Introduction
Wood, a renewable and biodegradable material, has been a cornerstone of human industry for centuries, serving critical roles in construction, paper production, and fuel. However, for much of modern history, the application of biotechnology in wood processing remained largely confined to paper manufacturing, with limited exploration into broader applications [
1,
2,
3]. Today, growing environmental awareness, scientific advancements, and stricter regulations have reshaped the field [
4], leading to innovations such as fungal enzymes for eco-friendly wood treatments, biological alternatives to chemical preservatives, and bio-based adhesives for engineered wood products [
5,
6,
7,
8].
Despite these advancements, the urgency of addressing climate change and reducing CO
2 emissions underscores the need for genuinely carbon-neutral or near-zero-carbon solutions. Deforestation, which claims approximately 10 million hectares of forest annually [
9], has accelerated dramatically in the past century, matching in just over 100 years what historically took millennia to occur [
10]. While industrial efforts often focus on recycling byproducts like fly ash and slag [
11,
13], these solutions typically address symptoms rather than root causes, offering temporary fixes rather than sustainable progress [
14]. In this context, nature-inspired strategies (particularly fungal-based wood modification) have emerged as promising alternatives [
15,
16,
17]. White-rot fungi, for instance, are valued for their ability to selectively degrade lignin while preserving cellulose, reducing energy consumption in pulping processes, and improving pulp quality [
18].
The use of fungi in human innovation is not new. Ancient civilizations employed fungi for fermentation, food preservation, and medicine over 9,000 years ago [
19,
20]. Early Neolithic Chinese communities, for example, developed
qu starters for alcohol production using microbial consortia of yeasts and molds, anticipating modern industrial fermentation techniques [
19,
21]. Fungi also fascinated ancient scholars, with Hippocrates documenting treatments for mushroom poisoning and Aristotle speculating about fungal growth mechanisms [
22]. By the Renaissance, Pier Antonio Micheli’s empirical work on fungal spore reproduction disproved the theory of spontaneous generation, laying the foundation for modern mycology [
23,
24]. Today, fungi are recognized as essential ecological agents, actively shaping ecosystems through lignocellulose decomposition, nutrient cycling, and carbon sequestration [
25].
This ecological role extends into material science, where MBCs revolutionize sustainable alternatives to conventional wood and plastics. A historically significant yet overlooked example of fungal innovation is mykoholz (also known as myco-wood), a bioengineered wood developed in mid-20th-century East Germany. Using controlled fungal decay, mykoholz selectively degraded lignin in hardwood beech like
Fagus sylvatica, producing lighter, more porous materials for applications ranging from pencil manufacturing to musical instruments [
26,
27,
28,
29]. Despite its potential, large-scale production ceased by the mid-1960s due to inconsistent material properties, scalability challenges, and a lack of automation [
30].
Walter Luthardt’s initial experiments were not industrial in nature but stemmed from a culinary motivation: he cultivated Brown Stew Fungus (
Kuehneromyces mutabilis) to support preparation of the traditional Thuringian dish Schwammbrühe mit Klös (mushroom broth with dumplings) [
26]. During these trials, he accidentally observed that the supporting wood had lost up to three-quarters of its weight and developed millions of microscopic pores, dramatically increasing absorbency [
26]. This serendipitous discovery laid the foundation for subsequent research into fungal-modified wood that later evolved into the industrial mykoholz process.
Revisiting mykoholz is particularly timely, as fungal-based materials gain momentum in sustainable industries [
16,
17]. Unlike modern MBCs that form entirely new substrates, mykoholz modified existing wood fibers, offering a low-energy, chemical-free alternative [
31]. Fungal action at the nanoscale altered hydroxyl availability, water uptake, and surface energy, enabling tunable, application-specific properties [
32]. Today, advancements in biotechnology, climate-controlled incubation, and AI-driven bio-fabrication provide solutions to the scalability and consistency challenges that once limited mykoholz [
33,
34].
This paper situates mykoholz within its historical and technological context, clarifying its methods, industrial relevance, and decline. By linking this mid-century innovation with current fungal bio-fabrication research, we show how early selective delignification strategies anticipated features of modern sustainable materials. The next section presents the study’s specific aims and outlines its contribution to contemporary discussions in bioengineered wood and MBCs.
Research Gap and Objectives
Fungal bio-fabrication has gained momentum in recent years, spanning MBC for packaging, construction, textiles, and acoustic panels, yet few studies have critically revisited early industrial-scale applications of selective fungal decay as a deliberate materials-engineering strategy.
Contemporary literature largely focuses on controlled mycelial growth in molds or enzymatic delignification under laboratory conditions, with minimal reference to historical precedents. This omission overlooks valuable process knowledge from ventures such as mykoholz, a mid-20th-century East German fungal wood-modification technology that achieved controlled property alteration without modern automation or biotechnology. Unlike much modern research, which tends to address scalability, environmental performance, and material standardization in isolation, mykoholz represents an integrated example in which process engineering, industrial application, and functional performance were developed concurrently. The lack of systematic comparisons between such historical approaches and current fungal material strategies constitutes a critical gap, one this study addresses by situating mykoholz within its historical context and evaluating its potential to inform climate-conscious, circular-economy-driven material innovation.
This study pursues three primary aims:
Reconstruct the historical trajectory of mykoholz through archival patents and production records.
Analyze its bioengineering methods, material properties, and industrial applications.
Assess its relevance to contemporary fungal bio-fabrication and circular-economy practices, linking early selective delignification to modern sustainable materials research.
The novelty lies in reframing mykoholz as an early industrial-scale example of fungal bio-modification of lignocellulosic materials, rather than as a mere historical curiosity. While it differs fundamentally from modern MBC in substrate, fabrication, and end properties, both approaches harness fungi’s enzymatic capabilities to enhance material performance. This diachronic perspective connects early “technical mycology” to emerging AI-assisted bio-fabrication and climate-controlled incubation methods, offering complementary insights for next-generation sustainable materials development.
Background on Fungal Materials
Fungal Lifecycle and Taxonomy
In the 19th century, German forester Robert Hartig (1839‐1901) laid the foundation for forest pathology by detailing fungal degradation mechanisms in his book titeled “Wichtige Krankheiten der Waldbäume” which was published in 1874 [
35]. His work remains central to modern wood preservation strategies. Hartig identified two primary types of fungal wood decay: white rot, which breaks down both lignin and cellulose through enzymes like lignin peroxidase and manganese peroxidase, and brown rot, which selectively degrades cellulose and hemicellulose while leaving modified lignin behind.
Building on this understanding of fungal degradation, the 20th century saw significant advancements in fungal taxonomy and ecology [
36,
37].
Meinhard Michael Moser (1924–2002) refined fungal classification and deepened knowledge of mycorrhizal fungi, emphasizing their symbiotic relationships with plant roots. His research highlighted how these fungi facilitate nutrient exchange, improving soil health and supporting plant growth [
38].
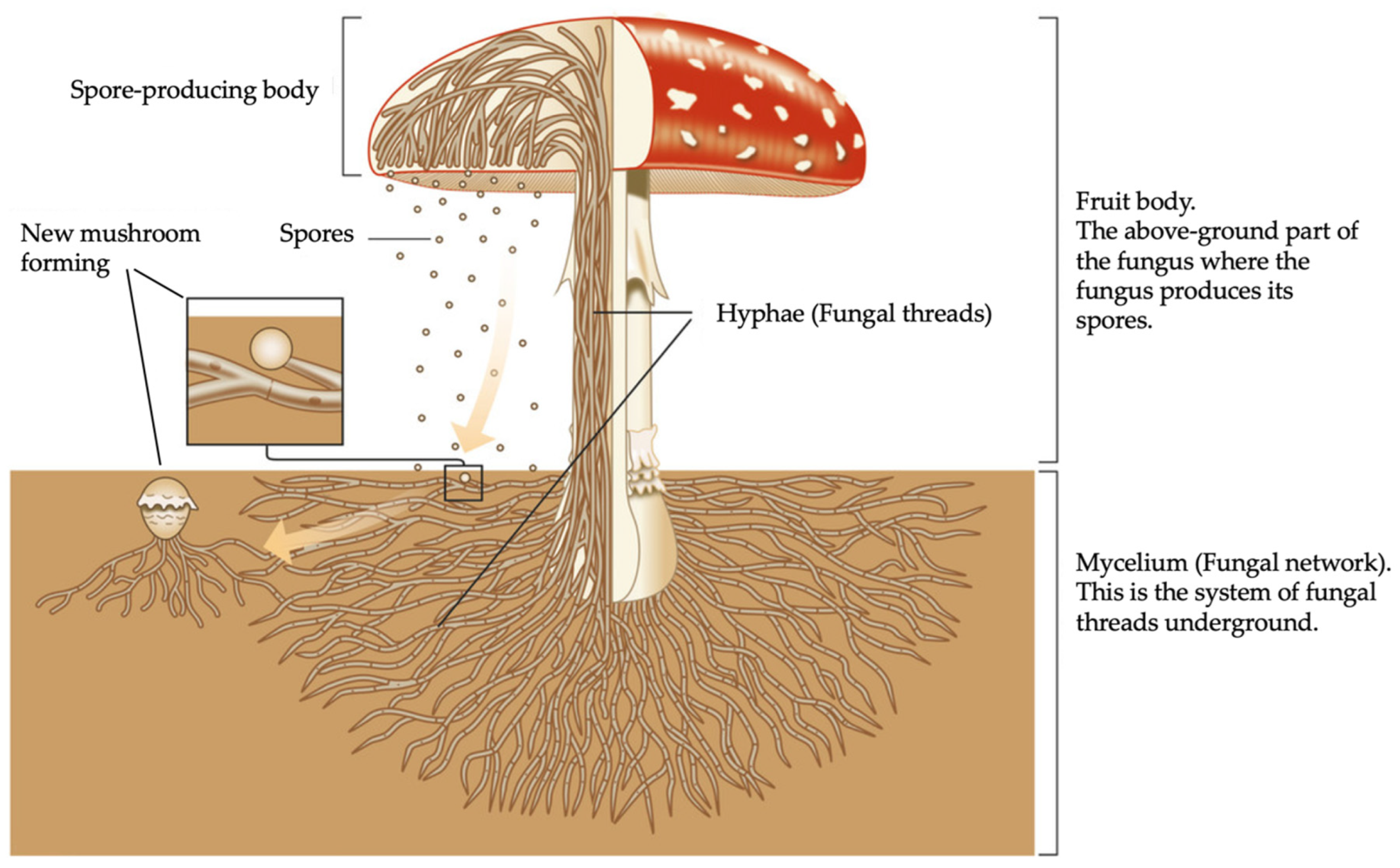
Beyond their roles in decay and symbiosis, fungi play a crucial role in ecosystem sustainability through their reproductive cycle. This life cycle progresses through several distinct phases, ensuring the continuous spread and function of fungal networks, see
Figure 1:
Spore Dispersal: Millions of microscopic spores travel through air currents, settling on a suitable substrate.
Spore Germination: Under favorable conditions, spores develop into hyphal strands, which extend and explore the environment.
Spore Mating Phase: Genetically compatible hyphae fuse, forming dikaryotic mycelium that enables further growth.
Nutrient Absorption: The fertile mycelium secretes enzymes to decompose organic matter and absorb vital nutrients.
Colonization Phase: Mycelium spreads extensively through the substrate, establishing a resilient fungal network.
Primordia Formation Phase: Small reproductive structures, or primordia, begin to emerge.
Sporulation Phase: Mature mushrooms release spores, completing the cycle and enabling further propagation.
From wood decomposition to plant symbiosis and reproduction, fungi are indispensable to natural ecosystems and industrial applications. Their diverse roles in decay, nutrient cycling, and biomass transformation continue to inspire scientific research and sustainable material innovation.
Fungal Lifecycle and Taxonomy
Mushrooms were traditionally cultivated on logs, see
Figure 2, but systemic fungal cultivation on wood substrates began in East Germany around 1930. During this time, Johannes Liese conducted experiments growing
Flammulina velutipes,
K. mutabilis,
Lentinula edodes, and
Pleurotus ostreatus ‘P. ostreatus’ on felled wood, primarily to address food shortages by introducing edible mushroom cultivation techniques [
28].
Around the same time, Hikosaburo Morimoto revolutionized mushroom cultivation by pioneering the use of sawdust as a substrate. In 1930, he secured a U.S. patent (No. 1833089), marking the earliest explicit reference to sawdust-based cultivation in the provided materials [
41]. Sawdust effectively replicates the natural wood-decaying environment necessary for mushroom growth, supplying key structural components such as lignin (33.29%), cellulose (47.82%), and hemicellulose, all essential for mycelial development [
42].
Due to its physicochemical compatibility with lignocellulose-degrading fungi, sawdust has since become one of the most widely used substrates in mushroom cultivation.
Fungal Bio-Modification in Material Science
During World War II, Walter Luthardt studied the growth of
K. mutabilis and other wood-decaying fungi, initially focusing on fungal cultivation for consumption. His research, however, was interrupted by the increased post-war demand for wood, which shifted industrial priorities [
28,
43].
In the aftermath of the war, East Germany banned cedarwood imports, creating significant challenges for industries reliant on cedar-particularly pencil manufacturers [
28,
43]. Beginning in 1948, Luthardt redirected his research toward the fungal treatment of residual hardwood to produce a lighter, more absorbent, and easily carved material suitable for pencil production [
28,
43].
Luthardt observed that fungal mycelium created millions of microscopic boreholes in the wood, reducing its weight by up to 75% and dramatically increasing its absorbency [
26]. This allowed easier impregnation with substances such as potassium chloride, water glass, and fire-retardant agents, thereby reducing flammability.
When treated with synthetic resins or paraffin-like substances, the wood gains hardness, dimensional stability, and excellent carving and sharpening properties [
26].
Despite these early advancements, large-scale production of MBC did not emerge until the 1950s. Before this, fungi-based materials were primarily limited to specific cultural practices, such as the use of fungal mats derived from fruiting bodies for fabric production among Indigenous groups in North America and parts of Eastern Europe [
44].
Table 1.
Fabrication details of mykoholtz compiled from [
26].
Table 1.
Fabrication details of mykoholtz compiled from [
26].
| State |
Process |
Details |
| Mycelium Preparation |
Cultivation of fungal strain (Schmetterlings-Spurling) |
Requires strict sterility; grown in climate-controlled flasks with constant shaking for several days until small pellets form. |
| Substrate Inoculation |
Inoculation of sawdust–nutrient mixture |
The pelletized mycelium is added to a mixture of sawdust and nutrients; over several weeks the fungus colonizes the mixture, producing the “Tharander Impfpaste.” |
| Wood Preparation |
Cutting beech logs into 42 cm blocks |
Beech logs are cut into uniform 42 cm blocks (two pencil lengths) to facilitate consistent processing and later applications. |
| Inoculation of Wood |
Application of Tharander Impfpaste to block ends |
Paste is applied to the end-grain surfaces of the blocks; fungal filaments penetrate from both ends, breaking down lignin and cellulose evenly. |
| Incubation |
Controlled growth in humid, temperature-regulated environment (e.g., unused mines) |
Blocks are incubated for 3–4 months; fungal activity creates porous Mykoholz with visible zones indicating progression of colonization. |
| Post-Treatment |
Sawn into planks and conditioned in climate chambers |
After transformation, blocks are sawn into planks and conditioned for 2 months in climate chambers; the fungus is killed through drying. |
| Impregnation & Finishing |
Treated according to intended application |
Impregnated with fire-retardants (potassium chloride, water glass) for flame resistance; resins for hardening; wax or paraffin for easier carving and finishing. |
| Applications |
End-use of Mykoholz |
Used for pencils, casting molds, polishing materials, fire-resistant textile machines, glassblowing molds, parquet flooring, and other industrial and everyday products. |
Mykoholtz Case Study
Fabrication
The fabrication process emphasized absolute sterility, with fungal cultures grown under controlled conditions in shaking flasks until small mycelial pellets formed [
26]. Freshly felled beech logs were cut into 42 cm segments, approximately two pencil lengths, a practical dimension optimized for pencil manufacturing [
26]. Incubation typically occurred in cellars, mine shafts, or earthen pits, where stable humidity and temperature favored uniform fungal growth [
26].
Mykoholz is made from beech bolts or other suitable woods by applying a paste-form inoculum to both ends and storing them in locations such as cellars, mineshafts, clamps, or under chips/chaff until softened, depending on humidity and temperature [
45], see
Figure 3. To facilitate fungal colonization, freshly felled small-diameter beech logs are typically cut into sections of 20-42 cm (two pencil lengths) or 60-80 cm [
26,
46], with the bark left intact to maintain moisture. These logs are kept under controlled climatic conditions (usually for 3-4 months) to achieve uniform, reproducible softening [
46]. During this period, white-rot fungi selectively decompose lignin, transforming the wood into a lightweight, porous material [
26].
The progression of fungal activity was visually apparent: darkened zones marked areas of advanced decay, while white zones indicated early loosening [
26]. Once colonization reached the desired level, the logs underwent a controlled drying phase of approximately two months to halt fungal growth, ensuring stability before impregnation and finishing [
26].
Walter Luthardt developed this controlled wood-decay method using selectively bred pure fungal cultures, resulting in reduced density, increased porosity, improved whittling, stress-free structure, and enhanced liquid impregnation [
47]. An air-drying phase was sometimes incorporated to optimize fungal activity. After 14 days to several months of exposure, mykoholz could be further processed chemically or through impregnation to deactivate the fungus, ideally with an air-drying phase [
46]. For pencil manufacturing, an incubation kiln temperature of approximately 23 °C was recommended [
48].
Once the desired transformation was achieved (marked by a darkened zone indicating fungal progression and a white region where loosening had begun) a two-month controlled drying phase halted fungal activity before the boards were transported to pencil factories for dye-bath impregnation [
26].
Besides beech, birch, horse chestnut, and aspen were occasionally used [
29]. White-rot fungi like
P. ostreatus and
Trametes versicolor degrade lignin first, producing a lighter-colored, low-density material that remains structurally intact, unlike brown-rot decay, which causes crumbling [
29]. In practice, logs of beech or other suitable hardwoods are kept in cellars, mineshafts, earthen pits, or beneath wood chips until sufficiently softened; this duration varies with humidity and temperature [
29,
45]. Crucially, the fungus must grow primarily along the wood fibers, creating a porous structure that readily absorbs resins, oils, flame retardants, and gases [
45]. As a result, the wood’s density and cutting resistance are reduced, while its capacity for impregnation with substances such as water glass, paraffin-like agents, or fire retardants is improved [
45].
Inoculation
Both ends of the wood sections are coated with a paste containing sawdust and pure cultures of
P. ostreatus,
K. mutabilis, or
T. versicolor [
46]. This paste ensures direct fungal contact with the wood, promoting uniform colonization. The inoculation paste was refined to include myco-wood shavings colonized by fungal mycelium, often supplemented with nutrients to promote growth [
29].
The invention described in the patent ref. DE932998C introduces a novel method for producing and handling inoculum in mushroom cultivation [
46]. Traditional inoculation approaches often use straw or grains, which are prone to contamination and necessitate extensive disinfection [
46]. This new technique employs cardboard, paper, or cellulose hydrate film as vaccine carriers
1 . These materials are placed on mycelium-colonized wooden discs and incubated for one to two days, allowing the fungus to grow through the foils [
46]. The colonized foils are then securely attached to logs or tree stumps-held in place by natural materials such as soil or grass-protecting the inoculation site from contaminants and maintaining a humid microenvironment that supports robust mycelial growth [
46]. This method greatly reduces the required amount of inoculum, streamlines production, and handling, and lowers overall costs [
46]. Furthermore, vaccine carrier materials can be prepared in rolls, inoculated with the desired fungus, briefly incubated in a brood chamber
2 to accelerate growth, and then applied to inoculation sites as needed [
46]. Initially, incubation took place in earthen pits, but as demand grew, underground wartime vaults were repurposed to maintain a stable climate conducive to fungal growth [
26,
29].
Characteristics
Gramss tested the characteristics of freshly felled Fagus sylvatica boles, ≤230 mm diameter, by end-grain inoculating them with K. mutabilis and P. ostreatus (used in mykoholz production) under four conditions:
- 1)
immediately after felling,
- 2)
after 5 months of indoor storage (sealed cut ends),
- 3)
after 5 months of unprotected outdoor storage, and
- 4)
after pasteurization at 47 °C for 24 hours to kill living parenchyma
Fungal growth in (a) and (b) was severely inhibited by fungistatic effects. In (c), growth was 2–4 times faster but hindered by pre-infection with stain fungi. In (d), growth was 5.1–20.8 times faster than in (a), indicating that pasteurization enhances uniform colonization by breaking the “vitality factor” and eliminating fungal antagonists, partially restoring dry heartwood permeability. Screening tests on six additional non-industrial timber species assessed their suitability for mykoholz production [
49].
Initially, incubation occurred in earthen pits; however, as industrial demand grew, underground wartime vaults and mine tunnels were repurposed to maintain stable climatic conditions conducive to fungal growth [
29].
Laboratory tests on small
Fagus sylvatica specimens evaluated three fungal strains: two of
T. versicolor and one of
P. ostreatus [
50]. The most virulent
T. versicolor strain caused 90% weight loss within 26 weeks, significantly exceeding the effects of the other two strains. Separate tests showed that after 12 weeks of incubation with
T. versicolor, mass loss reached 30% [
28]. Pentosan content initially increased up to 15% weight loss, but by 40% weight loss, cellulose, lignin, and pentosan levels declined proportionally. At advanced decay stages, pentosans were nearly depleted, and lignin’s relative proportion increased. Other wood constituents, which originally made up 18–54% of sound wood, rose to 47% of the residual mass during decay [
50].
Density reduction in mykoholz varies depending on fungal strain, wood species, and incubation conditions. Reported density losses range from 75% [
26,
51] to 90% [
29], likely influenced by intended uses such as pencils or mold-making.
The untreated mykoholz exhibited several advantageous characteristics due to controlled white-rot fungal decay, including a lightweight, highly porous, and tension-free structure, excellent carving and sharpening characteristics, superior soundproofing and thermal insulation, and high moisture absorption [
27,
28,
29]. Density reductions ranged from 50-90%, and mechanical stiffness was reduced by approximately 60%, with an E-modulus of 6,700 MPa compared to untreated beech at 16,000 MPa. These properties were particularly beneficial for applications such as the glass industry [
27,
28,
29].
When impregnated with flame retardants or other chemicals, mykoholz demonstrated enhanced fire resistance, water repellency, acid resistance, and increased abrasion durability [
26,
29].
Laboratory tests on pressure cooking in 0.2–0.5% HCl solutions with a plasticizer at >100 °C showed optimal results with 0.3% HCl and 20% “Tallosan” plasticizer at 120 °C and 10 atm for 5 hours, significantly reducing cutting resistance to 4.5-9.5 kg without excessive weight or strength loss. Sharpening quality, though inferior to
Juniperus virginiana, was superior to untreated mykoholz. Redheart had minimal effect, while tylose formation had none. Treatment with hot water and plasticizer at 140 °C also softened the wood but resulted in darkening and an unpleasant odor [
52].
Industrial Applications
3.4.1. From 1958 to 1965: Peak Production
Mykoholz undergoes fungal modification techniques that significantly enhance its absorbency, allowing effective impregnation with flame retardants and other chemicals [
28]. Subsequent treatments with synthetic resins or paraffin provide dimensional stability, increased hardness, and improved carving and sharpening properties [
26,
28,
29,
47,
53]. These enhanced properties make mykoholz suitable for various applications, including [
26,
28,
29,
45,
47,
53]:
Pencil manufacturing as a substitute for imported red cedar (Juniperus virginiana).
Musical instruments.
Drafting tools and aids (rulers, measuring sticks, drawing boards).
Wood polishing, particularly advantageous in watchmaking.
Stage set construction due to ease of workability, minimal swelling/shrinkage, and absence of internal stress.
Pattern-making and mold construction in the glass industry.
Laundry products impregnated with paraffin.
Thermal and electrical insulation.
Filter media and battery separators.
Artificial limbs.
Candle production by dipping mykoholz sticks in paraffin.
Activated charcoal for the chemical industry.
Mykoholz shavings for drying and polishing galvanized components in watchmaking and mechanical industries.
Bath mats (Baderosten) that reduce the spread of foot fungus in public facilities.
Hardened casting molds for foundries and additional drafting tools resistant to abrasive sand.
Fire-retardant mykoholz slats in cotton spinning mills to improve worker safety.
Schmidt estimated that between 1958 and 1961, around 120 million mykoholz-based pencils were manufactured in East Germany, while Mai et al. placed total pencil production at 55 million for the broader period of 1958–1965. These differences likely stem from variations in data sources and the scope or definitions used by each author. However, Archival records indicate that the German Democratic Republic produced approximately 4,000 cubic meters (Festmeter) of mykoholz annually during peak operations, primarily for pencil manufacturing [
26].
This significantly reduced reliance on imported Eastern red cedar, enabling a process that not only met industrial demand but also saved millions in foreign currency [
26].
The superior water absorption and desorption properties of mykoholz proved particularly advantageous in glass molding applications. These properties facilitated the formation of a steam cushion between mykoholz molds and molten glass, which extended mold longevity and boosted production efficiency from 800 to 12,000 goblets per mold [
26,
28].
Fungal modification techniques have been successfully applied to other materials, including
Fagus sylvatica, tropical woods [
54], and bamboo [
28].
3.4.2. 1965: End of Production
Refining its production, Luthardt patented mykoholz in 1951, following an earlier 1944 patent on edible mushroom cultivation, both registered in East Germany [
27].
Luthardt obtained multiple patents to enhance fungal colonization and bio-engineered wood [
27], including DE932998C (1956), available on Google Patents, which detailed improved methods for producing inoculation material in fungal culture. This was followed by a patent from West Germany in 1957 [
28]. Production of mykoholz continued until 1964/1965 in six plants in East Germany [
30,
52], and Luthardt’s research influenced further studies on fungal-treated wood in Hungary, Czechoslovakia, and West Germany [
53].
In 1965, the Council for Mutual Economic Assistance (CMEA) transferred pencil manufacturing from East Germany to Czechoslovakia, leading to the closure of the Tröbach (Steinach) factory that same year [
30]. However, inconsistent material quality rendered mykoholz unreliable for pencil production in Czechoslovakia, resulting in defective batches and necessitating the use of high-quality beech wood instead [
30]. These quality issues ultimately made Mykoholz economically unviable, prompting Czechoslovakia to discontinue its use in pencil manufacturing.
Had more attention been given to optimizing mykoholz production, better outcomes could have been achieved through the automated regulation of temperature and humidity in concrete chambers [
30].
3.4.3. From 2000s till Today: Revival Attempts
According to Mai et al. [
2], some pencil manufacturers in Germany considered reviving this technology in the early 2000s; however, there is no evidence of its current use, aside from the experimental application by Schwarze et al. in violin production in Switzerland, which is present here.
Antonio Stradivari’s violins from the late 17th and early 18th centuries are renowned for their exceptional tonal qualities. Studies indicate that he used Norway spruce that grew during the Maunder Minimum, a period of low solar activity and colder temperatures [
55]. These climatic conditions produced wood with narrow annual rings, resulting in a high modulus of elasticity and low density-ideal characteristics for violin construction [
55].
To replicate these acoustic properties, Schwarze et al. investigated whether specific decay fungi could reduce wood density without altering elasticity. Their study, summarized in
Table 2, involved incubating resonance wood samples of Norway spruce (
Picea abies) and sycamore (
Acer pseudoplatanus) with
Physisporinus vitreus (a white-rot fungus) and
Xylaria longipes (a soft-rot fungus). These fungi were selected for their ability to selectively degrade wood while preserving the compound middle lamella
3 in the early stages of decay. The findings demonstrated that fungal treatment effectively reduced density by 10–14.8% while maintaining the modulus of elasticity (bending strength) and sound velocity. This led to an increased radiation ratio, enhancing sound projection and aligning the wood’s properties with those of superior resonance wood traditionally used in violin-making.
The fungal mycelium modifies the wood structure by breaking down hemicellulose and selectively delignifying secondary cell walls while keeping the compound middle lamella intact. This structural modification allows for greater vibration and resonance without compromising the wood’s integrity. Ideal violin wood requires low density, high speed of sound, and a high modulus of elasticity. Stradivari’s wood (1645–1715) naturally developed these traits due to slow growth during a cold climatic period. The fungal treatment mimics these properties by creating evenly degraded cell walls, avoiding the typical reduction in sound wave speed seen in decayed wood.
For violin fabrication and similar musical instruments, particularly for soloists, this enhanced wood offers greater projection, dynamic range, and tonal modulation-key attributes of high-quality instruments. To test the effectiveness of this fungal modification, Schwarze collaborated with Swiss violin maker Michael Rohnheimer in 2009 for a blind experiment, with violinist Matthew Trusler performing. During the test (n = 180), 50% of participants favored the fungus-treated violin, ranking it above the Stradivarius, which received 21.7% of the votes [
56]. 62.8% of participants mistakenly identified the fungus-treated violin, crafted from wood with nine months of fungal treatment, as the Stradivarius [
56].
Post-treatment, the wood is sterilized with ethylene oxide gas to halt fungal activity, ensuring that the material remains stable for violin-making. By successfully modifying wood density without compromising its bending strength, Schwarze validated Walter Luthardt’s hypothesis. Luthardt’s hypothesis suggests that structural alterations in wood could enhance its acoustic properties for violin-making, aiming to reduce weight and potentially modify the sound characteristics of musical instruments [
29].
More recently, Rachel Rosenkrantz, in collaboration with Ecovative, built an electric guitar using mycocomposite material [
57]. However, no data is currently available regarding the musical performance of this instrument.
Context and Contemporary Relevance
3.5.1. Traditional Roots to Innovative Technologies
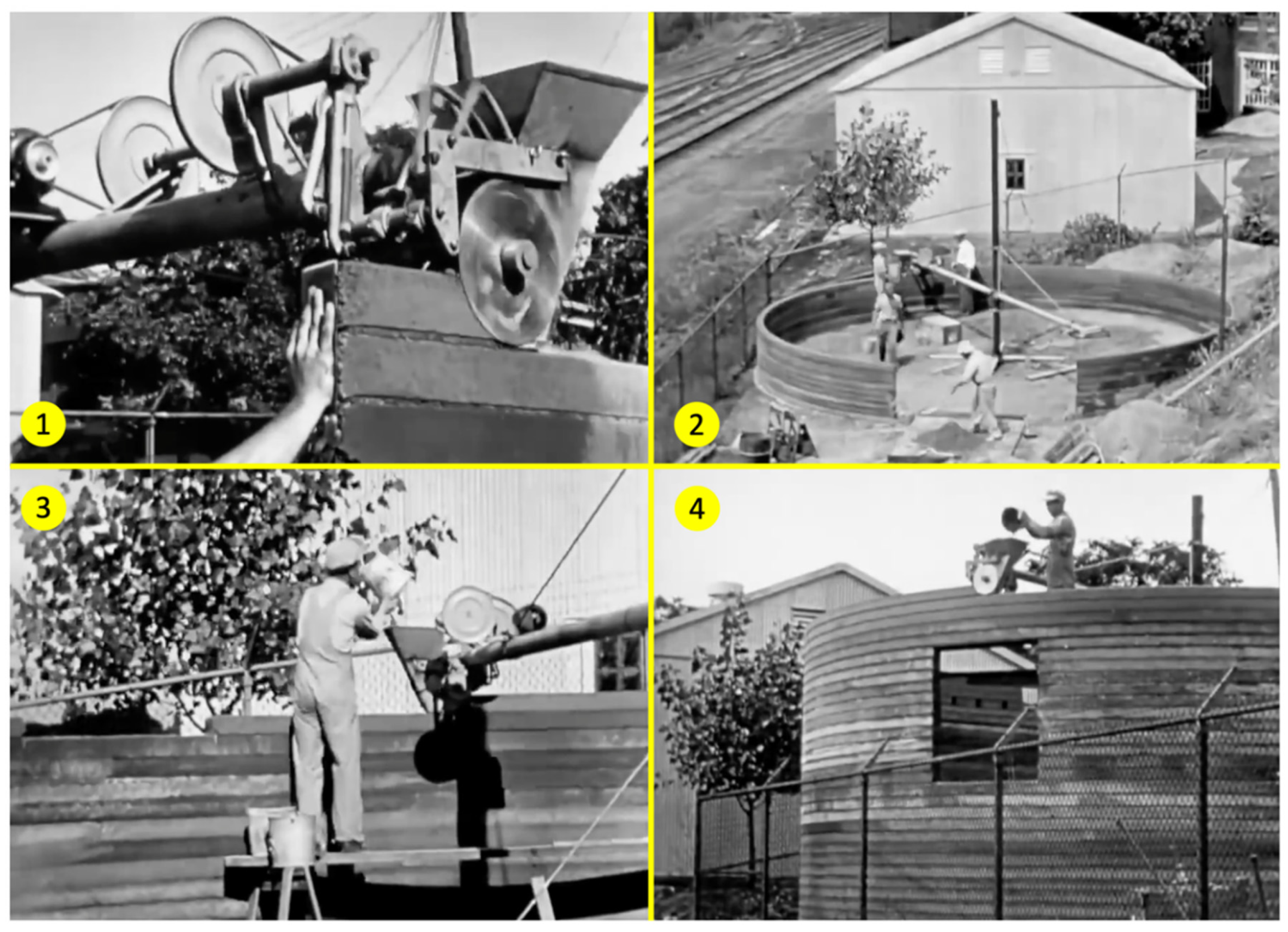
Modern technologies often adapt principles from historical practices [
58]. Traditional methods, such as Luthardt’s mykoholz, demonstrate early large-scale fungal bio-modification of wood, which, while not a direct precursor, shares conceptual parallels with modern MBC that deliver substantial sustainability benefits across multiple industries. Similarly, contemporary 3D-printed housing technologies trace their origins back to William E. Urschel’s 1930s “Wall Building Machine” [
59]. Urschel’s innovative system, see
Figure 4, enabled the construction of multistory concrete structures featuring integrated reinforcement and self-supporting domes without traditional formwork [
60].
His exploration of geometric design freedom, reinforcement integration, variable extrusion methods, and material compaction laid critical foundations for current additive manufacturing practices.
Digital fabrication technologies have significantly transformed traditional construction approaches.
By integrating bioengineering with 3D printing, researchers have developed extrudable MBCs using waste materials as substrates [
15,
61,
62]. These renewable materials offer significant environmental advantages, such as a reduced carbon footprint. MBCs have been successfully adapted for diverse applications, including construction materials, sustainable packaging alternatives, leather substitutes, and acoustic materials [
63]. Customization of MBCs is achieved by selecting specific fungal species, substrate combinations, growth conditions, and post-processing techniques. One of the most ambitious applications of MBCs is extraterrestrial construction. NASA has proposed utilizing these composites to construct habitats on Mars and the Moon, where biological structures could potentially be grown on-site with minimal transported materials, binding effectively with local regolith to form an innovative habitat [
64].
Creativity stands at the intersection of scientific knowledge [
65], technological innovation, and artistic expression [
66,
67,
68]. In the realm of bio-fabrication, it fosters synergy among diverse fields, enabling the reimagining of conventional materials and techniques [
11,
12,
14,
65,
66,
67,
68,
69,
70]. By daring to question familiar processes and experiment with uncharted territory, creativity catalyzes breakthroughs that can reshape entire industries. Whether we are designing new fungal composites or refining historical methods like mykoholz, an inventive spirit ensures that fresh perspectives drive progress. Ultimately, creativity transforms limitations into opportunities, offering solutions that are both functional and purposeful. Remaining deeply rooted in ecological harmony it ensures alignment with future-minded thinking.
AI-Driven Optimization in Mycelium Composites
AI-driven systems are transforming the fabrication of MBCs by automating monitoring, predicting material behavior, and optimizing process parameters. In the FungAI project, machine learning models analyze standardized image data to determine whether a composite has fully developed or requires further incubation, ensuring consistent quality and reducing production time [
77]. These systems also correlate visual growth characteristics with mechanical properties like strength and flexibility. Integration of Digital Image Correlation (DIC) and biochemical data is expected to further enhance the precision of these predictions [
77].
Optimization techniques such as Design of Experiments (DoE), Response Surface Methodology (RSM), and Artificial Neural Networks (ANN) allow researchers to systematically refine variables including fungal species, substrate types, environmental conditions (temperature, humidity, CO
2 levels, light exposure), and post-processing techniques like hot and cold pressing [
78]. This data-centric approach reduces the need for physical experimentation, increases reproducibility, and shortens development cycles.
In manufacturing settings, AI governs climate-controlled growth chambers, dynamically adjusting internal conditions to maintain optimal fungal development. Simultaneously, robotic 3D printers equipped with AI can manage deposition, compensate for substrate compaction, and enable the creation of complex geometries without rigid molds [
79,
80]. These applications enhance precision, scalability, and design versatility.
Historical practices offer valuable insights for present-day material optimization [
81,
82]. CIA records document Mykoholz as a fungal-treated wood developed in East Germany to improve the pliability and workability of beech and spruce for use in furniture and housing components [
83]. Despite limitations such as extended incubation times and inconsistent results, Mykoholz represents one of the earliest industrial-scale efforts in fungal wood modification. The CIA’s interest likely arose from its potential for both civilian and military applications, reflecting broader Cold War surveillance of alternative material technologies behind the Iron Curtain.
Mykoholz, as a biologically modified, low-resource material, held promise for lightweight construction, soundproofing, and radar absorption, making it strategically significant. Its development illustrated East Germany’s innovation under economic constraints, especially in circumventing petroleum-based synthetics. The CIA classified and analyzed its properties under Technical Intelligence (TECHINT), viewing it as a case of resource-efficient innovation particularly relevant during oil crises and trade embargoes. The documentation reflects not only an interest in fabrication methods but also early evaluations of scalability, durability, and economic viability [
83]. Retrospectively, Mykoholz can be seen as a precursor to today’s bio-based circular design, identified by intelligence services before such concepts were formally established.
Today, early techniques from Mykoholz, such as selective delignification, acoustic tuning, and adaptive post-processing, can inform substrate preparation and enzymatic control in AI-enhanced mycelium-based systems [
77,
83]. AI contributes by transforming lignocellulosic waste into biodegradable composites, supporting local resource use, and optimizing energy efficiency [
77,
80,
84]. It also enables the development of advanced bioreactors for large-scale production, improving metabolite yields while reducing energy demands [
78].
Nevertheless, challenges remain. These include the high upfront costs of AI infrastructure, the need for extensive high-quality datasets, and the biological variability of fungi, which complicates full automation. Consumer hesitation toward biofabricated products also hinders widespread adoption [
77,
80].
Despite these barriers, integrating AI with historical knowledge and culturally reframed practices presents a resilient pathway for sustainable material innovation. By bridging past experiments with current technologies, researchers can recover undervalued strategies and enhance them for modern applications.
Limitations and Future Directions
This study integrates both historical records and contemporary literature to reassess mykoholz as an early industrial example of fungal bio-modification.
However, several limitations must be acknowledged:
Archival gaps: Many records on mykoholz production are either lost or remain non-digitized, limiting the precision of process reconstruction.
Testing disparities: Historical testing methods may not align with modern ISO or ASTM standards, complicating direct comparisons with contemporary fungal-based materials.
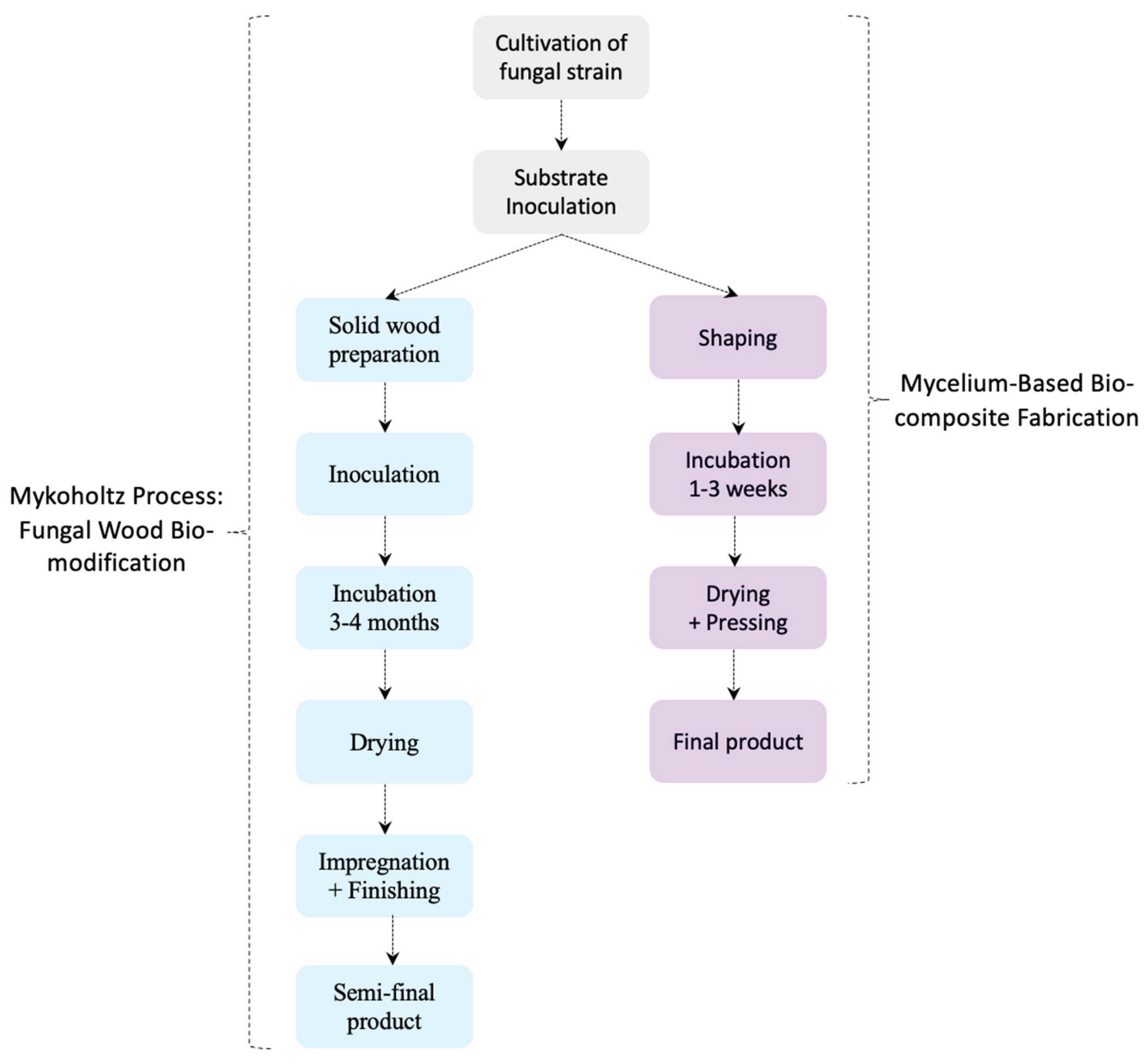
Fundamental differences: Mykoholz and modern MBCs differ in substrates, processes, and applications; conceptual links are interpretative rather than directly analogous, see
Table 3 and
Figure 5.
Language barriers: Technical German documentation may have been partially inaccessible to non-native researchers, narrowing the literature base.
Lack of experimental validation: This study does not replicate or test historical fungal bio-modification methods under present-day regulatory or environmental conditions.
Future research should aim to replicate mykoholz processes under controlled laboratory and pilot-scale settings using standardized metrics, enabling more direct comparisons with MBCs. Comparative life cycle assessments could evaluate environmental trade-offs, while hybrid approaches that blend historical fungal techniques with modern AI-assisted optimization may lead to novel materials with improved acoustic, thermal, or mechanical performance.
Interdisciplinary collaborations, spanning materials science, mycology, heritage studies, and industrial engineering, are essential for evaluating the feasibility of reintroducing these techniques at scale. Recontextualizing mykoholz within a circular bioeconomy framework offers a pathway not only to sustainability but also to cultural continuity, preserving the ingenuity of pre-petrochemical innovation.
The rediscovery and reinvention of forgotten fungal technologies, combined with contemporary AI and bio-fabrication, can help close the innovation gap in sustainable materials research and shift the dominant narrative from high-tech novelty to biological intelligence and ecological resilience.
Conclusions
This study demonstrates that mykoholz is more than a historical curiosity, it is a pioneering case of eco-compatible fungal wood treatment that predates the modern sustainability movement by over 90 years. Developed in mid-20th-century East Germany, mykoholz represents one of the earliest industrial-scale examples of fungal bioengineering. By selectively degrading lignin through controlled white-rot decay, it transformed dense hardwood into a lightweight, porous, and versatile material without synthetic chemicals or energy-intensive processing.
Three key contributions emerge from this study:
Historical validation of fungal bio-modification as an early sustainable technology,
Technical insights into scalable bio-fabrication processes, and
Lessons in circular design that remain relevant to contemporary material science.
Despite its industrial success, production ceased in 1965 due to issues of reproducibility, climate control, and material inconsistency. Yet these very limitations anticipated challenges now being addressed through fungal genomics, climate-controlled incubation, synthetic biology, and AI-optimized fabrication. The resurgence of interest in fungal materials reflects not only technological readiness but also a growing shift toward regenerative, carbon-conscious manufacturing.
Mykoholz offers critical insights into substrate softening, improved impregnation, and reduced material stress, concepts that resonate strongly with modern mycelium-based composites. While MBCs are typically moldable and biodegradable, mykoholz illustrates how solid wood can be transformed in situ for applications demanding dimensional precision, acoustic tuning, or thermal control. These complementary paradigms open the door to hybrid fabrication approaches that combine historical process knowledge with modern biotechnological capabilities.
It is a forgotten but historically significant innovation that deserves further study and critical reassessment. While it may not be replicable in its original form, it offers valuable insights that can inform future bio-fabrication research.
The re-evaluation of mykoholz contributes to a broader rethinking of material innovation, one that bridges past ingenuity with future needs. As researchers continue to explore fungal composites across packaging, construction, acoustics, and aerospace, the unanswered questions from mykoholz’s early days serve as both a challenge and a catalyst. This work underscores that decay, far from being destructive, can be reimagined as a tool of transformation, and that fungi, nature’s oldest recyclers, may help shape the architecture of the next sustainable era.
| 1 |
Vaccine carriers is a material used to transfer and apply fungal inoculum onto wood or other substrates in a clean and efficient manner. |
| 2 |
A brood chamber is a controlled environment used to stimulate and accelerate fungal growth, typically maintaining optimal humidity and temperature for incubation. |
| 3 |
The compound middle lamella is a lignin‐rich layer binding adjacent plant cells, providing structural adhesion and strength in wood. |
| 4 |
An isotropic material or property is one that is identical in all directions. This means that its physical properties (such as strength, conductivity, elasticity, or refractive index) do not change regardless of the direction in which they are measured. |
| 5 |
An anisotropic material or property varies depending on the direction in which it is measured. This means the material exhibits different values of a property (e.g., thermal conductivity, strength) in different directions. |
References
- T. K. Kirk, T. W. Jeffries, and G. F. Leatham, “Biotechnology: Applications and implications for the pulp and paper industry,” Tappi Journal, vol. 66, pp. 45–51, 1983.
- C. Mai, U. Kües, and H. Militz, “Biotechnology in the wood industry,” Applied Microbiology and Biotechnology, vol. 63, pp. 477–494, 2004.
- H. X. Cao, D. Michels, G. T. H. Vu, and O. Gailing, “Applications of CRISPR technologies in forestry and molecular wood biotechnology,” International Journal of Molecular Sciences, vol. 25, Art. no. 11792, 2024. [CrossRef]
- IEA Bioenergy, The Science-Policy Interface on the Environmental Sustainability of Forest Bioenergy: A Strategic Discussion Paper, Technical Report Ref. ExCo:2013:03, Paris, France: International Energy Agency, 2013. [Online]. Available: https://www.ieabioenergy.com/wp-content/uploads/2013/10/The-Science-Policy-Interface-on-the-Environmental-Sustainability-of-Forest-Bioenergy-a-Strategic-Discussion-Paper.pdf.
- V. Hemmilä, S. Adamopoulos, O. Karlsson, and A. Kumar, “Development of sustainable bio-adhesives for engineered wood panels—A review,” RSC Advances, vol. 7, pp. 38604–38630, 2017. [CrossRef]
- T. Teng et al., “Conventional technology and nanotechnology in wood preservation: A review,” BioResources, vol. 13, 2018. [CrossRef]
- M. Schubert, G. Panzarasa, and I. Burgert, “Sustainability in wood products: A new perspective for handling natural diversity,” Chemical Reviews, vol. 123, pp. 1889–1924, 2022. [CrossRef]
- M. Siahkamari, S. Emmanuel, D. B. Hodge, and M. Nejad, “Lignin-glyoxal: A fully biobased formaldehyde-free wood adhesive for interior engineered wood products,” ACS Sustainable Chemistry & Engineering, vol. 10, pp. 3430–3441, 2022. [CrossRef]
- UNEP (United Nations Environment Programme), Deforestation, 2021. [Online]. Available: https://wedocs.unep.org/bitstream/handle/20.500.11822/35851/DF.pdf.
- H. Ritchie, “The world has lost one-third of its forest, but an end of deforestation is possible,” Our World in Data, 2021. [Online]. Available: https://ourworldindata.org/world-lost-one-third-forests.
- D. Ben Ghida, “Geopolymeric cross-linking compressed lateritic soil-based bricks: An innovative eco-friendly building material with 60 MPa of compressive strength,” in Springer Proceedings in Materials, vol. 32, Singapore: Springer, 2023. [CrossRef]
- D. Ben Ghida, “Next-generation rammed earth architecture: A systematic review of geopolymer stabilization techniques,” in Springer Proceedings in Materials, vol. 60, Singapore: Springer, 2024. [CrossRef]
- P. Gaikwad and S. Sathe, “Effect of fly ash on compressive strength, carbonation and corrosion resistance of reinforced concrete: A systematic review,” World Journal of Engineering, vol. 22, pp. 40–60, 2025. [CrossRef]
- D. Ben Ghida, “Organic stabilization in earthen plaster: Eco-compatible architecture and ancient techniques in Tata Somba homes,” Frontiers of Architectural Research, vol. 13, pp. 625–638, 2024. [CrossRef]
- D. Luo, J. Yang, and N. Peek, “3D-printed mycelium biocomposites: Method for 3D printing and growing fungi-based composites,” 3D Printing and Additive Manufacturing, 2025. [CrossRef]
- M. A. Shakir et al., “From waste to wealth: Converting rubber wood sawdust into green mycelium-based composite,” Biomass Conversion and Biorefinery, vol. 15, pp. 739–757, 2025. [CrossRef]
- N. M. Majib et al., “Fungal mycelium-based biofoam composite: A review in growth, properties and application,” Progress in Rubber, Plastics and Recycling Technology, vol. 41, pp. 91–123, 2024. [CrossRef]
- E. Bari et al., “Fungal behavior and recent developments in biopulping technology,” World Journal of Microbiology and Biotechnology, vol. 40, Art. no. 207, 2024. [CrossRef]
- L. Liu et al., “The origins of specialized pottery and diverse alcohol fermentation techniques in early Neolithic China,” Proceedings of the National Academy of Sciences of the United States of America, vol. 116, pp. 12767–12774, 2019. [CrossRef]
- A. De Obeso Fernandez Del Valle and C. Q. Scheckhuber, “From past to present: Biotechnology in Mexico using algae and fungi,” Plants, vol. 10, Art. no. 2530, 2021. [CrossRef]
- Y. He, Long-Term Human-Plant Relationship: Food Practices, Environmental Dynamics, and Societal Processes in the Northern Zone, China During the Neolithic Period [Ph.D. thesis Ref. 31643314], Stanford University, Stanford, CA, 2024.
- L. Chowdhury, J. Jogi, and M. P. A. Dey, Introduction to Microbiology, Bhopal, India: Addition Publishing House, 2024.
- G. C. Ainsworth, Introduction to the History of Mycology, Cambridge, UK: Cambridge University Press, 1976.
- G. Sumbali and B. M. Johri, The Fungi, Oxford, UK: Alpha Science International, 2005.
- E. Elsacker, E. Peeters, and L. De Laet, “Mycelium-based materials at the dawn of the Anthropocene,” in Structures and Architecture—Bridging the Gap and Crossing Borders, CRC Press, 2019, pp. 1083–1090. [CrossRef]
- H. Emuth, DEFA, Mykoholz—Ein Neuer Werkstoff der DDR [YouTube video], 1964. [Online]. Available: https://www.youtube.com/watch?v=afUf_6n1BAc&t=721s.
- S. Stange and A. Wagenführ, “70 years of wood modification with fungi,” Fungal Biology and Biotechnology, vol. 9, Art. no. 7, 2022. [CrossRef]
- O. Schmidt, Wood and Tree Fungi, Berlin, Germany: Springer-Verlag, 2006.
- V. Krackler, D. Keunecke, and P. Niemz, Verarbeitung und Verwendungsmöglichkeiten von Laubholz und Laubholzresten [Technical Report], Zurich, Switzerland: ETH Zurich, 2010. [CrossRef]
- G. Müller, Die Luthardtsche Technische Mykologie [Technical Report], 2015. [Online]. Available: https://www.tham-thueringen.de/wp-content/uploads/7_Luthardt_und-das-Mykoholz.pdf.
- T. Balaeș, B. M. Radu, and C. Tănase, “Mycelium-composite materials—A promising alternative to plastics?” Journal of Fungi, vol. 9, Art. no. 210, 2023. [CrossRef]
- N. Z. Plaza, S. V. Pingali, and R. E. Ibach, “Nanostructural changes correlated to decay resistance of chemically modified wood fibers,” Fibers, vol. 10, Art. no. 40, 2022. [CrossRef]
- M. Filippi, M. Mekkattu, and R. K. Katzschmann, “Sustainable biofabrication: From bioprinting to AI-driven predictive methods,” Trends in Biotechnology, vol. 43, pp. 290–303, 2025. [CrossRef]
- E. Camilleri, S. Narayan, D. Lingam, and R. Blundell, “Mycelium-based composites: An updated comprehensive overview,” Biotechnology Advances, vol. 79, Art. no. 108517, 2025. [CrossRef]
- R. Hartig, Wichtige Krankheiten der Waldbäume: Beiträge zur Mycologie und Phytopathologie für Botaniker und Forstmänner, Berlin, Germany: Springer, 1874.
- J. Guarro, J. Gené, and A. M. Stchigel, “Developments in fungal taxonomy,” Clinical Microbiology Reviews, vol. 12, pp. 454–500, 1999. [CrossRef]
- D. Moore, V. Ahmadjian, and C. J. Alexopoulos, “Fungus,” Encyclopedia Britannica, 2025. [Online]. Available: https://www.britannica.com/science/fungus.
- A. Sportes et al., “A historical perspective on mycorrhizal mutualism emphasizing arbuscular mycorrhizas and their emerging challenges,” Mycorrhiza, vol. 31, pp. 637–653, 2021. [CrossRef]
- Blankevoort/Naturalis, “Schimmels: Tussen Planten en Dieren In,” 2019. [Online]. Available: https://natuurwijzer.naturalis.nl/leerobjecten/schimmels-tussen-planten-en-dieren-in.
- Kimikel, “Trametes versicolor growing on a rotting log,” 2024. [Online]. Available: https://en.wikipedia.org/wiki/Polypore#/media/File:Trametes_versicolor_Bear_Creek.jpg.
- S. Hirao et al., “Japanese ‘Nameko’ mushrooms (Pholiota microspora) produced via sawdust-based cultivation exhibit severe genetic bottleneck associated with a single founder,” Mycoscience, vol. 63, pp. 79–87, 2022. [CrossRef]
- K. B. Boadu et al., “Influence of the chemical content of sawdust on the levels of important macronutrients and ash composition in pearl oyster mushroom (Pleurotus ostreatus),” PLoS ONE, vol. 18, Art. no. e0287532, 2023. [CrossRef]
- O. Tackmann, O. Schmidt, and W. Liese, “Geschichte der Mykologie und Holzpathologie,” Zeitschrift für Mykologie, vol. 75, p. 13, 2009.
- R. A. Blanchette, D. T. Haynes, B. W. Held, J. Niemann, and N. Wales, “Fungal mycelial mats used as textiles by Indigenous people of North America,” Mycologia, vol. 113, pp. 261–267, 2021. [CrossRef]
- W. Luthardt, “Mykoholz: Its production, properties, and uses,” in Holzzerstörung durch Pilze: Internationales Symposium Eberswalde 1962, Berlin, Germany: Akademie-Verlag, 1963, pp. 83–88.
- W. Luthardt, Verfahren zur Herstellung und Handhabung von Impfmaterial in der Pilzkultur, Patent DE932998C, 1953.
- R. Trautvetter, “‘Mykoholz’—A material with new properties,” Holzindustrie, vol. 9, pp. 238–241, 1956.
- W. Luthardt, “From cultivation of edible fungi on wood to ‘biological stump disposal’ and manufacture of ‘Myko-Holz,’” Forst- und Jagdzeitung, vol. 6, pp. 429–437, 1956.
- G. Gramss, “Rationalization in ‘Mykoholz’ production by breaking the vitality factor in freshly felled stemwood of European beech prior to inoculation with wood-decay fungi,” Material und Organismen, vol. 24, pp. 104–119, 1989.
- E. Jahn, G. Patscheke, and G. Kerner, “Changes in wood composition in the production of ‘Mykoholz,’” in Holzzerstörung durch Pilze: Internationales Symposium Eberswalde 1962, Berlin, Germany: Akademie-Verlag, 1963, pp. 89–96.
- H. Unbehaun, B. Dittler, G. Kühne, and A. Wagenführ, “Investigation into the biotechnological modification of wood and its application in the wood-based material industry,” Acta Biotechnologica, vol. 20, pp. 305–312, 2000. [CrossRef]
- G. Patscheke and G. Kerner, “Chemical softening of beech wood, especially for use as pencil wood,” Holztechnologie, vol. 7, pp. 93–96, 1966.
- G. Gramss, “Kuehneromyces mutabilis,” in The Biology and Cultivation of Edible Mushrooms, S. T. Chang and W. A. Hayes, Eds. New York, NY, USA: Academic Press, 1978, pp. 423–443. [CrossRef]
- C. Stührk, Tomographische Visualisierung des Hyphensystems des Bioincising Pilzes Physisporinus vitreus in Fichtenholz (Picea abies), Ph.D. dissertation, ETH Zurich, Zurich, Switzerland, 2012.
- F. W. M. R. Schwarze, M. Spycher, and S. Fink, “Superior wood for violins—Wood decay fungi as a substitute for cold climate,” New Phytologist, vol. 179, pp. 1095–1104, 2008. [CrossRef]
- EMPA, Empa-Geige Übertrifft Stradivari, Medienmitteilung, Dübendorf, Switzerland: EMPA, 2009.
- Ecovative, “Meet the Luthier Growing Guitars with Mycelium,” 2022. [Online]. Available: https://shop.ecovative.com/blogs/blog/meet-the-woman-growing-guitars-with-mycelium.
- T. P. Hughes, “The evolution of large technological systems,” in The Social Construction of Technological Systems: New Directions in the Sociology and History of Technology, W. E. Bijker, T. P. Hughes, and T. Pinch, Eds. Cambridge, MA, USA: MIT Press, 1987, pp. 51–82.
- Urschel, Urschel Wall Building Machine [YouTube video], 1930. [Online]. Available: https://www.youtube.com/watch?v=QXqwnJTVSsE&t=119s.
- W. E. Urschel, US2339892A—Machine for Building Walls, 1941. [Online]. Available: https://patents.google.com/patent/US2339892A/en.
- E. Soh, J. H. Teoh, B. Leong, T. Xing, and H. Le Ferrand, “3D printing of mycelium engineered living materials using a waste-based ink and non-sterile conditions,” Materials & Design, vol. 236, Art. no. 112481, 2023. [CrossRef]
- E. Özdemir, A. Rossi, and P. Eversmann, “MycoCurva: Stay-in-place fabric formworks for curved veneer-reinforced mycelium building components,” Architecture, Structures and Construction, vol. 5, Art. no. 16, 2025.
- H. J. Shin et al., “Review on mushroom mycelium-based products and their production process: From upstream to downstream,” Bioresources and Bioprocessing, vol. 12, Art. no. 3, 2025. [CrossRef]
- L. Rothschild, Mycotecture Off Planet, NASA, 2021. [Online]. Available: https://www.nasa.gov/general/mycotecture-off-planet/.
- D. Ben Ghida, “Nanomaterials’ application in architectural façades in Italy,” Applied Science and Engineering Progress, 2022. [CrossRef]
- D. Ben Ghida and S. Ben Ghida, “Investment in agrophotovoltaics: Efficient solutions from Switzerland,” International Journal of Innovative Technology and Exploring Engineering, vol. 8, p. 12, 2019.
- D. Ben Ghida, “Prospects and challenges in the Korean construction industry: An economic overview,” International Journal of Civil Engineering and Technology, vol. 8, pp. 1338–1346, 2017.
- M. Schellini, S. BenGhida, D. Ben-Ghida, and F. Romanelli-Assumpção, “Academic Philistinism? The challenges of contemporary artistic research inside academia. Semi-structured interviews with visual art students in Brazil,” Arte, Individuo y Sociedad, vol. 35, p. 181, 2023.
- D. Ben Ghida, “Indoor radon mitigation in South Korea,” International Journal of Applied Engineering Research, vol. 11, pp. 8521–8523, 2016.
- D. BenGhida, “INSA balconies: A parasitic architecture,” AIP Conference Proceedings, vol. 2799, no. 1, Art. no. 020101, 2024. [CrossRef]
- W. Aiduang et al., “Amazing fungi for eco-friendly composite materials: A comprehensive review,” Journal of Fungi, vol. 8, no. 8, Art. no. 842, 2022. [CrossRef]
- R. Volk et al., “Life cycle assessment of mycelium-based composite materials,” Resources, Conservation and Recycling, vol. 205, Art. no. 107579, 2024. [CrossRef]
- Franklin Associates, Cradle-to-Gate Life Cycle Analysis of Polyether Polyol for Rigid Foam Polyurethanes, Technical Report Ref. 4031.00.002, American Chemistry Council (ACC) Plastics Division, 2023. [Online]. Available: https://www.americanchemistry.com/better-policy-regulation/plastics/resources/cradle-to-gate-life-cycle-analysis-of-polyether-polyol-for-rigid-foam-polyurethanes.
- E. Y. Osman, “Economic assessment of mycelia-based composite in the built environment,” M.S. thesis, Kansas State University, Manhattan, KS, USA, 2023.
- E. Soh and H. L. Ferrand, “Woodpile structural designs to increase the stiffness of mycelium-bound composites,” Materials & Design, vol. 225, Art. no. 111530, 2022. [CrossRef]
- Yang, L., & Qin, Z. (2023). Mycelium-based wood composites for light weight and high strength by experiment and machine learning. Cell Reports Physical Science, 4(6), 101424. [CrossRef]
- MNEXT, “FungAI: Smart growth of sustainable materials.” [Online]. Available: https://www.mnext.nl/en/projects/fungai/.
- E. Camilleri, S. Narayan, D. Lingam, and R. Blundell, “Mycelium-based composites: An updated comprehensive overview,” Biotechnology Advances, vol. 79, Art. no. 108517, 2025. [CrossRef]
- D. Luo, J. Yang, and N. Peek, “3D-Printed mycelium biocomposites: Method for 3D printing and growing fungi-based composites,” 3D Printing and Additive Manufacturing, vol. 12, pp. 98–111, 2025. [CrossRef]
- N. Wattanavichean, J. Phanthuwongpakdee, P. Koedrith et al., “Mycelium-based breakthroughs: Exploring commercialization, research, and next-gen possibilities,” Circular Economy and Sustainability, 2025. [CrossRef]
- D. BenGhida, S. BenGhida, and S. BenGhida, “Rethinking higher education post-COVID-19: Innovative design studio teaching to architecture students,” AIP Conference Proceedings, vol. 2799, no. 1, Art. no. 020107, 2024. [CrossRef]
- D. Ben Ghida, “Complexity in simplicity: Darel Carey on Op Art and the Responsive Eye,” Arte, Individuo y Sociedad, vol. 37, no. 3, pp. 671–678, 2025. [CrossRef]
- CIA, “Development of Myco-Wood in East Germany,” Secret Information Report Ref. CIA-RDP80-00810A006100380003-3, 1955. [Online]. Available: https://www.cia.gov/readingroom/document/cia-rdp80-00810a006100380003-3.
- C. Madusanka, D. Udayanga, R. Nilmini et al., “A review of recent advances in fungal mycelium based composites,” Discover Materials, vol. 4, Art. no. 13, 2024. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).