Submitted:
10 November 2025
Posted:
11 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Units and Notation
3. Results
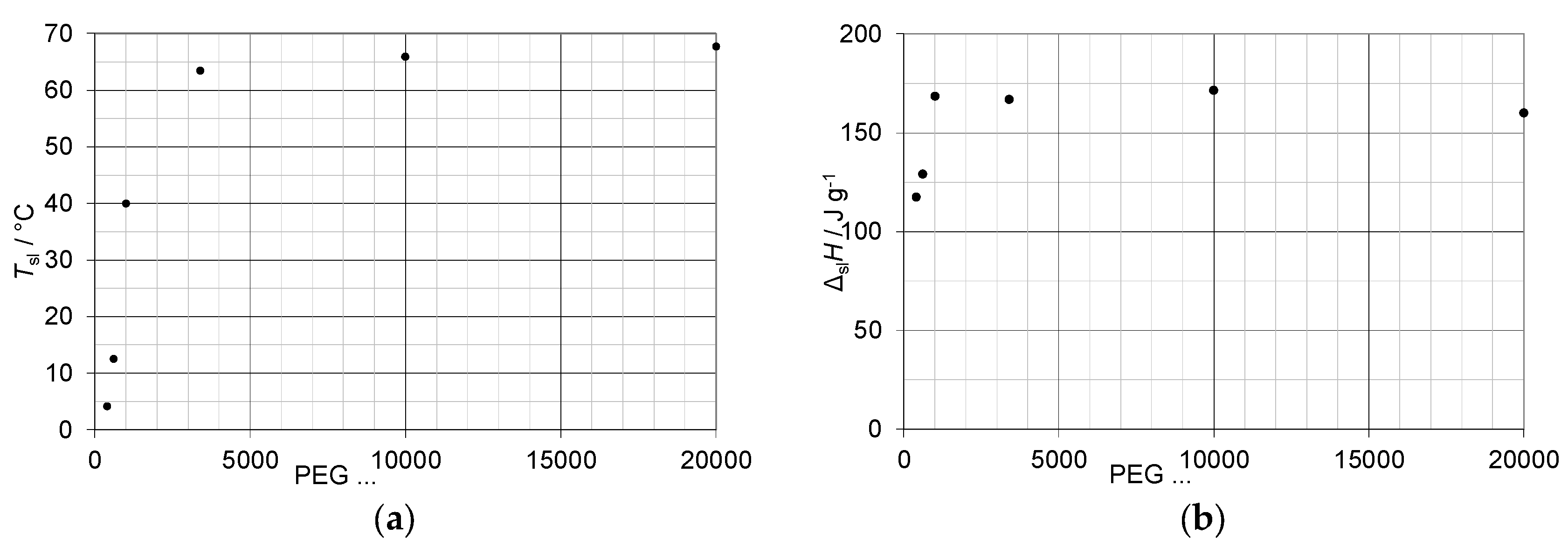
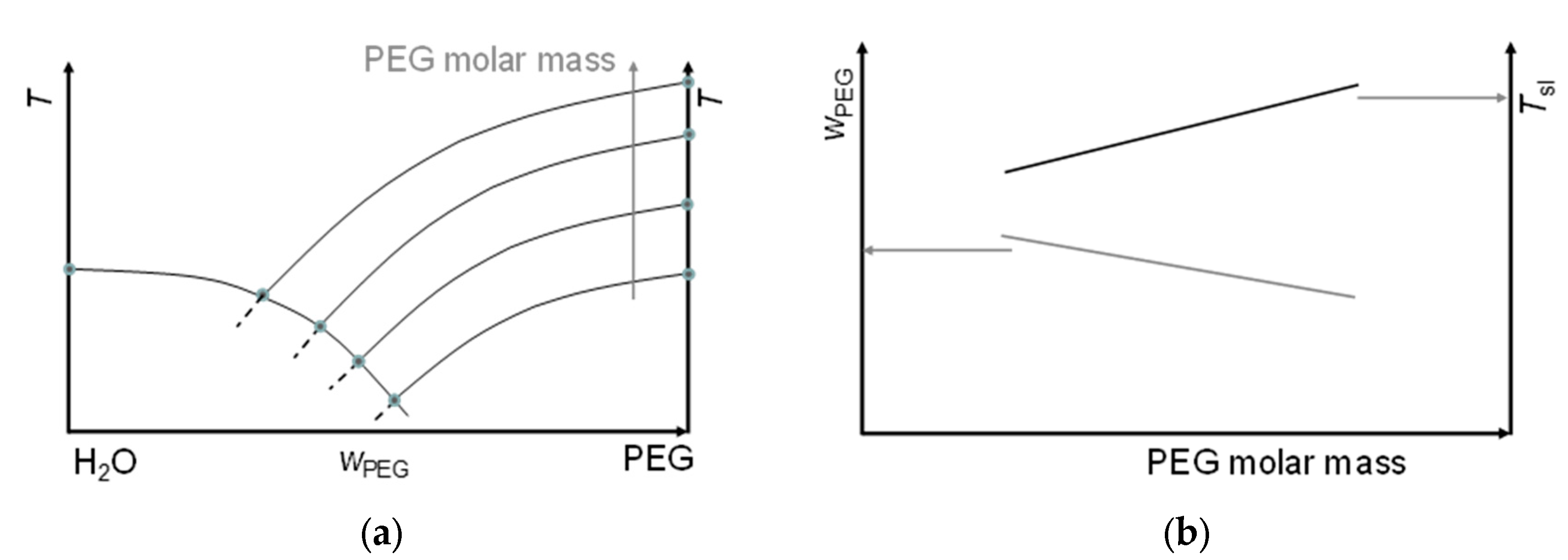
3.1. Mixtures of Water with PEG
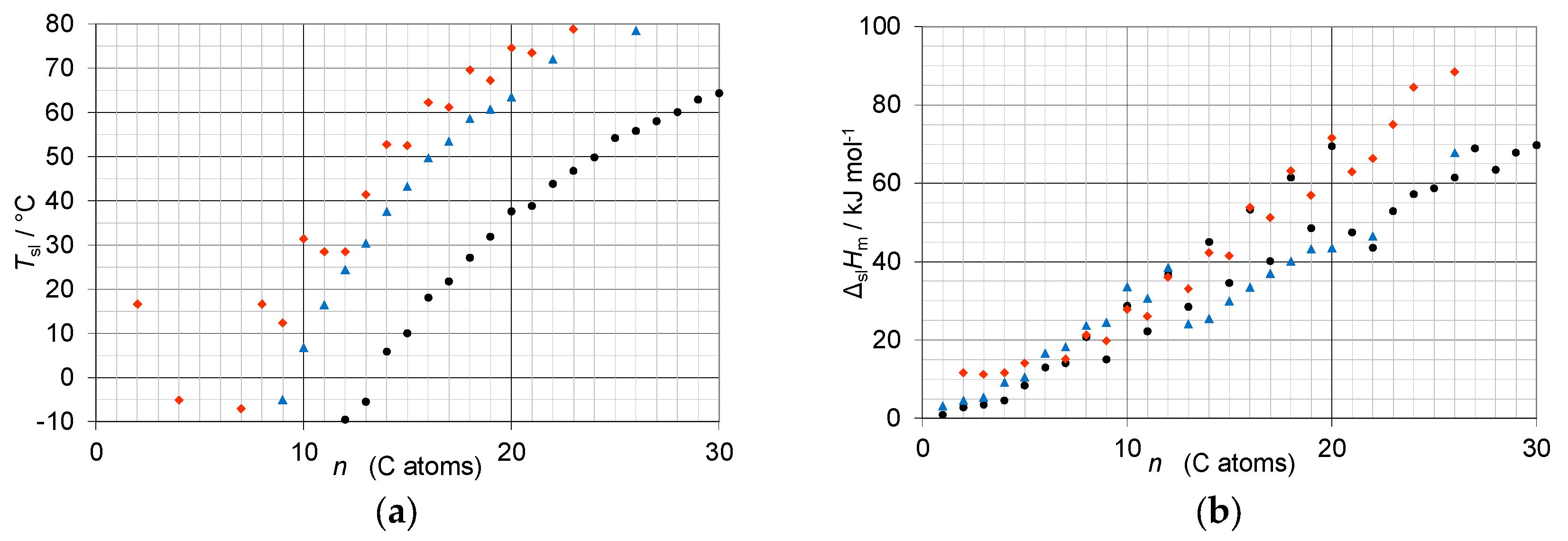
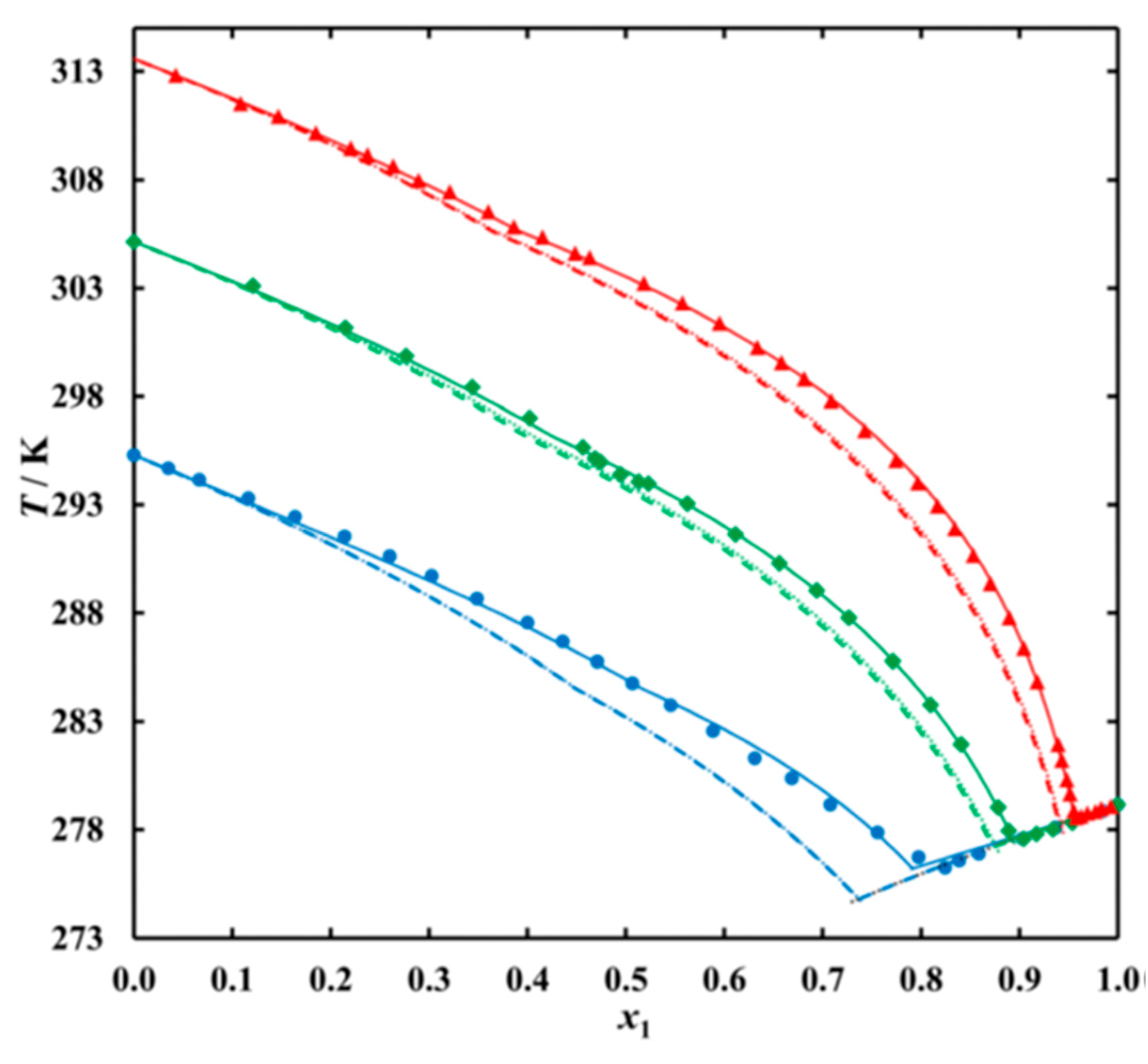
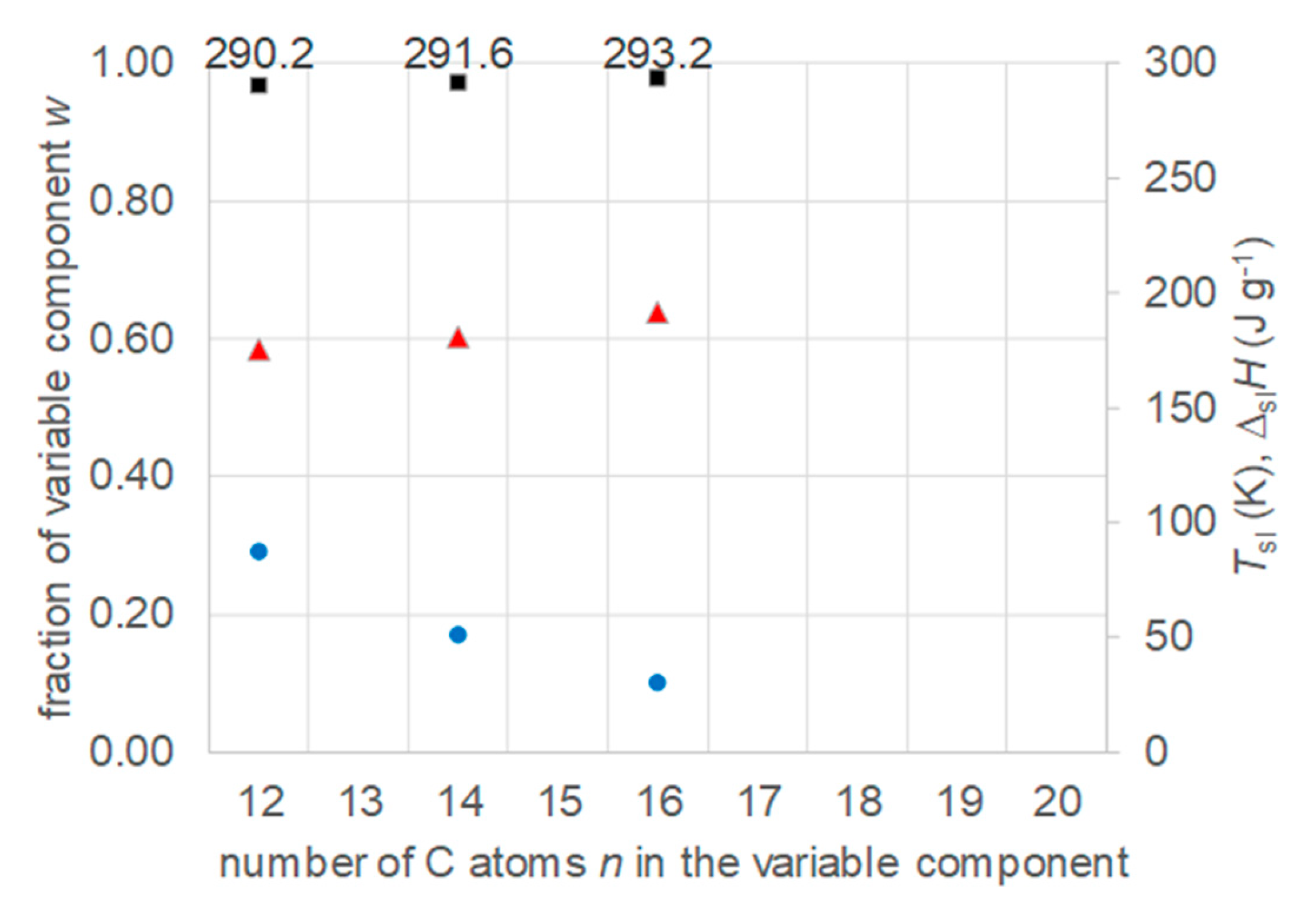
3.2. Mixtures of n-Alkanes with n-Alkanes
3.3. Mixtures of n-Alkanols with n-Alkanols
3.4. Mixtures of n-Alkanoic acids with n-Alkanoic Acids
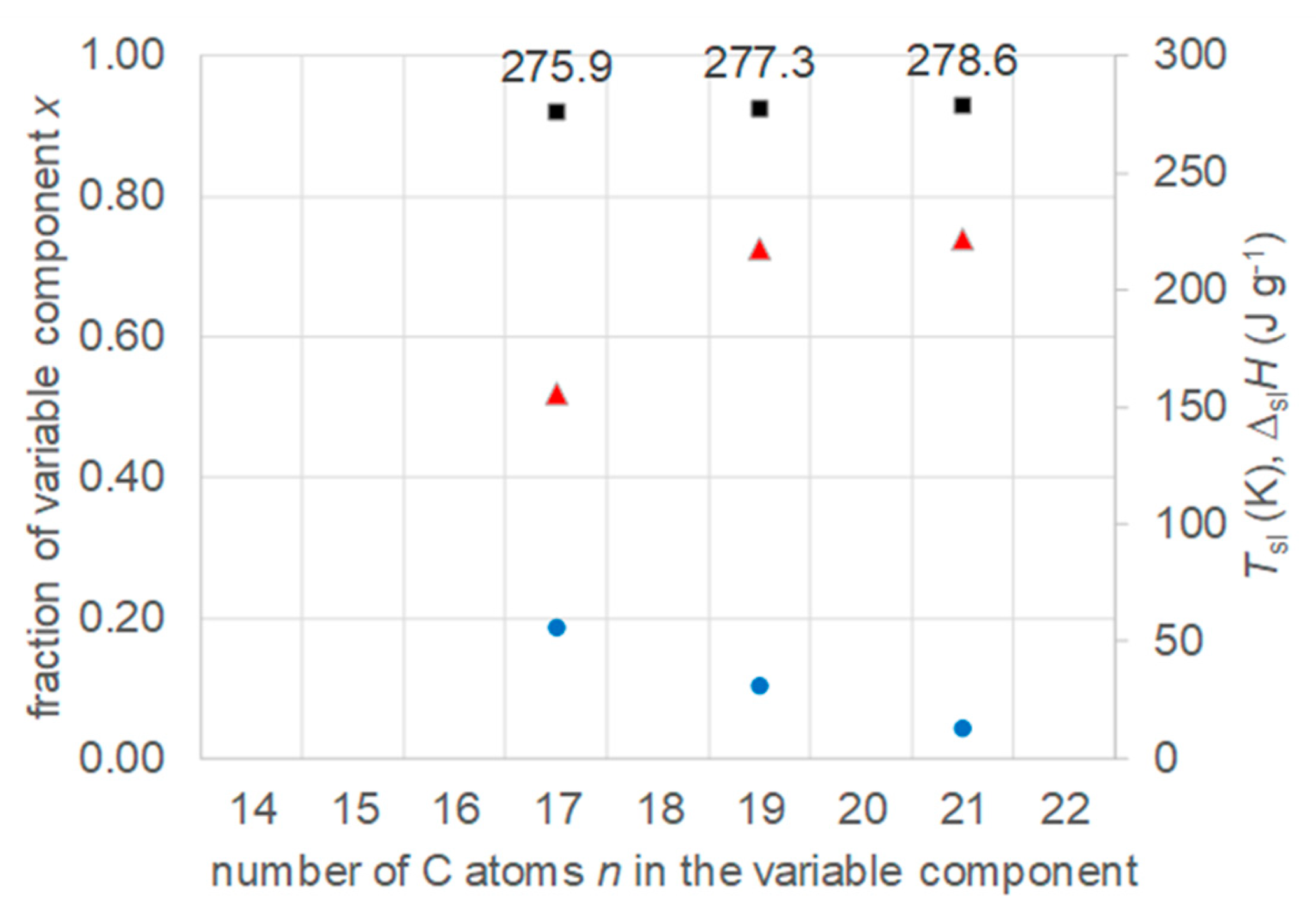
3.5. Mixtures of n-Alkanoic acids with n-Alkanes
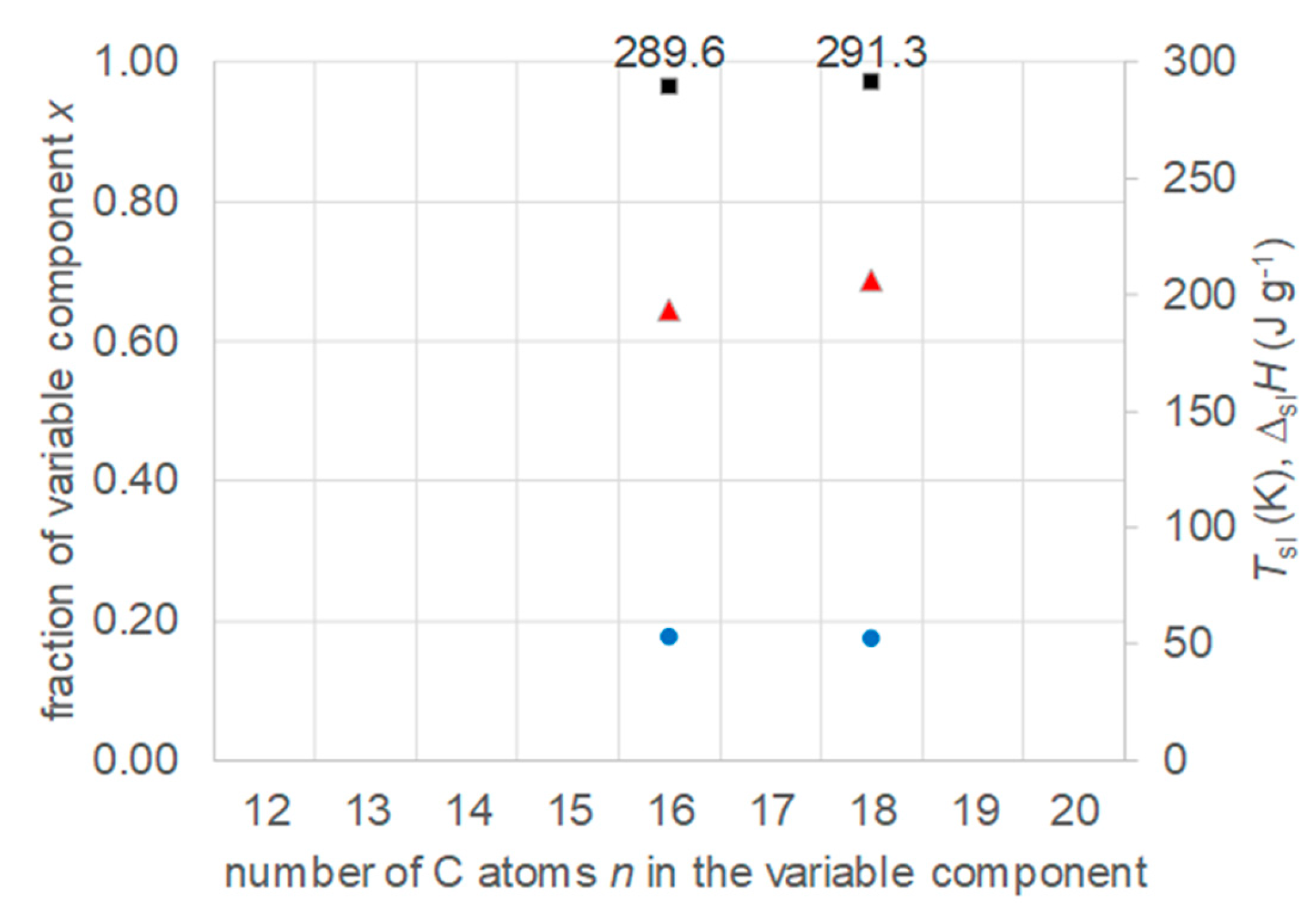
3.6. Mixtures of n-Alkanols with n-Alkanes
3.7. Mixtures of n-Alkanols with n-Alkanoic Acids
4. Discussion
4.1. Mixtures of Water with PEG
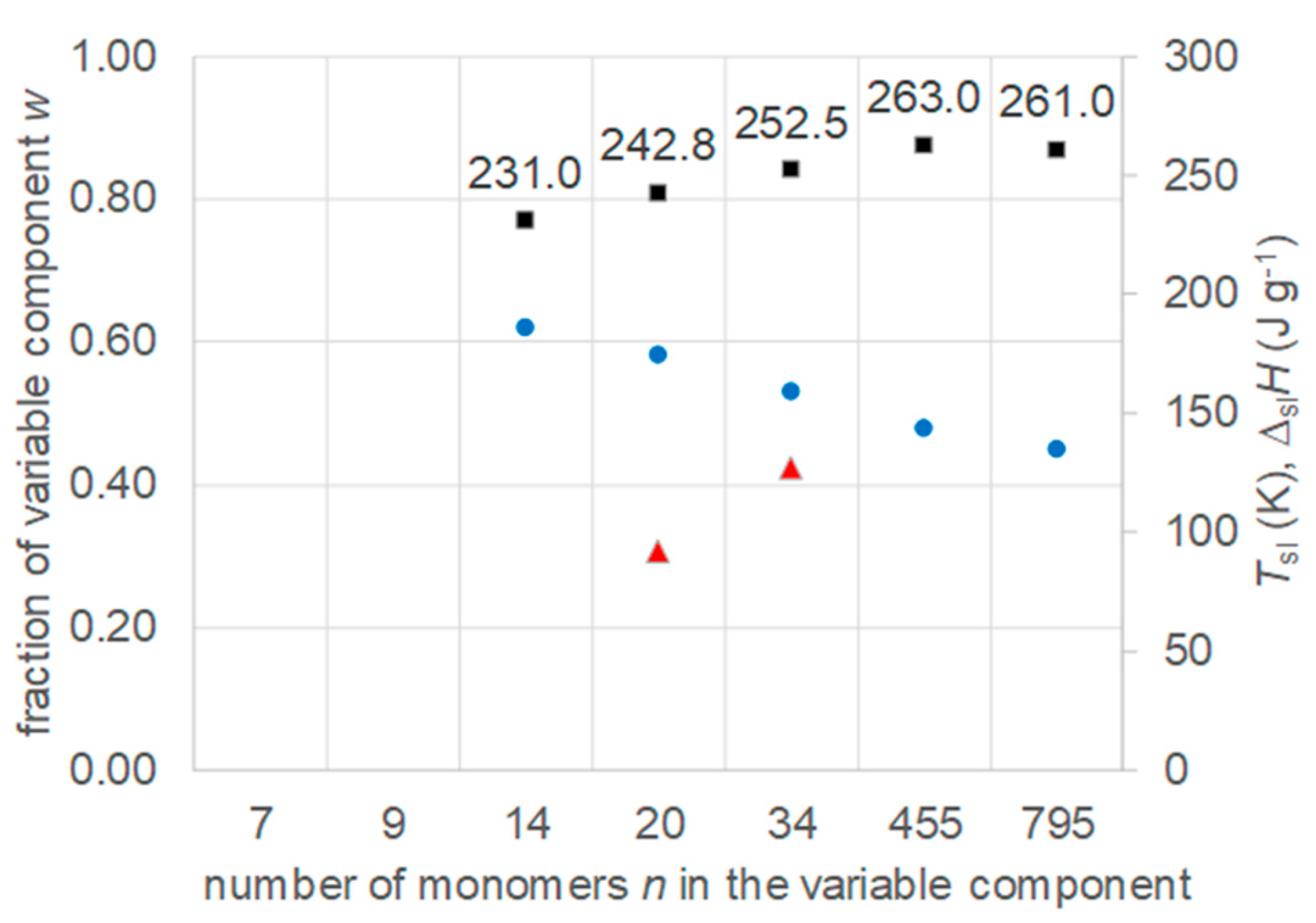
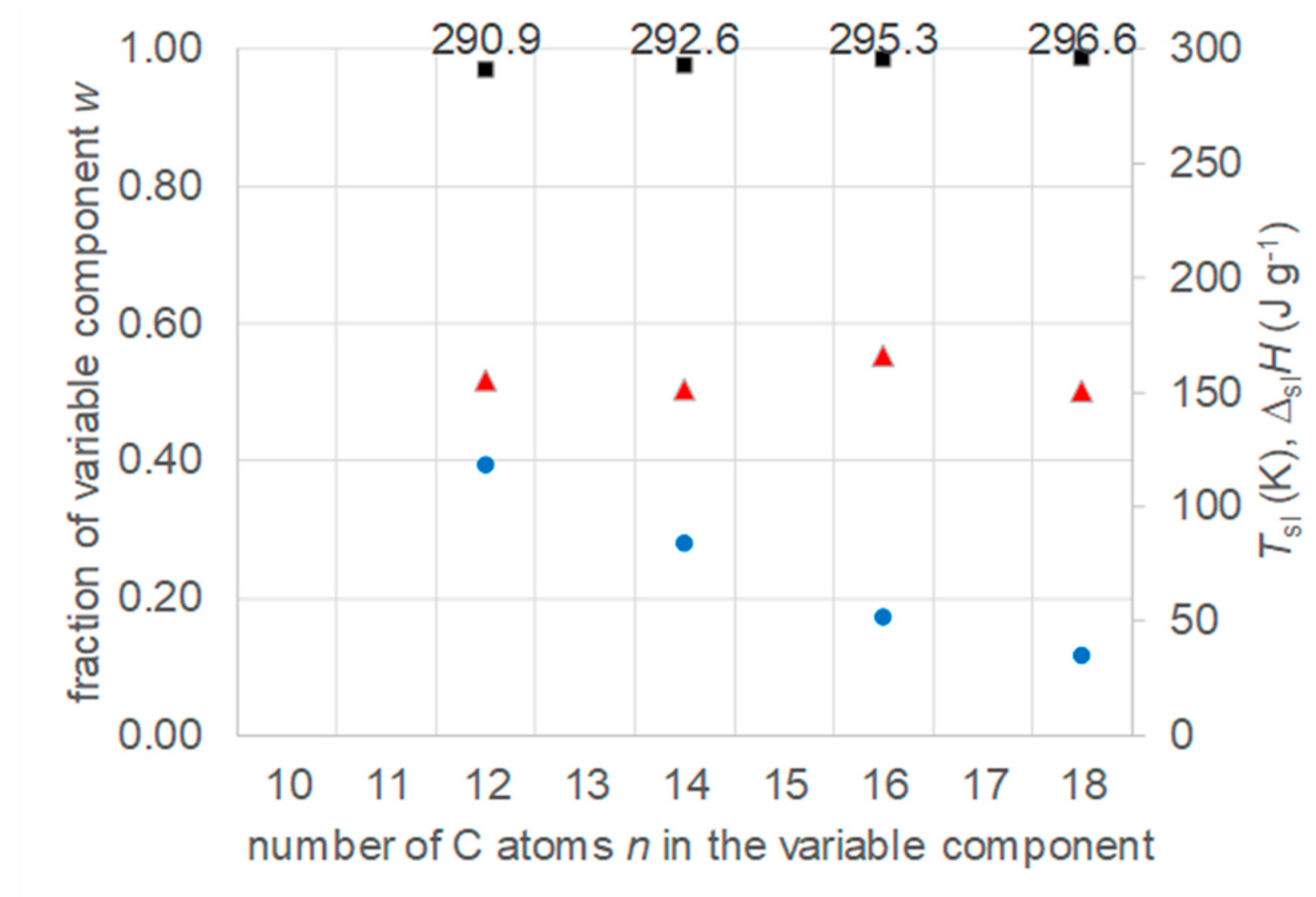
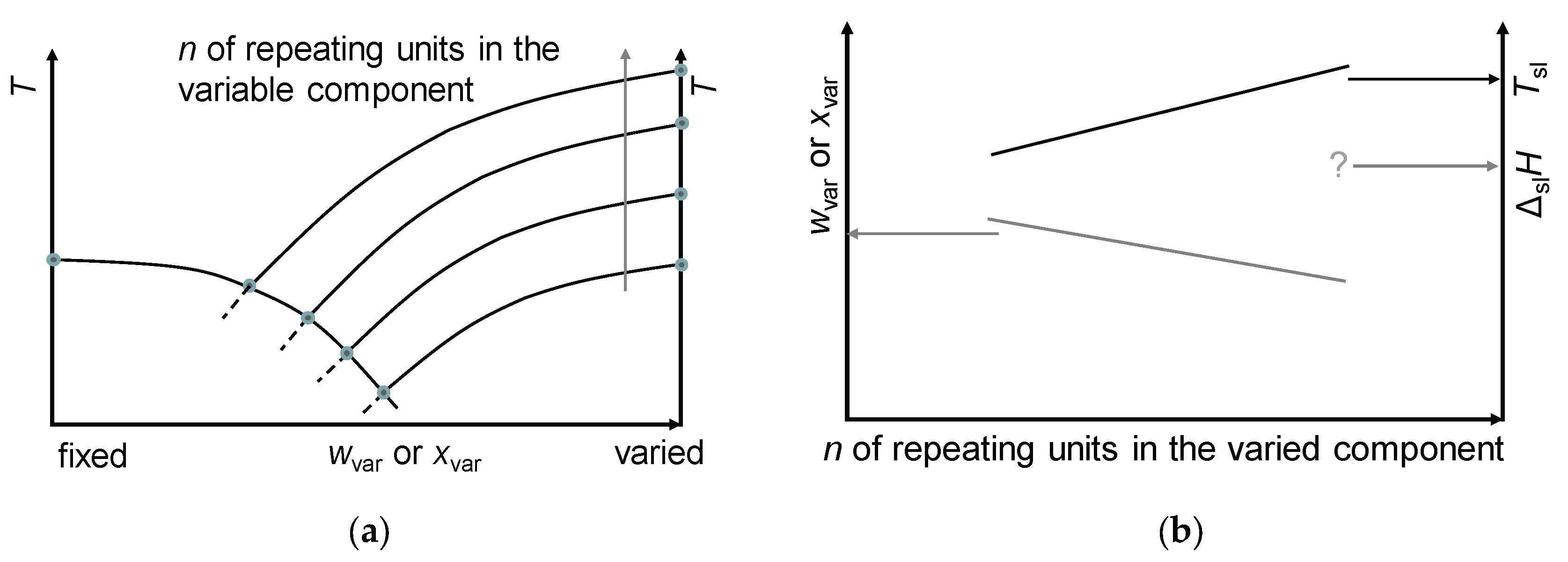
4.2. Other Mixtures with a Fixed Component and a Varied Component from a Homologous Series
4.3. Investigation of the Physical and Chemical Basis
5. Conclusions
5.1. Applications of Systematic Trends
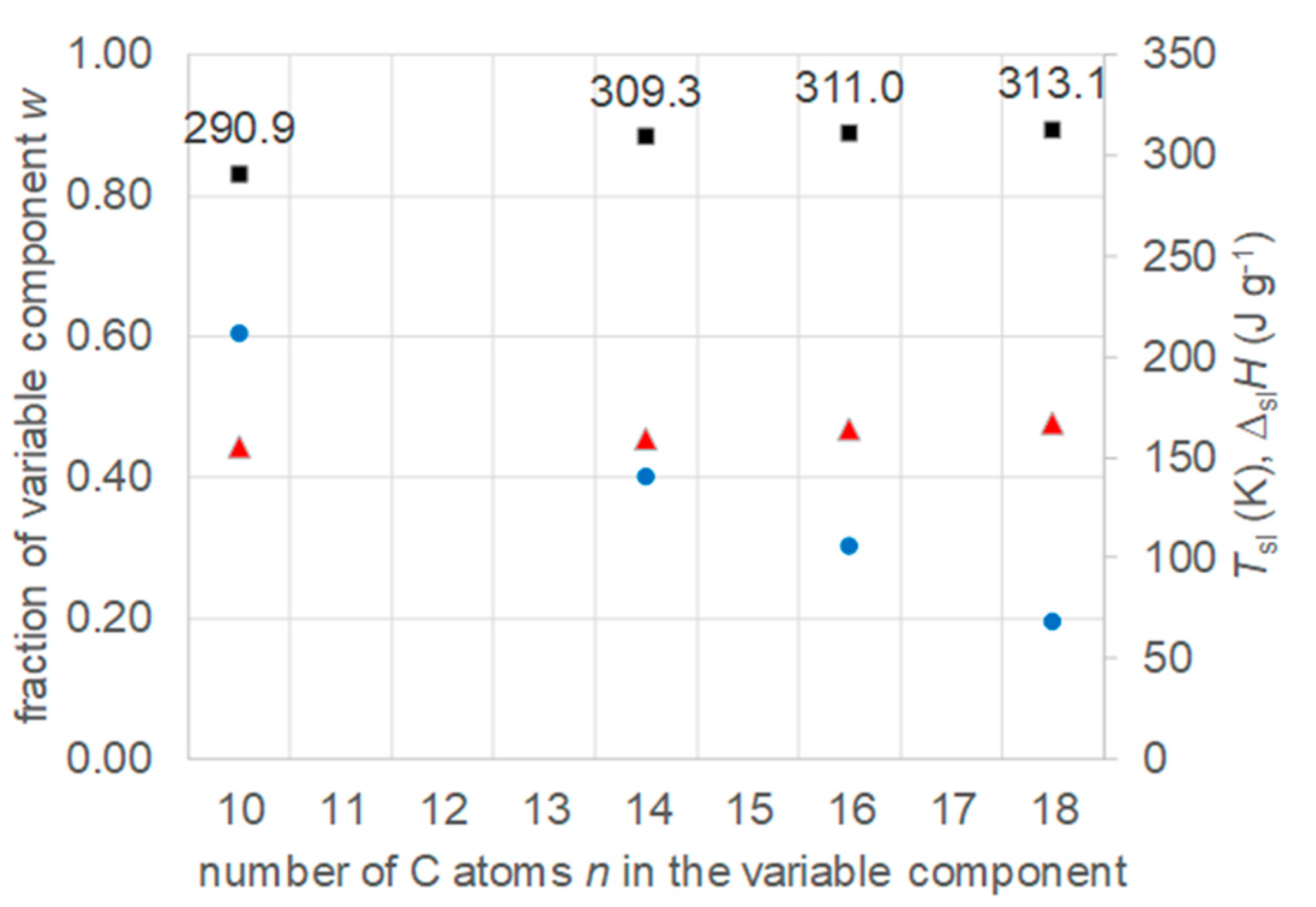
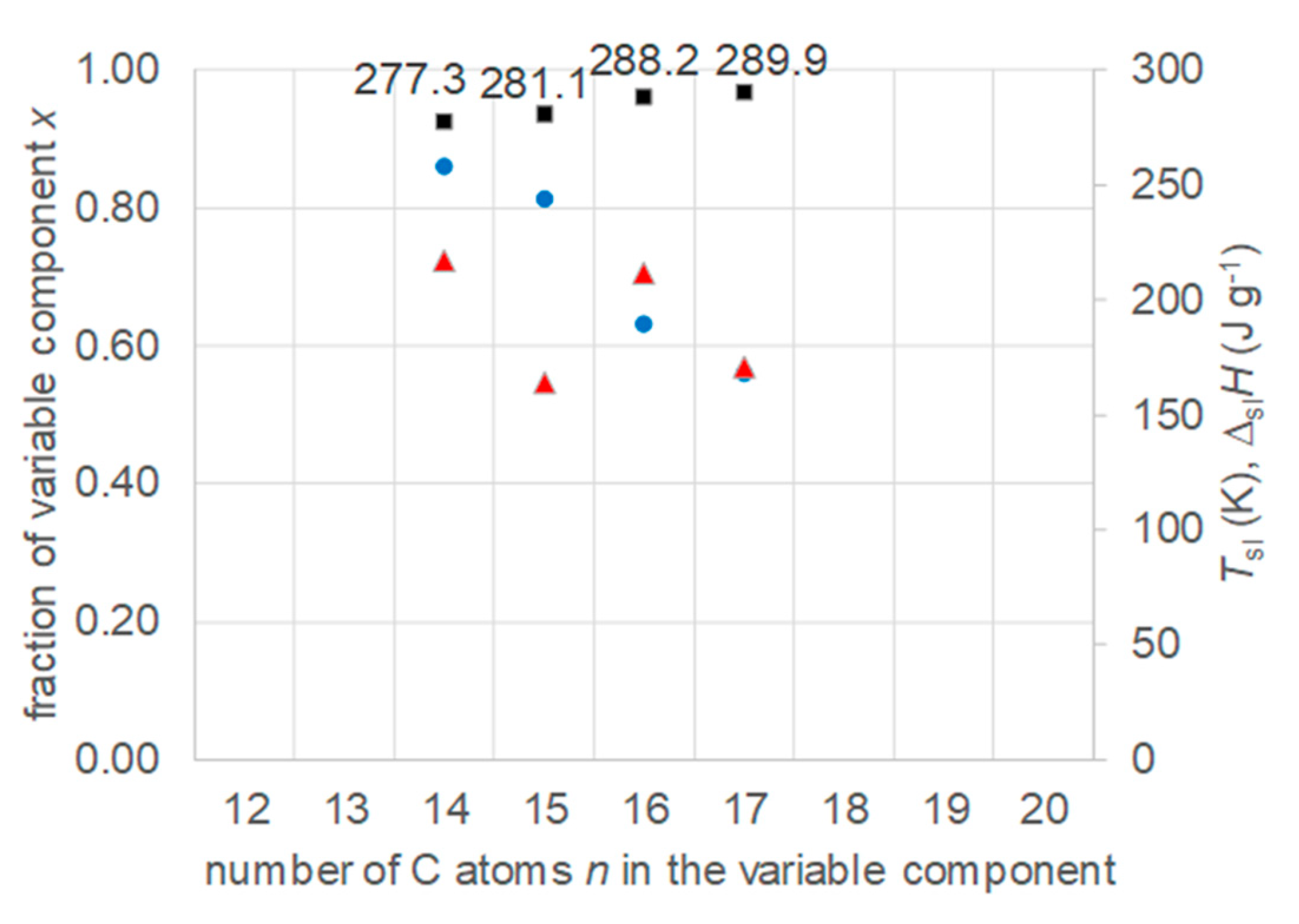
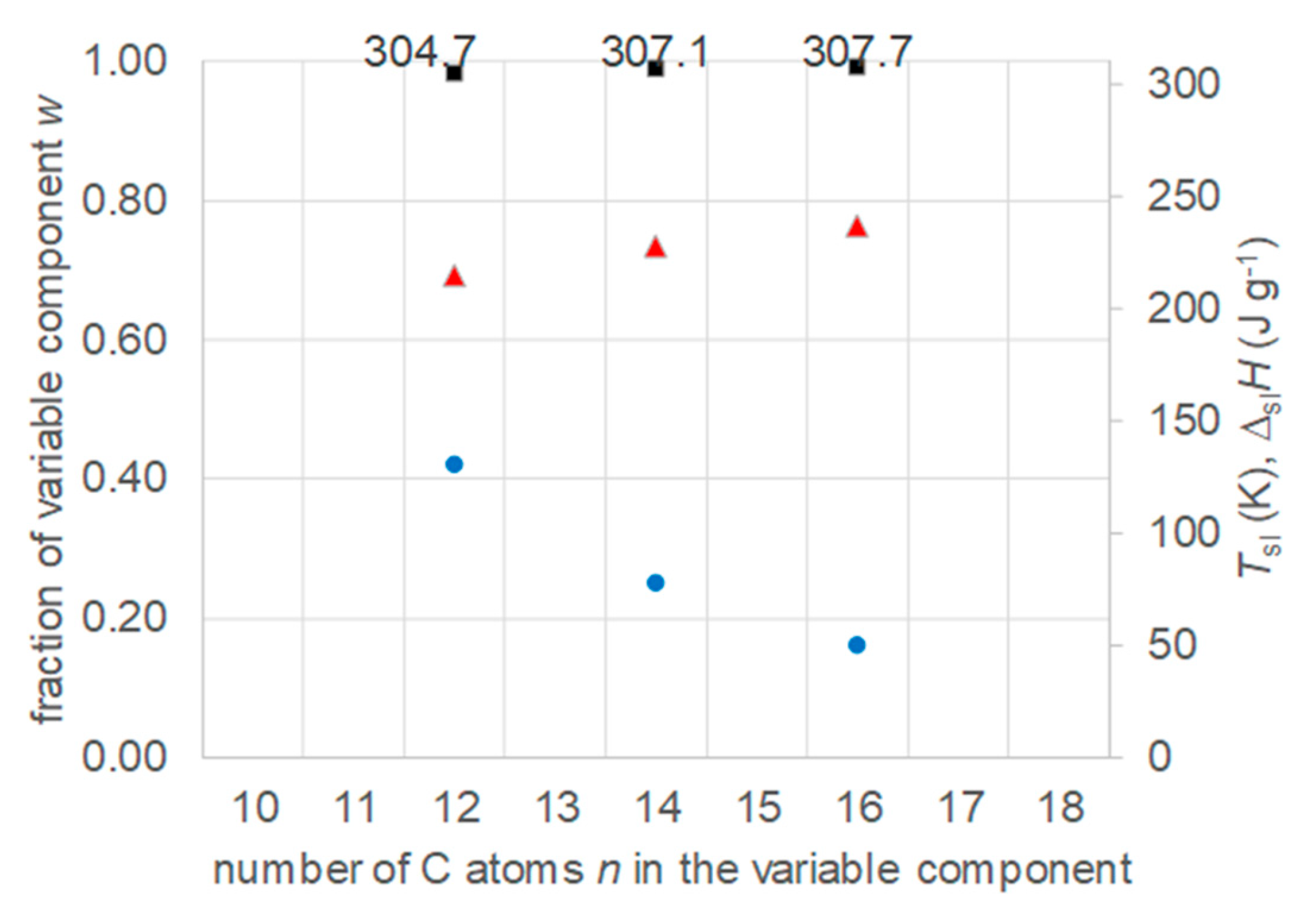
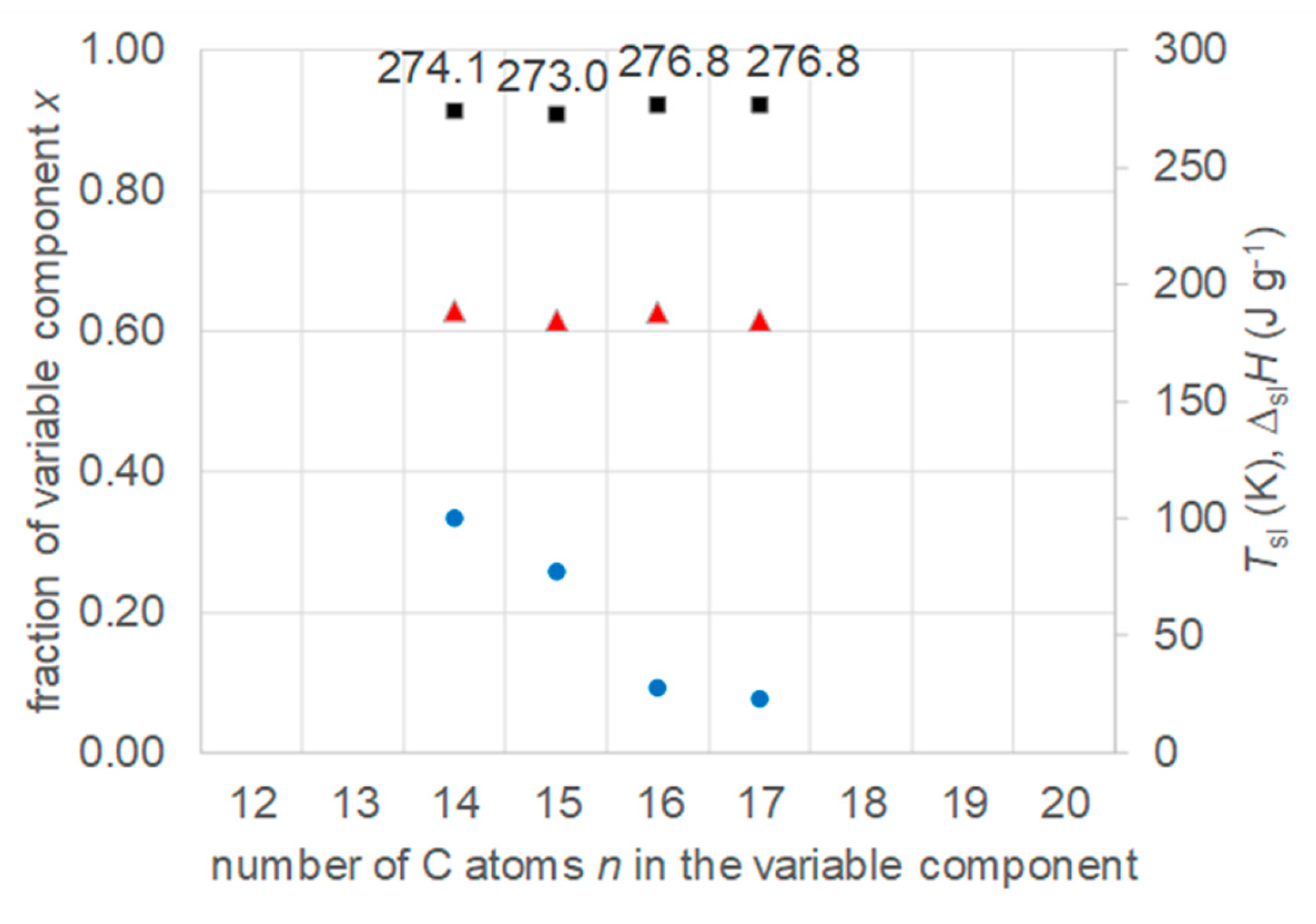
- Regarding PCM selection, the use of eutectic mixtures with one component fixed and the other a varying member from a homologous series allows to vary and adjust Tsl, due to the discovered systematic trends, while other properties stay rather constant. The latter is the crucial difference to other mixtures. For example, for pure n-alkanes microencapsulation is an established process, widely used for thermal management in clothes, and composites with graphite are used for thermal management, e.g. of batteries. In both cases the compatibility of the materials and processes used with pure n-alkanes is established, and consequently also for eutectic mixtures of them. To adjust Tsl to an application, pure n-alkanes or eutectic mixtures of them are thus the primary choice. While pure n-alkanes have however limited choice of Tsl, e.g., between 0°C and 10°C only 5.9°C and 10°C (Figure 1), their eutectic mixtures already with n=14 as fixed basis offer 2.8°C, 4.1°C, and 5.4°C (Figure 6).
- 2.
- Regarding PCM development, a systematic trend in Tsl and in the fraction w or x of eutectics (Figure 14) allows to predict probable Tsl and w or x of new eutectics from a few known ones. For example, knowing Tsl for mixtures of n-alkane n=14 with n=17, 19, and 21 (Figure 6), it is likely that mixtures with other odd n outside that n range have higher Tsl for higher n, and lower Tsl for lower n, or to fill gaps with regard to n. This can be done as well by theoretical models. Theoretical models work for any mixture, can even predict what can be mixed, the phase diagram which allows to assess the phase change behavior, even if a eutectic exists at all. However, specifically regarding Tsl the trends seem to be more precise as some models. Even more, theoretical models require at least knowledge of Tsl and ΔslH of the components, thus literature data or own measurements. However, if a component decomposes before melting, these data cannot be determined at all.
5.2. Future R&D
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCM | Phase Change Material |
| PSE | Periodic System of Elements |
| PE | PolyEthylene |
| PEG | PolyEthyleneGlycol |
| IL | Ionic Liquid |
| IUPAC | International Union of Pure and Applied Chemistry |
| NRTL | Non-Random-Two-Liquid-modell |
| UNIFAC | UNIversal quasichemical Functional group Activity Coefficients |
References
- Mehling, H.; White, M. A. Analysis of trends in phase change enthalpy, entropy and temperature for alkanes, alcohols and fatty acids. Chemical Physics Impact 2023, 6, 100222. [Google Scholar] [CrossRef]
- Soodoo, N.; Poopalam, K. D.; Bouzidi, L.; Narine, S. S. Fundamental structure-function relationships in vegetable oil based phase change materials: A critical review. Journal of Energy Storage 2022, 51, 104355. [Google Scholar] [CrossRef]
- Mishra, D. K.; Bhowmik, S.; Pandey, K. M. Polyethylene Glycol based form stable composite Phase Change Material: a review. J. Phys.: Conf. Ser. 2020, 1455, 012025. [Google Scholar] [CrossRef]
- Jablonski, P. Kalorimetrische Untersuchungen des Systems Polyethylenglykol/Wasser. PhD Thesis, Fakultät für Naturwissenschaften der Gerhard-Mercator-Universität – Gesamthochschule, Duisburg, 2002. [Google Scholar]
- Faden, M.; Höhlein, S.; Wanner, J.; König-Haagen, A.; Brüggemann, D. Review of Thermophysical Property Data of Octadecane for Phase-Change Studies. Materials 2019, 12, 2974. [Google Scholar] [CrossRef] [PubMed]
- Renner, T. Quantities, Units, and Symbols in Physical Chemistry, IUPAC Green Book, 3rd ed.; Editors Cohen, E.R.; Cvitas, T.; Frey, J.G.; Holmstrom, B.; Kuchitsu, K.; Marquardt, R.; Mills, I.; Pavese, F.; Quack, M.; Stohner, J.; Strauss, H.; Takami, M.; Thor, A.J. RSC Publishing, 2007. [CrossRef]
- Huang, L.; Nishinari, K. Interaction Between Poly(ethylene glycol) and Water as Studied by Differential Scanning Calorimetry. Journal of Polymer Science: Part B: Polymer Physics 2001, 39, 496–506. [Google Scholar] [CrossRef]
- Bo, H.; Gustafsson, M.; Setterwall, F. Paraffin Waxes and Their Binary Mixture as Phase Change Materials (PCMs) for Cool Storage in District Cooling System. Workshop paper, IEA Annex 10, Phase Change Materials and Chemical Reactions for Thermal Energy Storage. First Workshop, 16-17 April 1998, Adana, Turkey.
- Mondieig, D.; Rajabalee, F.; Metivaud, V.; Oonk, H. A. J.; Cuevas-Diarte, M. A. n-Alkane Binary Molecular Alloys. Chem. Mater. 2004, 16, 786–798. [Google Scholar] [CrossRef]
- Więckowski, M.; Krolikowski, M.; Scheller, Ł.; Dzida, M. Alkane-based eutectic phase change materials doped with carbon nanomaterials. Phys. Chem. Chem. Phys. 2023, 25, 16979–16990. [Google Scholar] [CrossRef] [PubMed]
- Ventola, L.; Calvet, T.; Cuevas-Diarte, M. A.; Ramírez, M.; Oonk, H. A. J.; Mondieig, D.; Negrier, P. Melting behaviour in the n-alkanol family. Enthalpy–entropy compensation. Phys. Chem. Chem. Phys. 2004, 6, 1786–1791. [Google Scholar] [CrossRef]
- Li, D.; Lenfant, T.; Landry, V.; Rodrigue, D.; Kaboorani, A.; Wang, X. Development and investigation of biobased binary eutectic phase change materials for low-temperature building applications: 1-Hexadecanol/1-Dodecanol and 1-Octadecanol/1-Dodecanol. Journal of Energy Storage 2024, 103, 114345. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, S.; Xiao, X.; Liu, Y. Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage. Separations 2023, 10, 49. [Google Scholar] [CrossRef]
- Więckowski, M.; Królikowski, M. Designing and Characterization of Low-Temperature Eutectic Phase Change Materials Based on Alkanes. J. Chem. Eng. Data 2022, 67, 727–738. [Google Scholar] [CrossRef]
- Bidiyasar, R.; Kumar, R.; Jakhar, N. Thermal Property Prediction of Eicosane-Fatty Acid Eutectic Phase Change Materials Using the Modified UNIFAC Model for Thermal Energy Storage Technology. Energy storage 2025, 7, e70277. [Google Scholar] [CrossRef]
- Kumar, R.; Vyas, S.; Dixit, A. Fatty acids/1-dodecanol binary eutectic phase change materials for low temperature solar thermal applications: Design, development and thermal analysis. Solar Energy 2017, 155, 1373–1379. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M. A. Prediction of the properties of eutectic fatty acid phase change materials. Thermochimica Acta 2018, 660, 94–100. [Google Scholar] [CrossRef]
- Mehling, H.; Thoen, J.; Glorieux, C.; White, M.A. High-Accuracy and High-Resolution Calorimetry Revealing New Correlations of Phase Change Enthalpy, Entropy, and Number of Carbon Atoms n in n-Alkanes. Molecules 2025, 30, 1300. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. C.; Sardo, M.; Rolemberg, M. P.; Ribeiro-Claro, P.; Meirelles, A.; Coutinho, J.; Krähenbühl, M.A. The solid–liquid phase diagrams of binary mixtures of consecutive, even saturated fatty acids: differing by four carbon atoms. Chemistry and Physics of Lipids 2009, 157, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. C. Rolemberg, M. P.; Meirelles, A.; Coutinho, J.; Krähenbühl, M.A. The solid–liquid phase diagrams of binary mixtures of even saturated fatty acids differing by six carbon atoms. Thermochimica Acta 2009, 496, 30–37. [Google Scholar] [CrossRef]
- Królikowski, M.; Więckowski, M.; Żółtańska, K.; Królikowska, M. Eutectic Phase Change Materials based on novel dicationic Isoquinoliunium Ionic Liquids: Synthesis and Characterization. J. Chem. Eng. Data 2024, 69, 958–972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




