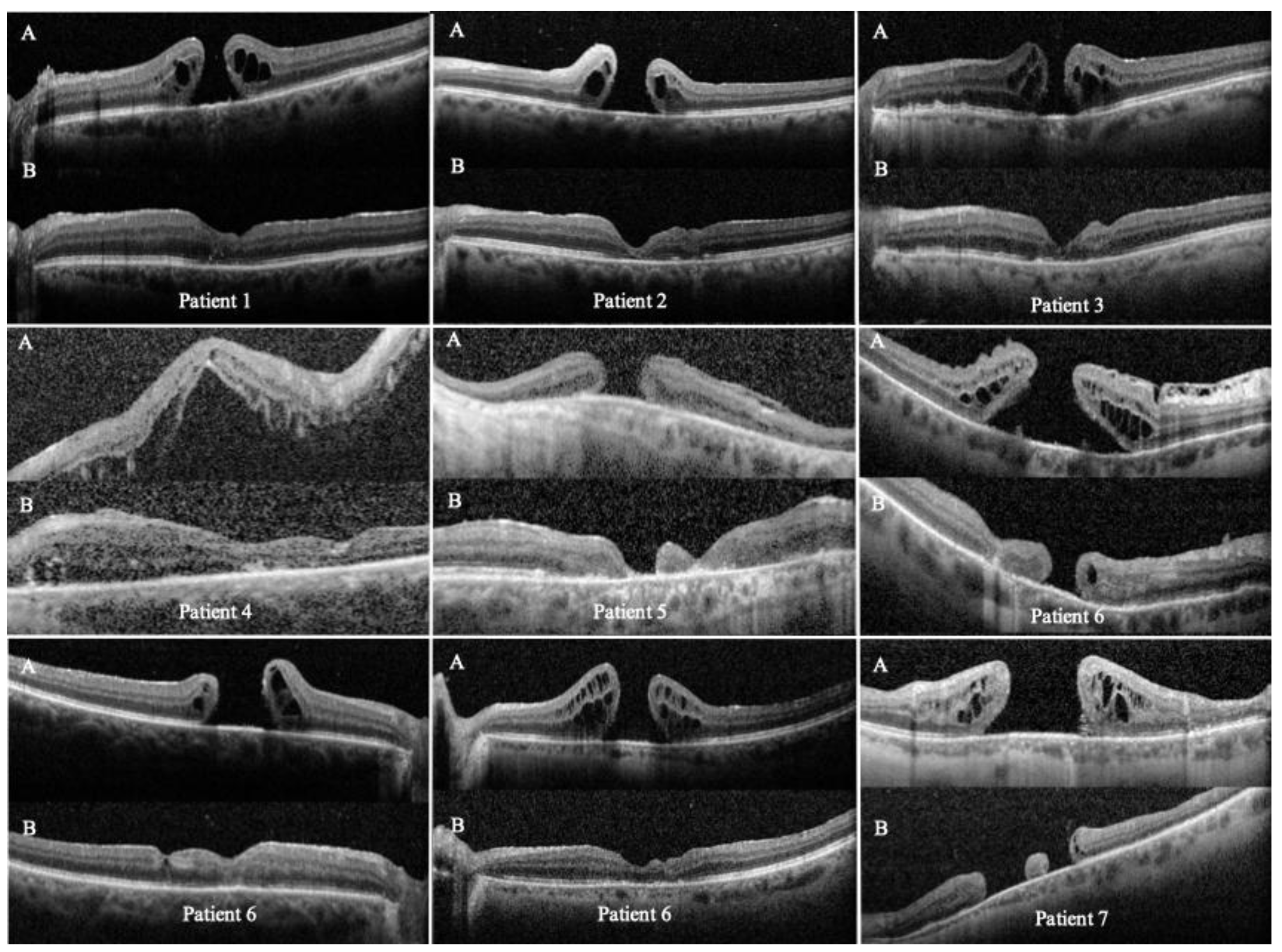
Successful FTMH closure was achieved in 7 of 9 eyes (closure rate, 78%;
Table 1), with an average postoperative follow-up of 10.4 months (range: 2 to 20 months). 4 eyes showed complete or partial restoration of the subfoveal ellipsoid zone. Some eyes with FTMH closure exhibited retinal thinning on postoperative OCT, likely owing to multiple surgical procedures, ILM peeling and tissue manipulation.
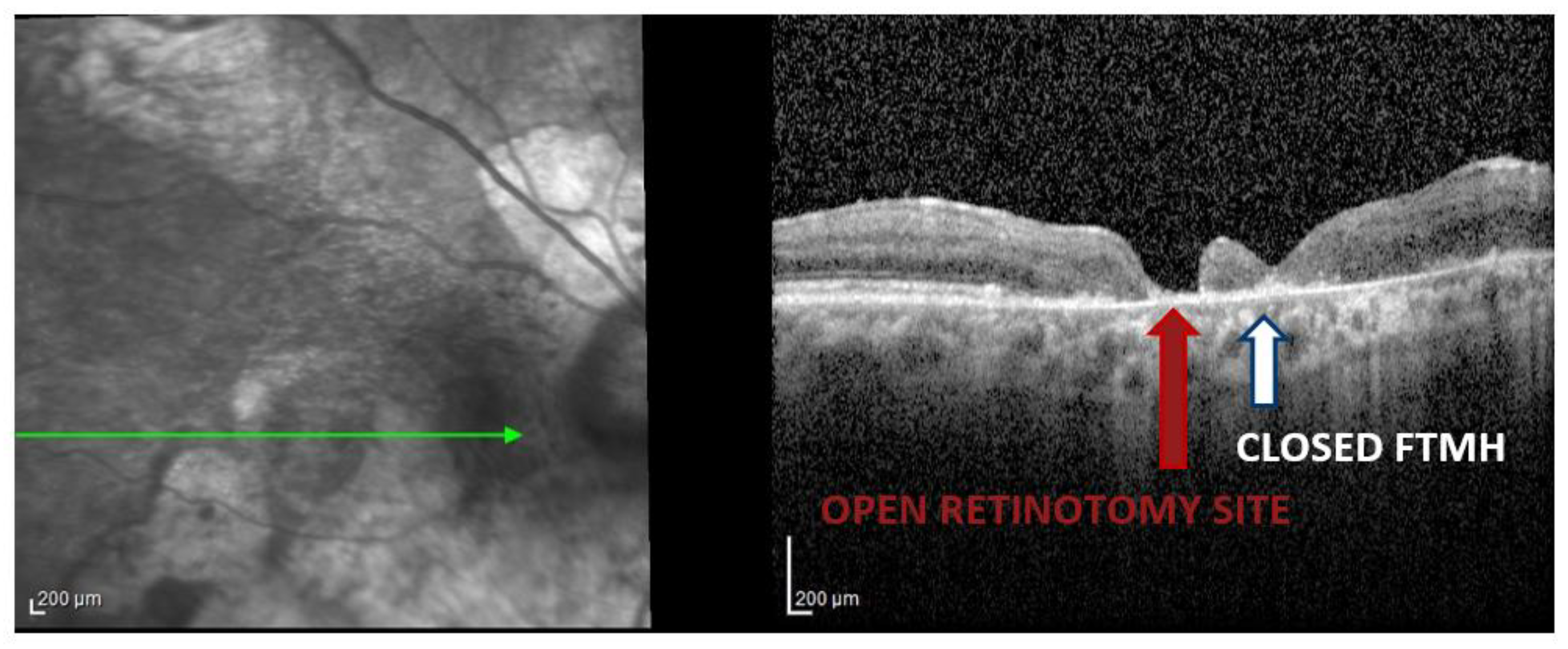
The temporal arcuate relaxing retinotomy successfully enabled closure even in one very large, long-standing FTMH (patient 5, expanded case study ahead). The retinotomy site remained open only in the two patients with chronic FTMHs (patients 5 and 9), for whom OCT data were available (
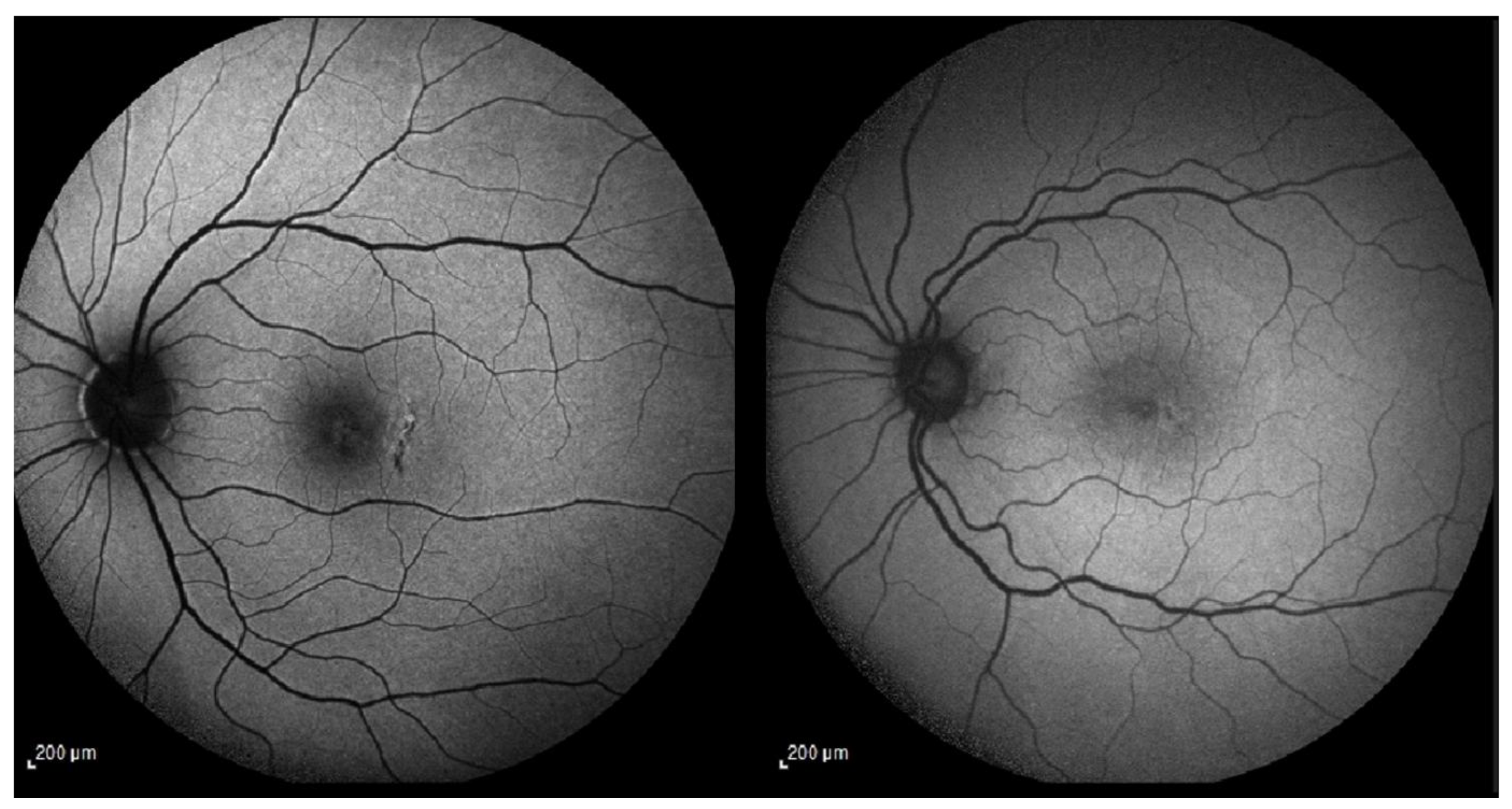
Figure 2); in these cases, postoperative enlargement of the open retinotomy or accumulation of subretinal fluid at the site was not observed.
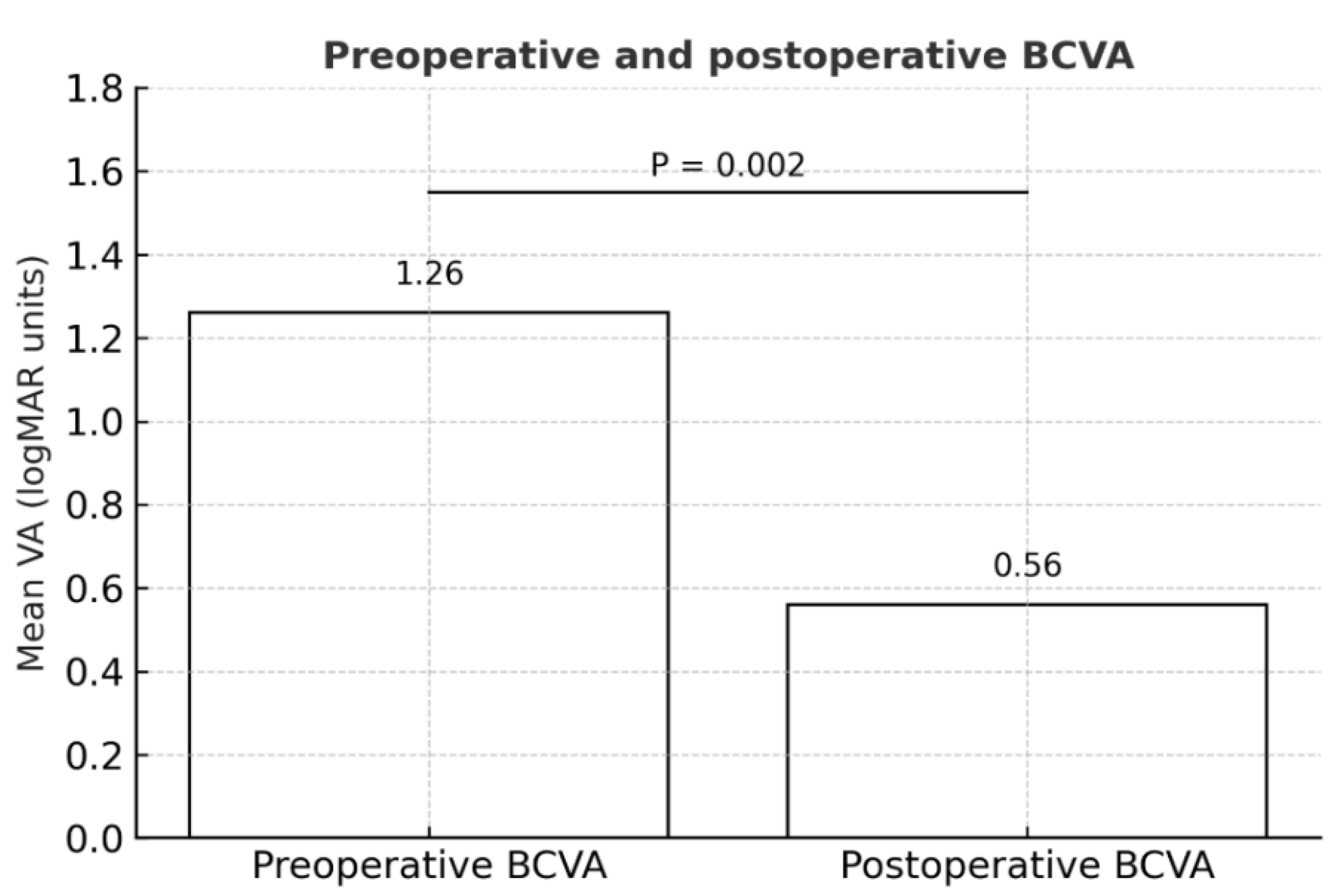
Eight of 9 eyes (89%) achieved BCVA improvement during postoperative follow-up, including the long-standing FTMH (see
Table 1). All the eyes were pseudophakic at baseline. Overall, mean BCVA (± SD) improved significantly from 1.26 ± 0.51 logMAR at baseline to 0.56 ± 0.27 logMAR during postoperative follow-up (P = 0.002;
Figure 3).
Only one patient reported a new paracentral nasal scotoma, but this was well tolerated. This patient experienced better functionality and quality of life with a postoperative paracentral scotoma than with a central scotoma due to FTMH.
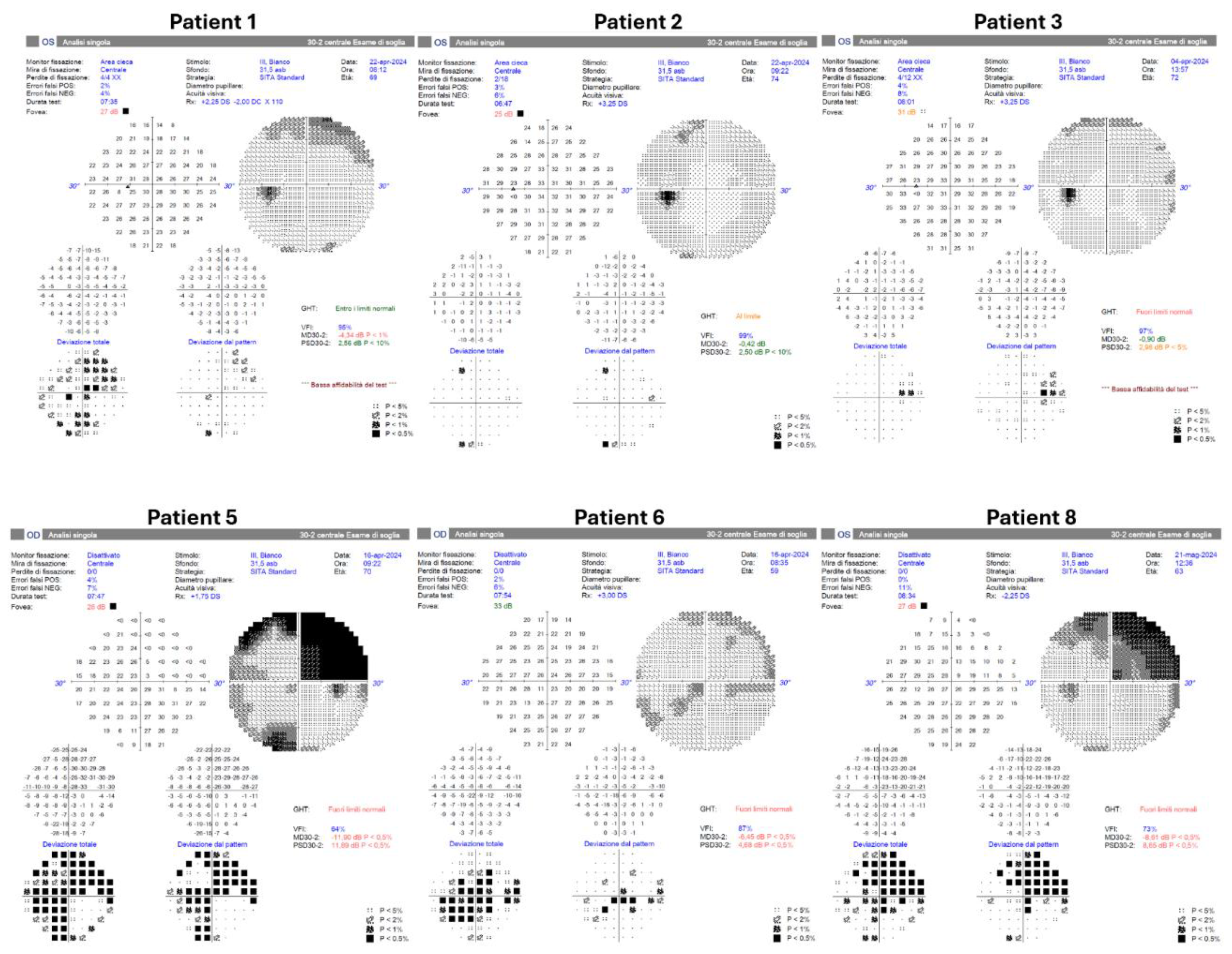
Formal Humphrey visual field testing was successfully performed on 6 patients (patients 1, 2, 3, 5, 6, and 8;
Figure 4) using the HFA3 (Carl Zeiss Meditec, Dublin, CA). The visual field (VF) examination of patients 1, 2, 3, and 6 showed no scotomas. However, the VF test of patient 5 revealed a supero-temporal scotoma but was deemed consistent with the infero-nasal chorioretinal atrophy (expanded case study forward). Patients 4, 7, and 9 have not undergone this assessment.
Expanded Case Study
Patient 5 was a 70-year-old man with a long-standing traumatic FTMH in his right eye following significant blunt trauma that occurred 20 years before our observation. The FTMH was chronic (lasting over 1 year), measuring 1037 µm in aperture diameter, with flat borders and an infero-nasal chorioretinal atrophy. Several years ago, this traumatised eye had already undergone a PPV with ILM peeling, without achieving FTMH closure.
We performed a 23G pars plana vitrectomy with extended ILM peeling and a temporal relaxing retinotomy to attempt to close this very large macular hole, followed by gas tamponade (SF6) and 7 days of face-down positioning.
During postoperative follow-up, complete FTMH closure was achieved and BCVA improved from counting fingers at 30 cm to 20/100. The retinotomy site remained open, but the most significant finding is that the macular hole completely closed, confirming the partial slippage of the retina to close the hole, considering the notable inferonasal chorioretinal atrophy (
Figure 5).
Conclusions
The promising outcomes of various available surgical techniques seem to support choosing to reoperate on recurrent and refractory FTMHs; however, a direct comparison between different procedures is difficult and challenging due to the limited number of randomised controlled trials and/or extensive prospective comparative studies.[
7] An evidence-based consensus on the best surgical approach has not yet been established, so a standardised approach for treating recurrent and refractory full-thickness macular holes is still to be determined.[
7]
The present study expands on previous research to demonstrate the effectiveness of temporal arcuate full-thickness relaxing retinotomy in a group of patients with persistent FTMHs, even without a prolonged follow-up period. In our case series, anatomical closure was achieved in 7 of 9 eyes (78%). All eyes had large FTMHs (aperture diameter > 400 µm), and 4 eyes showed complete or partial restoration of the subfoveal ellipsoid zone. Our technique helped resolve one long-standing, very large FTMH. As previously stated by Tsipursky et al. [
33] regarding nasal parafoveal retinotomy, our findings confirm that the chronicity of an FTMH should not prevent a surgeon from attempting closure with a temporal arcuate full-thickness retinotomy.
All our patients experienced improvements in vision during postoperative follow-up: mean BCVA (± SD) in these eyes significantly increased from 1.26 ± 0.51 logMAR at baseline to 0.56 ± 0.27 logMAR during postoperative follow-up.
Advantages of the relaxing retinotomy technique for recurrent and refractory FTMHs repair include preservation of central visual acuity, rapid reduction of hole size confirmed on intraoperative OCT, its use of surgical techniques commonly performed by vitreoretinal surgeons, and feasibility in previously vitrectomised eyes. [
6]
In contrast, relaxing retinotomy is a “destructive” surgical technique that involves sacrificing tissue to promote macular hole closure. Performing a retinotomy may potentially cause traumatic injury to the underlying RPE due to the difficulty in judging the depth of penetration under the retina, which is fully adherent at the time of incision.[
6] Still, in our case series, we did not observe significant complications. Only one retinotomy site remained open, while all other cases showed a closed retinotomy site. The open retinotomy might be due to the large diameter of the hole and, consequently, the substantial retinal displacement between the retinotomy and the hole.
We decided to perform a temporal arcuate relaxing retinotomy, unlike Tsipursky et al[
20], to avoid the papillomacular bundle. In Tsipursky’s study[
20], suction at the retinotomy site using a soft tip cannula followed the retinotomy to drain the FTMH. Intraoperatively, Tsipursky noticed that suction immediately permitted the borders of the FTMH to get closer and shrank the width of the defect, advising that this method may ultimately facilitate FTMH closure by further relaxing the adjacent retinal tissue[
20]. In our cases, we didn’t drain the fluid through the retinotomy.
The main reason for performing relaxing retinotomies in FTMH repair is to increase retinal compliance and reduce the tangential traction exerted by the adjacent retina on the FTMH [
14,
19,
31,
32], thereby promoting hole closure.[
19] Additionally, the retinal gliosis may be stimulated by the surgical trauma associated with the retinotomy, which can also contribute to hole closure.[
18]
The body of literature on relaxing retinotomy for recurrent and refractory FTMHs indicates that this surgical practice achieves high rates of anatomical closure, with varying degrees of functional success. Our case series supports this context, demonstrating a 78% FTMH closure rate and an 89% improvement in visual acuity, which suggests that relaxing retinotomies may be an effective procedure for facilitating anatomical closure and improving visual outcomes in patients with recurrent and refractory FTMHs.
Despite the reported high closure rate, the relaxing retinotomy technique carries a risk of inducing scotomas. Nevertheless, Tsipursky’s study[
20] found that the development of small temporal and nasal scotomas generally had less impact than the improvements in functional visual acuity seen in most patients during postoperative follow-up. In our case series, we performed a postoperative formal Humphrey.
We examined the visual field (HVF) in 6 patients, and during all postoperative follow-up checks, we consistently asked our patients if they experienced any new scotomas or visual field defects. Only one patient reported a new paracentral nasal scotoma, but this was well tolerated. The patient reported better function and quality of life with the paracentral scotoma than with a central scotoma caused by FTMH.
Next, it is essential to note that, in our case series, as well as in previous studies,[
6,
18,
19,
20] we excluded patients with significant macular pathologies (e.g., severe diabetic retinopathy, retinal vein occlusions, or advanced age-related macular degeneration). We agree with Tsipursky and coworkers[
20] that such patients should not undergo relaxing retinotomy, along with those with severe glaucoma who may potentially experience postoperative RNFL thinning.
Limitations of our case series include a very small sample size of 9 patients, so our positive findings need confirmation in larger comparative studies. Although a circumferential peel of residual ILM at the retinotomy site was also performed in 4 of 9 eyes, we believe that a relaxing retinotomy facilitated FTMH closure, since all eyes in this study had previously failed one or more standard repair procedures with ILM peeling.
Furthermore, pre- and postoperative HVF, pre- and post-peripapillary RNFL thickness, and microperimetry were not performed, nor was ganglion cell layer thickness assessed. Our study did not include a control or comparator group, so we did not compare the effectiveness of temporal arcuate relaxing retinotomy with alternative techniques for persistent FTMHs.
Compared to other proposed techniques for managing recurrent and refractory FTMHs, we consider relaxing retinotomies a “natural” surgical method that does not involve filling the hole with any materials (iris, tenon, amniotic membrane, aPRP…) and does not require tapping the hole. Using this approach, we successfully closed large persistent FTMHs without disturbing the macular hole (neither the margin of the FTMH nor the RPE beneath the hole), aiming to maximise visual acuity. However, a subsequent procedure remains possible with one of the aforementioned techniques.
In conclusion, the currently available studies on the secondary repair of refractory or persistent FTMH have several limitations, including small sample sizes, retrospective designs, heterogeneity in surgical procedures, and methods. These studies, including our case series, involve a limited number of patients.
It is crucial to undertake further studies with a large sample size, a consistent protocol, and standardised surgical procedures to better assess the anatomical and functional success rates of the relaxing retinotomy, the ideal location of the incision, its potential complications, and the patient population in which this procedure is most suitable. Additionally, future research is necessary to thoroughly compare the various surgical techniques available for the secondary repair of recurrent and refractory FTMHs in terms of both anatomical and functional outcomes.
Authors’ Note
Data from this study were presented in part at the International Edition of XXIII Congress Italian Society of Vitreoretinal Surgery, Trieste, Italy, June 8-10, 2023. Luca Ventre and Erik Mus are co-first authors for this study. All authors have read and agreed to the published version of the manuscript.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Abdelkader, E.; Lois, N. Internal Limiting Membrane Peeling in Vitreo-retinal Surgery. Surv. Ophthalmol. 2008, 53, 368–396. [Google Scholar] [CrossRef]
- Ch'NG, S.W.; Patton, N.; Ahmed, M.; Ivanova, T.; Baumann, C.; Charles, S.; Jalil, A. The Manchester Large Macular Hole Study: Is it Time to Reclassify Large Macular Holes? Arch. Ophthalmol. 2018, 195, 36–42. [Google Scholar] [CrossRef]
- La Cour, M.; Friis, J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol. Scand. 2002, 80, 579–587. [Google Scholar] [CrossRef]
- Lois, N.; Burr, J.; Norrie, J.; Vale, L.; Cook, J.; McDonald, A.; Boachie, C.; Ternent, L.; McPherson, G. ; Full-thickness Macular Hole and Internal Limiting Membrane Peeling Study (FILMS) Group Internal Limiting Membrane Peeling versus No Peeling for Idiopathic Full-Thickness Macular Hole: A Pragmatic Randomized Controlled Trial. Investig. Opthalmology Vis. Sci. 2011, 52, 1586–1592. [Google Scholar] [CrossRef]
- the BEAVRS Macular hole outcome group; Steel, D. H.; Donachie, P.H.J.; Aylward, G.W.; Laidlaw, D.A.; Williamson, T.H.; Yorston, D. Factors affecting anatomical and visual outcome after macular hole surgery: findings from a large prospective UK cohort. Eye 2020, 35, 316–325. [Google Scholar] [CrossRef]
- Ventre, L.; Mus, E.; Maradei, F.; Imparato, R.; Pintore, G.; Parisi, G.; Marolo, P.; Reibaldi, M. Relaxing Retinotomy in Recurrent and Refractory Full-Thickness Macular Holes: The State of the Art. Life 2023, 13, 1844. [Google Scholar] [CrossRef]
- Romano, M.R.; Rossi, T.; Borgia, A.; Catania, F.; Sorrentino, T.; Ferrara, M. Management of refractory and recurrent macular holes: A comprehensive review. Surv. Ophthalmol. 2022, 67, 908–931. [Google Scholar] [CrossRef]
- Abbey, A.M.; Van Laere, L.; Shah, A.R.; Hassan, T.S. RECURRENT MACULAR HOLES IN THE ERA OF SMALL-GAUGE VITRECTOMY. Retina 2017, 37, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Hillenkamp, J.; Kraus, J.; Framme, C.; Jackson, T.L.; Roider, J.; Gabel, V.-P.; Sachs, H.G. Retreatment of full-thickness macular hole: predictive value of optical coherence tomography. Br. J. Ophthalmol. 2007, 91, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.A.M.; McDonagh, N.B.; Wright, D.M.B.; Yek, J.T.O.B.; Essex, R.W.M.; Lois, N.M. FIRST FAILED MACULAR HOLE SURGERY OR REOPENING OF A PREVIOUSLY CLOSED HOLE. Retina 2020, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Valldeperas, X.; Wong, D. Is It Worth Reoperating on Macular Holes? Ophthalmology 2008, 115, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.J.M.; Steel, D.H.M.; Yorston, D.F.; Hind, J.F.; El-Faouri, M.F.; Jalil, A.F.; Tyagi, P.F.; Wickham, L.F.; Laidlaw, A.H.M. OUTCOME OF REVISION PROCEDURES FOR FAILED PRIMARY MACULAR HOLE SURGERY. Retina 2020, 41, 1389–1395. [Google Scholar] [CrossRef]
- D'SOuza, M.J.J.; Chaudhary, V.; Devenyi, R.; Kertes, P.J.; Lam, W.-C. Re-operation of idiopathic full-thickness macular holes after initial surgery with internal limiting membrane peel. Br. J. Ophthalmol. 2011, 95, 1564–1567. [Google Scholar] [CrossRef]
- Tam, A.L.C.; Yan, P.; Gan, N.Y.; Lam, W.-C. THE CURRENT SURGICAL MANAGEMENT OF LARGE, RECURRENT, OR PERSISTENT MACULAR HOLES. Retina 2018, 38, 1263–1275. [Google Scholar] [CrossRef]
- Figueroa, M.S.; Cantallops, A.M.; Virgili, G.; Govetto, A. Long-term results of autologous plasma as adjuvant to pars plana vitrectomy in the treatment of high myopic full-thickness macular holes. Eur. J. Ophthalmol. 2020, 31, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.M.; El-Faouri, M.M.; Ivanova, T.; Patton, N.M.C.; Ch'Ng, S.W.F.; Dhawahir-Scala, F.F.; Jalil, A.M. MANCHESTER REVISIONAL MACULAR HOLE STUDY. Retina 2021, 41, 908–914. [Google Scholar] [CrossRef]
- Meyer, C.H.; Szurman, P.; Haritoglou, C.; Maier, M.; Wolf, A.; Lytvynchuk, L.; Priglinger, S.; Hillenkamp, J.; Wachtlin, J.; Becker, M.; et al. Application of subretinal fluid to close refractory full thickness macular holes: treatment strategies and primary outcome: APOSTEL study. Graefe's Arch. Clin. Exp. Ophthalmol. 2020, 258, 2151–2161. [Google Scholar] [CrossRef]
- Reis, R.; Ferreira, N.; Meireles, A. Management of Stage IV Macular Holes: When Standard Surgery Fails. Case Rep. Ophthalmol. 2012, 3, 240–250. [Google Scholar] [CrossRef]
- Charles, S.; Randolph, J.C.; Neekhra, A.; Salisbury, C.D.; Littlejohn, N.; Calzada, J.I. Arcuate Retinotomy for the Repair of Large Macular Holes. Ophthalmic Surgery, Lasers Imaging Retin. 2013, 44, 69–72. [Google Scholar] [CrossRef]
- Tsipursky, M.S.; Byun, M.; Jager, R.D.; Sheth, V.S. Anatomical and Functional Outcomes of Relaxing Parafoveal Nasal Retinotomy for Refractory Macular Hole Repair. J. Vitr. Dis. 2021, 5, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Wang, N.-K.; Chen, Y.-P.; Hwang, Y.-S.; Chuang, L.-H.; Liu, I.-C.; Chen, K.-J.; Wu, W.-C.; Lai, C.-C. Outcomes of Outpatient Fluid-Gas Exchange for Open Macular Hole After Vitrectomy. Arch. Ophthalmol. 2013, 156, 326–333.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, M.; Di, R.; Qi, Y.; Pei, C.; Gao, S.; Liu, S.-W.; Xie, A.-M.; Cheng, Y.-H. Parafoveal retinal massage combined with autologous blood cover in the management of giant, persistent or recurrent macular holes. Int. J. Ophthalmol. 2020, 13, 1773–1779. [Google Scholar] [CrossRef]
- Hejsek, L.; Dusova, J.; Stepanov, A.; Rozsival, P. Re-operation of idiopathic macular hole after failed initial surgery. Biomed. Pap. 2014, 158, 596–599. [Google Scholar] [CrossRef]
- Mura, M.; Iannetta, D.; Buschini, E.; de Smet, M.D. T-shaped macular buckling combined with 25G pars plana vitrectomy for macular hole, macular schisis, and macular detachment in highly myopic eyes. Br. J. Ophthalmol. 2016, 101, 383–388. [Google Scholar] [CrossRef]
- Grewal, D.S.; Charles, S.; Parolini, B.; Kadonosono, K.; Mahmoud, T.H. Autologous Retinal Transplant for Refractory Macular Holes: Multicenter International Collaborative Study Group. Ophthalmology 2019, 126, 1399–1408. [Google Scholar] [CrossRef]
- Rizzo, S.; Caporossi, T.; Tartaro, R.; Finocchio, L.; Franco, F.; Barca, F.; Giansanti, F. A Human Amniotic Membrane Plug to Promote Retinal Breaks Repair and Recurrent Macular Hole Closure. Retina 2019, 39, S95–S103. [Google Scholar] [CrossRef]
- Cao, J.L.; Kaiser, P.K. Surgical Management of Recurrent and Persistent Macular Holes: A Practical Approach. Ophthalmol. Ther. 2021, 10, 1137–1153. [Google Scholar] [CrossRef] [PubMed]
- Morizane, Y.; Shiraga, F.; Kimura, S.; Hosokawa, M.; Shiode, Y.; Kawata, T.; Hosogi, M.; Shirakata, Y.; Okanouchi, T. Autologous Transplantation of the Internal Limiting Membrane for Refractory Macular Holes. Arch. Ophthalmol. 2014, 157, 861–869.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-N.; Yang, C.-M. LENS CAPSULAR FLAP TRANSPLANTATION IN THE MANAGEMENT OF REFRACTORY MACULAR HOLE FROM MULTIPLE ETIOLOGIES. Retina 2016, 36, 163–170. [Google Scholar] [CrossRef]
- Peng, J.; Chen, C.; Zhang, H.; Zhang, L.; Liu, J.; Ren, J.; Zhao, P. LONG-TERM SURGICAL OUTCOMES OF LENS CAPSULAR FLAP TRANSPLANTATION IN THE MANAGEMENT OF REFRACTORY MACULAR HOLE. Retina 2020, 41, 726–734. [Google Scholar] [CrossRef]
- Knight, D.; Yu, J.J.B.; Adrean, S.D. RELAXING NASAL RETINOTOMY TECHNIQUE FOR CLOSURE OF A MACULAR HOLE THAT REOPENED AFTER PRIMARY VITRECTOMY. Retin. Cases Brief Rep. 2019, 15, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Karacorlu, M.; Muslubas, I.S.; Hocaoglu, M.; Arf, S.; Ersoz, M.G. DOUBLE ARCUATE RELAXING RETINOTOMY FOR A LARGE MACULAR HOLE. Retin. Cases Brief Rep. 2019, 13, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Tsipursky, M. Creation of Paracentral Retinotomy to Facilitate Closure of Persistent Macular Holes. Paper Presented at: American Society of Retina Specialists 35th Annual Meeting; -15, 2017; Boston. 11 August.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).