Submitted:
08 November 2025
Posted:
11 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Patients and Methods
2.1. Study Design and Patient Selection
2.2. Outcome Measurements
2.3. Statistical Analysis
3. Results
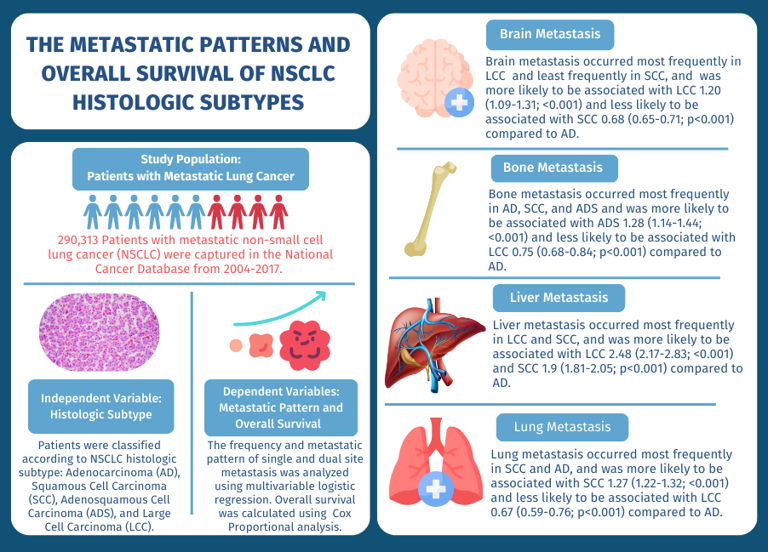
3.1. Patients
3.2. Sites of Metastasis
3.3. Effect of Histologic Subtype
3.4. Dual Site Metastasis
3.5. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Informed Patient Consent and Consent for Publication
Data Availability Statement
Conflicts of Interest Statement
Glossary of Abbreviations
| AD | Adenocarcinoma |
| ADS | Adenosquamous cell carcinoma |
| aHR | Adjusted Hazard Ratio |
| CDCC | Charlson Deyo Comorbidity Score |
| CT | Computed Tomography |
| HR | Hazard Ratio |
| ICD-O-3 | International Classification of Diseases for Oncology, 3rd edition |
| LCC | Large Cell Carcinoma |
| NCDB | National Cancer Database |
| NSCLC | Non-Small Cell Lung Cancer |
| OR | Odds Ratio |
| OS | Overall Survival |
| SCC | Squamous Cell Carcinoma |
| scRNA | Single-Cell-RNA |
| SD | Standard Deviation |
| TNM | Tumor, Node, Metastasis |
References
- MacConaill, L. E. et al. Profiling critical cancer gene mutations in clinical tumor samples. PLoS ONE 4, (2009).
- Dang, A. T. H. et al. Actionable Mutation Profiles of Non-Small Cell Lung Cancer patients from Vietnamese population. Scientific Reports 10, 1–11 (2020).
- Questions, G. Lung Cancer Biomarkers Guideline. Hematol Oncol Clin North Am. 2017 31, 13–29 (2017).
- Xiao, W. et al. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: A population-based study. Cancer Management and Research (2018). [CrossRef]
- Dudani, S. et al. Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association with Survival. JAMA Network Open 4, 1–11 (2021).
- Ren, Y. et al. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget (2016). [CrossRef]
- Siegel, R. L., Miller, K. D., Wagle, N.S., Jemal, A. Cancer statistics. CA: A Cancer Journal for Clinicians (2023). [CrossRef]
- Morgensztern, D., Ng, S. H., Gao, F. & Govindan, R. Trends in stage distribution for patients with non-small cell lung cancer: A national cancer database survey. Journal of Thoracic Oncology (2010). [CrossRef]
- Detterbeck, F. C., Boffa, D. J. & Tanoue, L. T. The new lung cancer staging system. Chest (2009). [CrossRef]
- Riihimäki, M. et al. Metastatic sites and survival in lung cancer. Lung Cancer 86, 78–84 (2014).
- Oikawa, A. et al. Application of conditional probability analysis to distant metastases from lung cancer. Oncology Letters 3, (2012).
- Quint, L. E. et al. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Annals of Thoracic Surgery 62, (1996).
- Hess, K. R. et al. Metastatic patterns in adenocarcinoma. Cancer 106, (2006).
- Kim, N. et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nature Communications 11, (2020).
- Weissleder, R. & Pittet, M. J. The expanding landscape of inflammatory cells affecting cancer therapy. Nature Biomedical Engineering 4, 489–498 (2020).
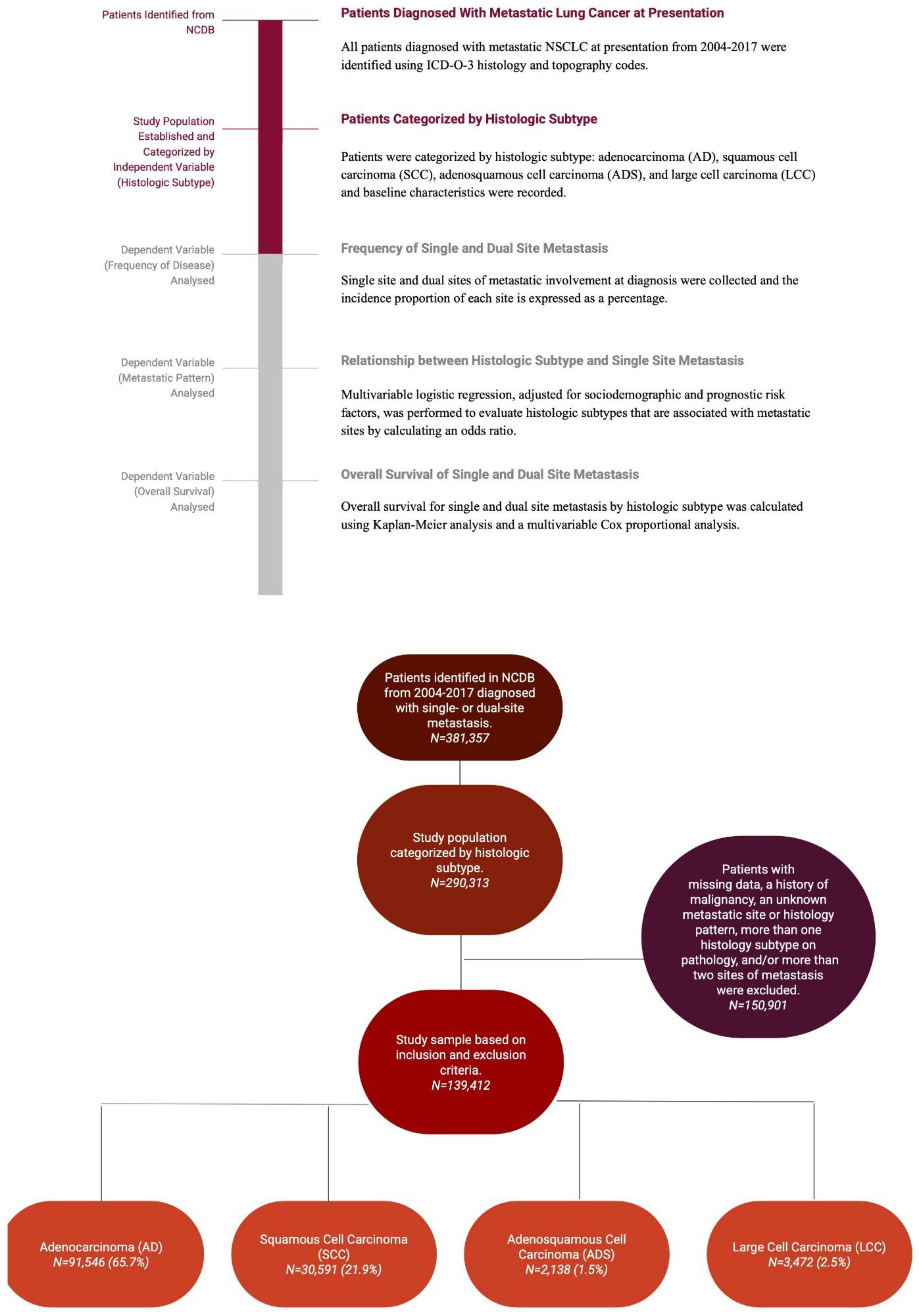
| Factor | Level | Adenocarcinoma (AD) | Squamous Cell Carcinoma (SCC) | Adenosquamous Carcinoma (ADS) | Large Cell Carcinoma (LCC) |
|---|---|---|---|---|---|
| N | 202,909 | 71,622 | 4,471 | 11,311 | |
| Age at Diagnosis, mean (SD) | 65.7 (11.4) | 68.0 (10.4) | 66.6 (11.2) | 65.1 (11.1) | |
| Sex | Male | 103,467 (51.0%) | 46,163 (64.5%) | 2,528 (56.5%) | 6,722 (59.4%) |
| Female | 99,442 (49.0%) | 25,459 (35.5%) | 1,943 (43.5%) | 4,589 (40.6%) | |
| Race | American Indian | 534 (0.3%) | 247 (0.3%) | 13 (0.3%) | 34 (0.3%) |
| Asian | 8,136 (4.0%) | 1,363 (1.9%) | 158 (3.5%) | 158 (1.4%) | |
| Black | 25,940 (12.8%) | 9,526 (13.3%) | 519 (11.6%) | 1,472 (13.0%) | |
| White | 165,586 (81.6%) | 59,687 (83.3%) | 3722 (83.2%) | 9,525 (84.2%) | |
| Other | 2,713 (1.3%) | 799 (1.1%) | 59 (1.3%) | 122 (1.1%) | |
| Median Income Quartile | 0-25th Percentile | 40,592 (21.3%) | 17,292 (25.6%) | 958 (22.6%) | 2,900 (26.8%) |
| 26-50th Percentile | 44,341 (23.2%) | 17,266 (25.5%) | 991 (23.3%) | 2,802 (25.9%) | |
| 51-75th Percentile | 44,887 (23.5%) | 15,784 (23.4%) | 1,006 (23.7%) | 2,488 (23.0%) | |
| 76-100th Percentile | 60,969 (32.0%) | 17,248 (25.5%) | 1,292 (30.4%) | 2,626 (24.3%) | |
| Insurance Status | Unknown | 3,401 (1.7%) | 1,172 (1.6%) | 88 (2.0%) | 208 (1.8%) |
| Not insured | 9,772 (4.8%) | 3,189 (4.5%) | 190 (4.2%) | 630 (5.6%) | |
| Private or Government Insurance | 68,154 (33.6%) | 17,989 (25.1%) | 1,398 (31.3%) | 3,636 (32.1%) | |
| Medicaid | 18,481 (9.1%) | 6,195 (8.6%) | 383 (8.6%) | 1,063 (9.4%) | |
| Medicare | 103,101 (50.8%) | 43,077 (60.1%) | 2,412 (53.9%) | 5,774 (51.0%) | |
| Facility Type | Community Cancer Program | 15,553 (7.7%) | 7,135 (10.0%) | 404 (9.1%) | 896 (8.0%) |
| Comprehensive Community Cancer Program | 80,972 (40.3%) | 30,510 (42.7%) | 2,094 (47.3%) | 5,141 (45.9%) | |
| Academic/Research Program | 65,426 (32.6%) | 20,160 (28.2%) | 1,216 (27.4%) | 2,561 (22.8%) | |
| Integrated Network Cancer Program | 38,806 (19.3%) | 13,582 (19.0%) | 717 (16.2%) | 2,613 (23.3%) | |
| Great Circle Distance, mean (SD) | 27.0 (108.1) | 23.8 (86.4) | 26.0 (100.9) | 24.9 (89.2) | |
| Charlson-Deyo Comorbidity Score | Total Score of 0 | 130,278 (64.2%) | 39,801 (55.6%) | 2,805 (62.7%) | 6,819 (60.3%) |
| Total Score of 1 | 48,076 (23.7%) | 20,076 (28.0%) | 1,094 (24.5%) | 3,037 (26.8%) | |
| Total Score of 2 | 16,222 (8.0%) | 7,870 (11.0%) | 388 (8.7%) | 999 (8.8%) | |
| Total Score ≥3 | 8,333 (4.1%) | 3,875 (5.4%) | 184 (4.1%) | 456 (4.0%) | |
| Year of Diagnosis | 2004 | 7,018 (3.5%) | 3,134 (4.4%) | 124 (2.8%) | 993 (8.8%) |
| 2005 | 7,374 (3.6%) | 3,260 (4.6%) | 133 (3.0%) | 966 (8.5%) | |
| 2006 | 7,680 (3.8%) | 3,317 (4.6%) | 141 (3.2%) | 859 (7.6%) | |
| 2007 | 8,430 (4.2%) | 3,562 (5.0%) | 172 (3.8%) | 916 (8.1%) | |
| 2008 | 10,922 (5.4%) | 4,454 (6.2%) | 234 (5.2%) | 990 (8.8%) | |
| 2009 | 12,756 (6.3%) | 5,157 (7.2%) | 301 (6.7%) | 920 (8.1%) | |
| 2010 | 15,134 (7.5%) | 5,629 (7.9%) | 373 (8.3%) | 905 (8.0%) | |
| 2011 | 16,057 (7.9%) | 5,669 (7.9%) | 365 (8.2%) | 796 (7.0%) | |
| 2012 | 17,256 (8.5%) | 5,884 (8.2%) | 452 (10.1%) | 728 (6.4%) | |
| 2013 | 18,823 (9.3%) | 6,210 (8.7%) | 442 (9.9%) | 684 (6.0%) | |
| 2014 | 19,645 (9.7%) | 6,397 (8.9%) | 441 (9.9%) | 692 (6.1%) | |
| 2015 | 20,121 (9.9%) | 6,351 (8.9%) | 437 (9.8%) | 651 (5.8%) | |
| 2016 | 20,728 (10.2%) | 6,241 (8.7%) | 468 (10.5%) | 624 (5.5%) | |
| 2017 | 20,965 (10.3%) | 6,357 (8.9%) | 388 (8.7%) | 587 (5.2%) | |
| T Stage | 1A | 3,545 (2.1%) | 595 (1.0%) | 46 (1.2%) | 185 (1.9%) |
| 1B | 11,439 (6.7%) | 1,752 (2.8%) | 177 (4.6%) | 555 (5.8%) | |
| 2A | 15,120 (8.9%) | 2,909 (4.7%) | 258 (6.7%) | 670 (7.0%) | |
| 2B | 22,249 (13.1%) | 5,888 (9.6%) | 476 (12.3%) | 1,129 (11.8%) | |
| 3A | 12,495 (7.4%) | 4,475 (7.3%) | 306 (7.9%) | 700 (7.3%) | |
| 3B | 28,709 (16.9%) | 12,517 (20.3%) | 832 (21.5%) | 1,648 (17.2%) | |
| 3C | 76,184 (44.9%) | 33,518 (54.4%) | 1,774 (45.9%) | 4,710 (49.1%) | |
| N Stage | N0 | 47,208 (23.3%) | 15,402 (21.5%) | 979 (21.9%) | 2,355 (20.8%) |
| N1 | 19,363 (9.5%) | 7,381 (10.3%) | 423 (9.5%) | 1,084 (9.6%) | |
| N2 | 89,248 (44.0%) | 33,615 (46.9%) | 2,012 (45.0%) | 5,328 (47.1%) | |
| N3 | 47,090 (23.2%) | 15,224 (21.3%) | 1,057 (23.6%) | 2,544 (22.5%) | |
| Tumor Grade | Well Differentiated | 5173 (2.5%) | 937 (1.3%) | 17 (0.4%) | 16 (0.1%) |
| Moderately Differentiated | 20,934 (10.3%) | 12,462 (17.4%) | 301 (6.7%) | 43 (0.4%) | |
| Poorly Differentiated | 48,835 (24.1%) | 22,411 (31.3%) | 1,723 (38.5%) | 3,161 (27.9%) | |
| Undifferentiated, Anaplastic | 891 (0.4%) | 433 (0.6%) | 52 (1.2%) | 2,069 (18.3%) | |
| Undetermined, High-Grade Dysplasia | 127,076 (62.6%) | 35,379 (49.4%) | 2,378 (53.2%) | 6,022 (53.2%) | |
| Treatment | Received Medical Treatment | 926 (0.5%) | 162 (0.2%) | 9 (0.2%) | 19 (0.2%) |
| No Medical Treatment Received | 201,983 (99.5%) | 71,460 (99.8%) | 4,462 (99.8%) | 11,292 (99.8%) | |
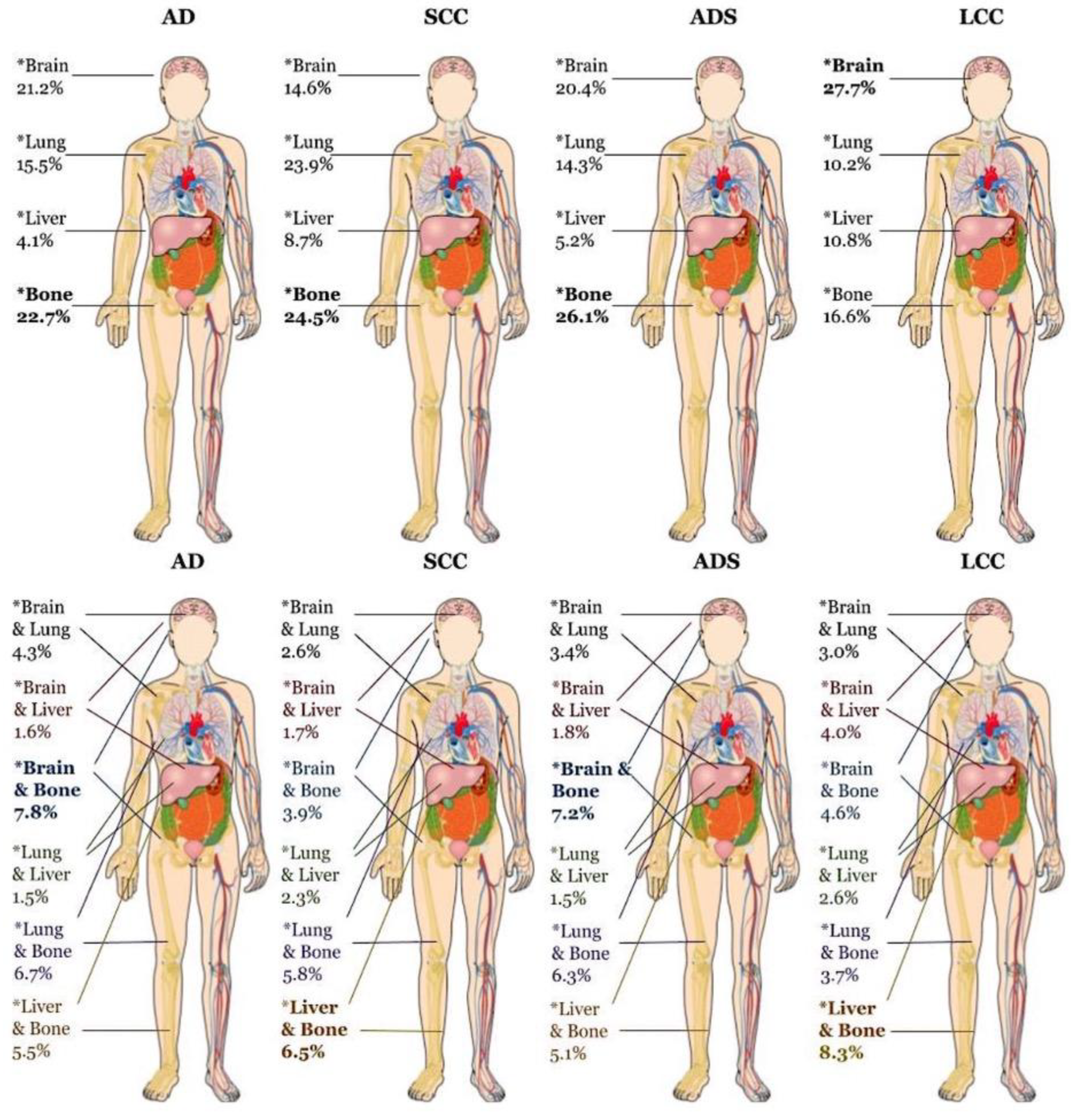
| Metastasis Pattern | Bone Only | 22,874 (22.7%) | 7,906 (24.4%) | 612 (26.1%) | 629 (16.6%) |
| Brain Only | 21,343 (21.2%) | 4,734 (14.6%) | 478 (20.4%) | 1,052 (27.7%) | |
| Liver Only | 4,115 (4.1%) | 2,820 (8.7%) | 121 (5.2%) | 409 (10.8%) | |
| Lung Only | 15,635 (15.5%) | 7,742 (23.9%) | 334 (14.3%) | 388 (10.2%) | |
| Brain & Lung | 4,329 (4.3%) | 853 (2.6%) | 80 (3.4%) | 115 (3.0%) | |
| Brain & Liver | 1,654 (1.6%) | 548 (1.7%) | 42 (1.8%) | 153 (4.0%) | |
| Brain & Bone | 7,843 (7.8%) | 1,249 (3.9%) | 169 (7.2%) | 174 (4.6%) | |
| Lung & Liver | 1,499 (1.5%) | 756 (2.3%) | 34 (1.5%) | 97 (2.6%) | |
| Lung & Bone | 6,737 (6.7%) | 1,884 (5.8%) | 148 (6.3%) | 140 (3.7%) | |
| Liver & Bone | 5,517 (5.5%) | 2,099 (6.5%) | 120 (5.1%) | 315 (8.3%) |
| *aOR (P-Value; 95% Confidence Intervals) | ||||
|---|---|---|---|---|
| Metastatic Site | ||||
| Histology Subtype | Bone | Brain | Lung | Liver |
| AD | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| SCC | 1.01 (0.76; 0.97-1.04) | 0.68 (<0.0001; 0.65-0.71) | 1.27 (<0.0001; 1.22-1.32) | 1.9 (<0.0001; 1.81-2.05) |
| ADS | 1.28 (<0.0001; 1.14-1.44) | 1.10 (0.16; 0.97-1.25) | 1.01 (0.84; 0.88-1.17) | 1.49 (<0.0001; 1.21-1.80) |
| LCC | 0.75 (<0.0001; 0.68-0.84) | 1.20 (<0.0001; 1.09-1.31) | 0.67 (<0.0001; 0.59-0.76) | 2.48 (<0.0001; 2.17-2.83) |
| *Adjusted for: age, sex, race, median income by quartile, insurance status, hospital facility type, living distance | ||||
| from hospital, CDCC score, year of diagnoses, t stage, n stage, and grade (medical treatment was excluded from | ||||
| model due to <0.5% of the population receiving any medical treatment) | ||||
| *aOR (P-Value; 95% Confidence Intervals) | ||||
|---|---|---|---|---|
| Initial Metastatic Site | ||||
| Concurrent Metastatic Site | Bone | Brain | Lung | Liver |
| Bone | -- | 0.95(<0.0001; 0.93-0.97) | 1.27 (<0.0001; 1.24-1.30) | 3.34 (<0.0001; 3.26- 3.43) |
| Brain | 0.95 (<0.0001; 0.93-0.98) | -- | 1.03 (0.014; 1.01-1.06) | 1.33 (<0.0001; 1.30-1.37) |
| Lung | 1.30 (<0.0001; 1.27-1.33) | 1.06 (<0.0001; 1.03-1.09) | -- | 1.56 (<0.0001; 1.52-1.61) |
| Liver | 3.37 (<0.0001; 3.28-3.46) | 1.32 (<0.0001; 1.28-1.35) | 1.51 (<0.0001; 1.47-1.56) | -- |
| *Adjusted for: age, sex, race, median income by quartile, insurance status, hospital facility type, living distance | ||||
| from hospital, CDCC score, year of diagnoses, t stage, n stage, and grade (medical treatment was excluded from | ||||
| model due to <0.5% of the population receiving any medical treatment) | ||||
|
*aHR (95% Confidence Intervals) Median Survival in Months (95% Confidence Intervals; Number of Patients) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metastatic Site and Overall Survival | ||||||||||||
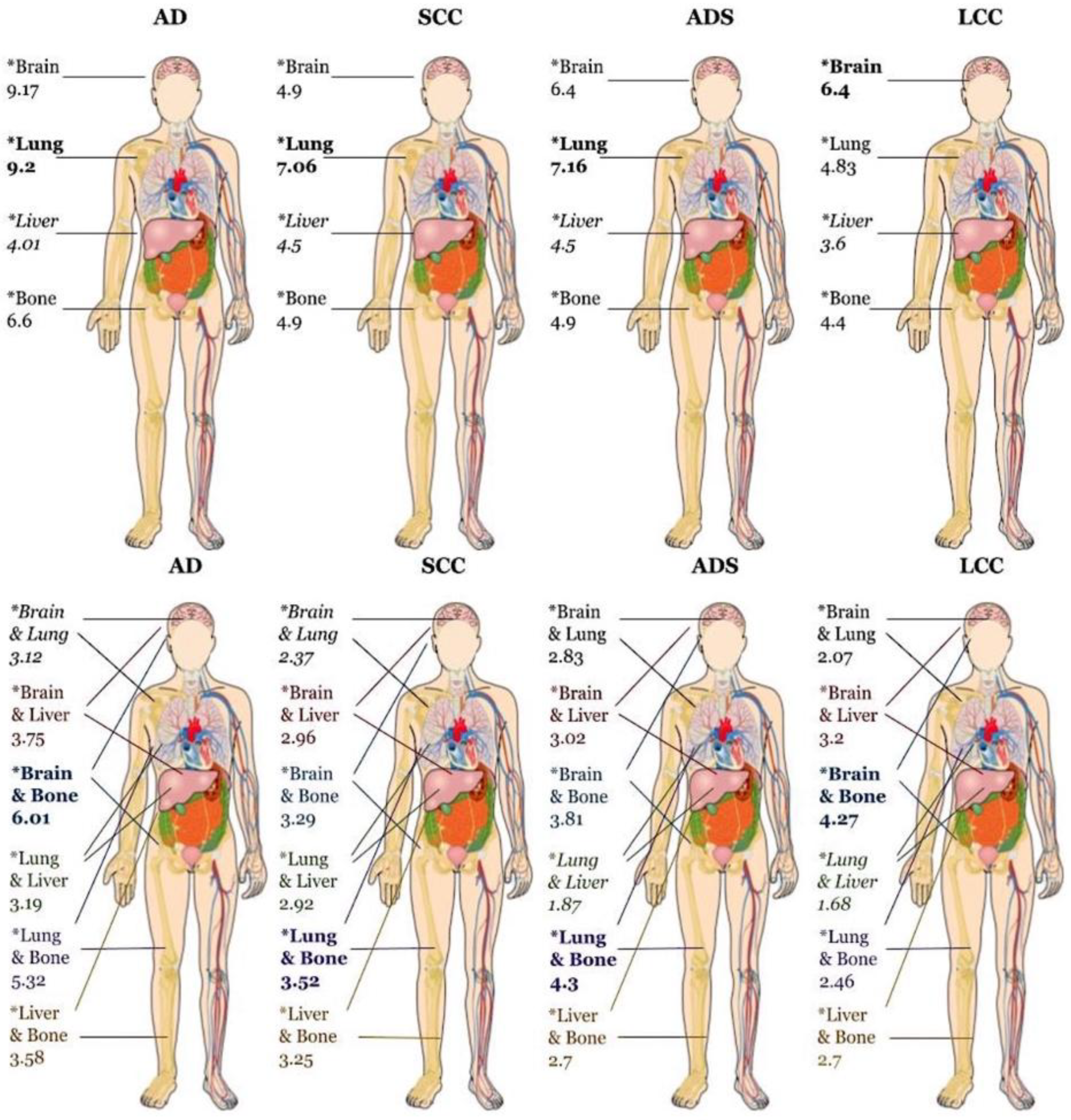
| Histologic Subtype | Bone | Median Survival | Brain | Median Survival | Lung | Median Survival | Liver | Median Survival | ||||
| AD | 1.08* (1.06-1.10) |
6.6* (6.44-6.77; 22,872) |
0.95* (0.93-0.97) |
9.17* (8.9-9.43; 21,340) | 0.79* (0.77-0.81) | 9.2* (8.87-9.53; 15,635) | 1.28* (1.23-1.33) | 4.01* (3.68-4.27; 4,113) |
||||
| SCC | 1.17* (1.13-1.21) |
4.9* (4.44-4.8; 7,906) |
1.18 (1.14-1.23) |
4.9* (4.7-5.13; 4,732) |
0.82* (0.80-0.85) | 7.06* (6.8-7.36; 7,742) | 1.19* (1.24-1.25) | 4.5* (4.24-4.93; 2,819) | ||||
| ADS | 1.73** (1.04-1.33) |
4.9** (4.2-5.49; 612) |
0.97 (0.85-1.11) |
6.4** (5.16-7.4; 477) | 0.78** (0.67-0.91) | 7.16** (5.75-8.11; 344) | 1.10 (0.88-1.37) | 4.5** (3.06-6.64; 121) | ||||
| LCC | 1.03 (0.93-1.14) | 4.4* (3.84-5.06; 629) |
0.93 (0.86-1.03) |
6.4* (5.59-7.2; 1,052) | 0.81*** (0.72-0.93) | 4.83* (3.84-6.21; 387) | 1.20*** (1.06-1.36) | 3.6* (2.6-5.06; 409) | ||||
|
*aHR (95% Confidence Intervals) Median Survival in Months (95% Confidence Intervals; Number of Patients) |
||||||||||||
| Metastatic Sites and Overall Survival | ||||||||||||
| Histologic Subtype | Brain & Lung | Median Survival | Brain & Liver | Median Survival | Brain & Bone | Median Survival | Lung & Liver | Median Survival | Lung & Bone | Median Survival | Liver & Bone | Median Survival |
| AD | 0.89* (0.88-0.91) |
3.12* (2.76-3.52; 781) |
1.02 (0.99-1.04) |
3.75* (3.38-4.24; 1,654) |
1.05* (1.03-1.07) | 6.01* (5.78-6.28; 7,843) | 0.89* (0.88-0.91) | 3.19* (2.79-3.68; 1,499) | 0.97* (0.96-0.97) | 5.32* (4.99-5.55; 6,737) | 1.17* (1.15-1.19) | 3.58* (3.4-3.81; 5,517) |
| SCC | 0.96** (0.93-0.98) | 2.37* (1.91-2.79; 211) |
1.22* (1.18-1.26) |
2.96* (2.66-3.3; 548) |
1.21* (1.18-1.24) | 3.29* (3.09-3.55; 1,249) | 0.91* (0.89-0.94) | 2.92* (2.6-3.22; 755) | 0.99 (0.97-1.02) | 3.52* (3.35-3.81; 1,884) | 1.23* (1.20-1.26) | 3.25* (3.06-3.4; 2,099) |
| ADS | 0.93 (0.84-1.03) | 2.83** (1.31-6.47; 13) |
1.03 (0.91-1.16) |
3.02** 2.1-4.4; 42) |
1.13*** (1.02-1.24) | 3.81** (2.99-5.32; 169) | 0.89 (0.79-1.02) | 1.87** (1.48-4.3; 34) | 1.01 (0.91-1.12) | 4.3** (3.35-5.52; 148) | 1.21* (1.09-1.36) | 2.7** (2.3-4.37; 120) |
| LCC | 0.91**** (0.84-0.99) | 2.07*** (1.54-3.52; 43) |
1.04 (0.96-1.13) |
3.2*** (2.53-4.67; 153) | 0.99 (0.993-1.07) | 4.27*** (3.4-5.03; 174) | 1.03 (0.94-1.13) | 1.68*** (1.28-2.86; 97) | 0.97 (0.89-1.05) | 2.46*** (2.0-3.38; 140) | 1.16* (1.07-1.26) | 2.7*** (2.23-3.35; 315) |
|
Hazard ratio compares involved vs noninvolved site of metastasis and is adjusted for by: age, sex, race, median income by quartile, insurance status, hospital facility type, living distance from hospital, CDCC score, year of diagnoses, T stage, N stage, and grade. *p-value <0.0001, **p-value <0.001, ***p-value <0.01, **** p-value <0.05 |
||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




