1. Background Theory
This analysis is based on the following foundational theories:
When a superconductor in a magnetic field enters the superconducting state, it expels the magnetic field, achieving electromagnetic shielding, i.e., the Meissner effect [
1,
2].
The superconducting phase transition occurs due to a reduction in quantum condensation energy levels, fundamentally triggered by temperature changes. According to energy conservation, after a complete phase transition cycle, the object returns to its initial state, and the cumulative heat exchange with the external environment is zero. Here, a phase transition cycle refers to the process: superconducting state → normal state → superconducting state, or vice versa.
Based on magnetic medium thermodynamics, for an object with magnetization intensity M in a magnetic field H (ignoring the excited field), the magnetization work done by the magnetic field on the object is:
[
1,
2,
3]. Here,
is the vacuum permeability (4π×10
−7H/m).
According to the first law of thermodynamics, , internal energy is a state function. Regardless of how the state changes during the process, if the object fully returns to its initial state (including temperature), its internal energy is conserved.
Analogous to an ideal gas with the equation of state , compressing or decompressing a fixed amount of gas in an adiabatic chamber can cause temperature changes. When the gas returns to its original state, the temperature resets, and no net energy is consumed, with zero net work done by the external environment.
For simplicity, this theoretical analysis temporarily neglects the following factors: assuming perfect magnetic shielding in the superconducting state; adiabatic phase transitions with no heat exchange; magnetic hysteresis losses and other dissipative effects; energy consumption due to force displacement; and thermodynamic volume work (e.g., pV work) from volume changes caused by temperature or magnetic field variations (e.g., magnetostriction). These are addressed collectively at the end of the article.
2. Analysis Under a Fixed Magnetic Field
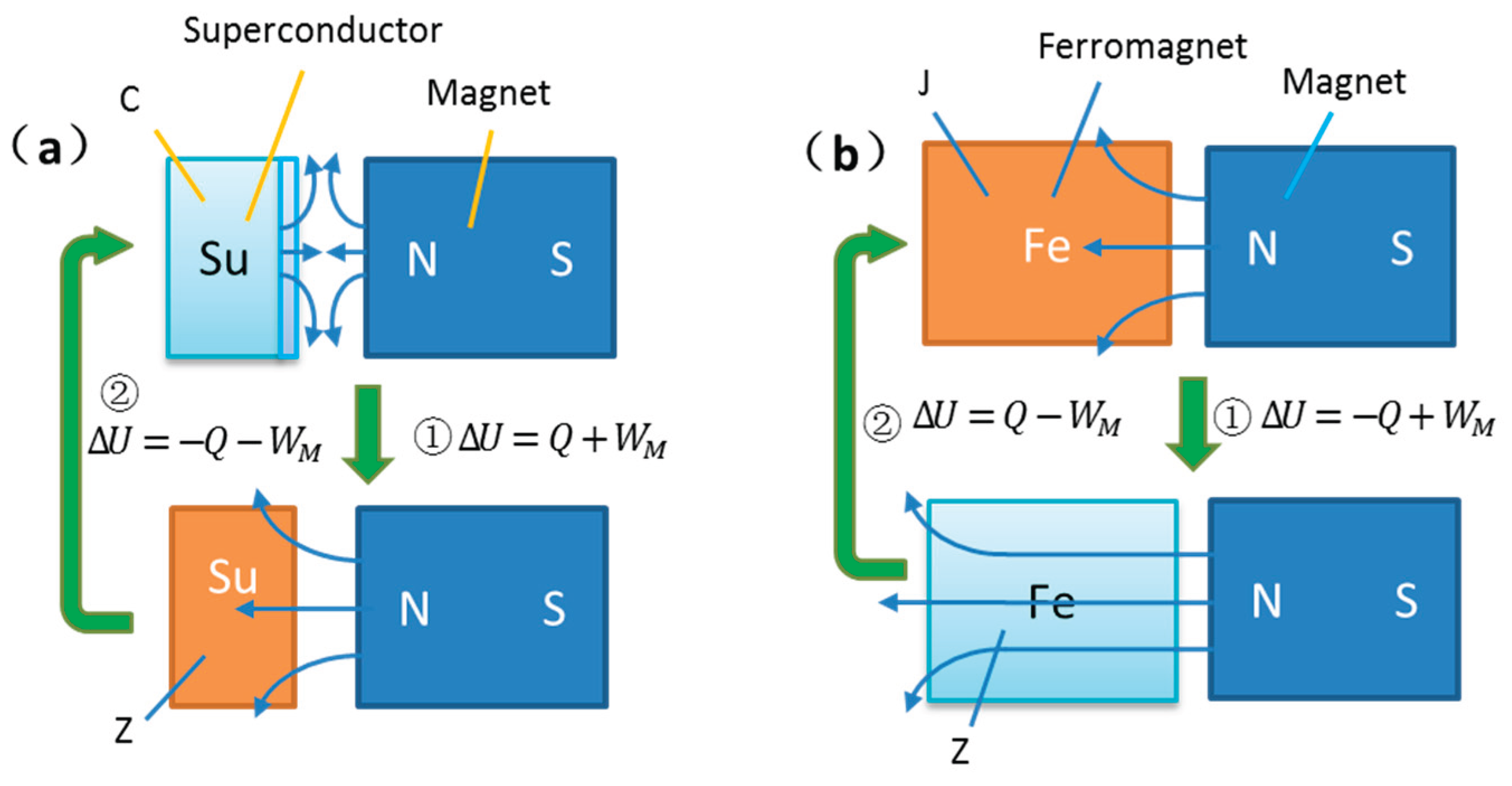
Figure 1 illustrates schematic diagrams of the two phase transition cycles, where (a) represents the superconducting phase transition and (b) represents the Curie phase transition.
First, based on magnetic medium thermodynamics and superconductivity thermodynamics, both phase transitions exhibit extremely similar characteristics: temperature changes induce phase transitions, and the internal magnetization state depends solely on the phase state, independent of the order of magnetic field application. Moreover, the magnetization work and heat absorption/release fully adhere to the thermodynamic properties of phase transitions. After a complete cycle, the total magnetization work is zero, the cumulative heat exchange with the environment is zero, and the first law of thermodynamics is satisfied.
3. Adiabatic Phase Transition Realized via Thermodynamic Work Conversion in a Piston-Equipped Adiabatic Chamber
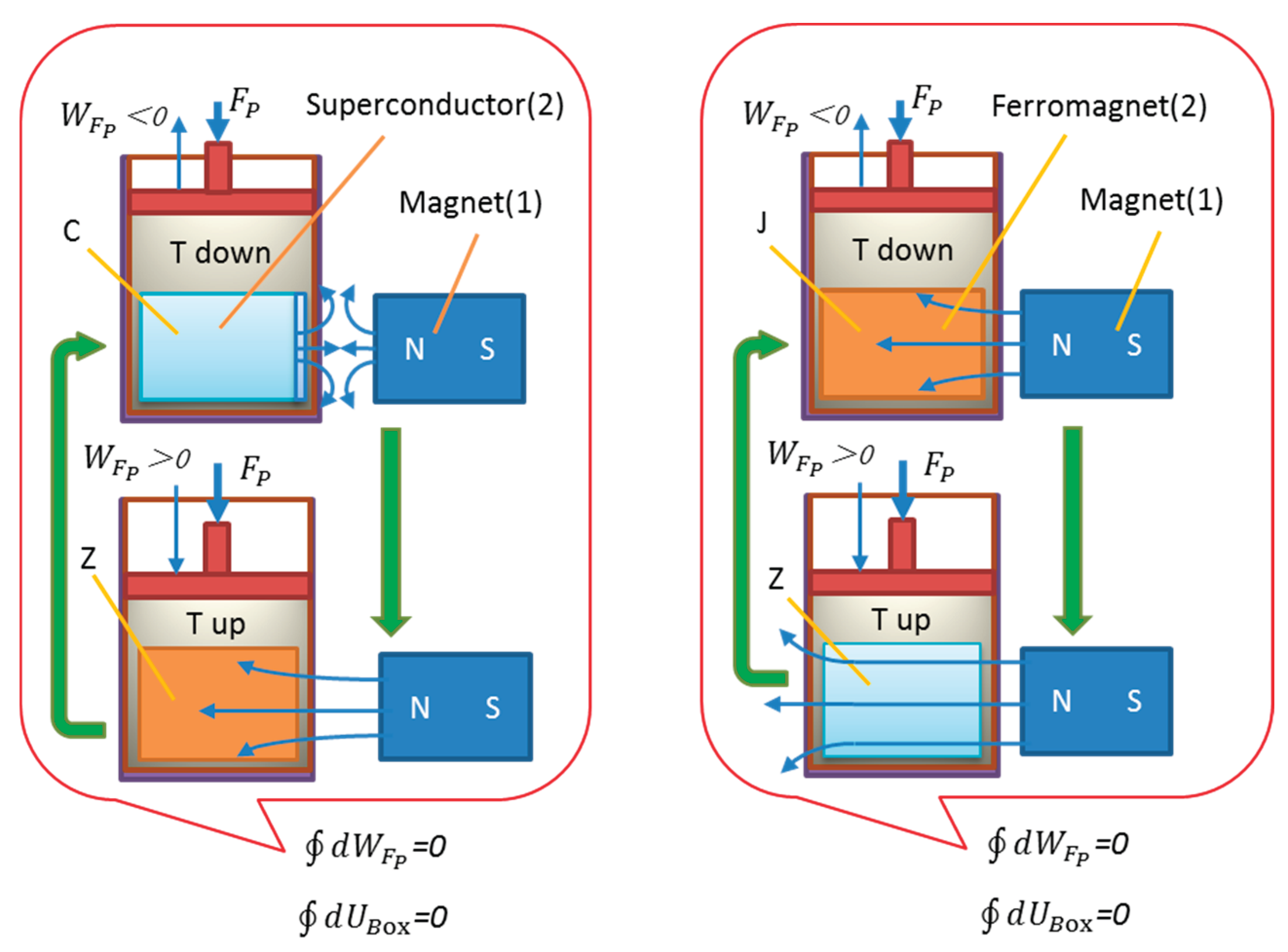
Although thermodynamics theory proves that no substance truly consumes energy during phase transitions—similarly for superconducting phase transitions, which are entirely temperature-driven and involve temporary heat exchange—a complete cycle results in no net energy consumption and conserved internal energy. In an adiabatic environment, the internal energy of the system remains unchanged after a cycle. To address skepticism, this paper introduces an adiabatic device model controlled by mechanical work to visually demonstrate that phase transitions are solely determined by temperature changes and do not truly consume energy.
Figure 2 intuitively displays the phase transition process using a piston-equipped adiabatic chamber model, where (a) represents the superconducting phase transition and (b) represents the Curie phase transition in a ferromagnet.
4. Thermodynamic Analysis of Phase Transitions
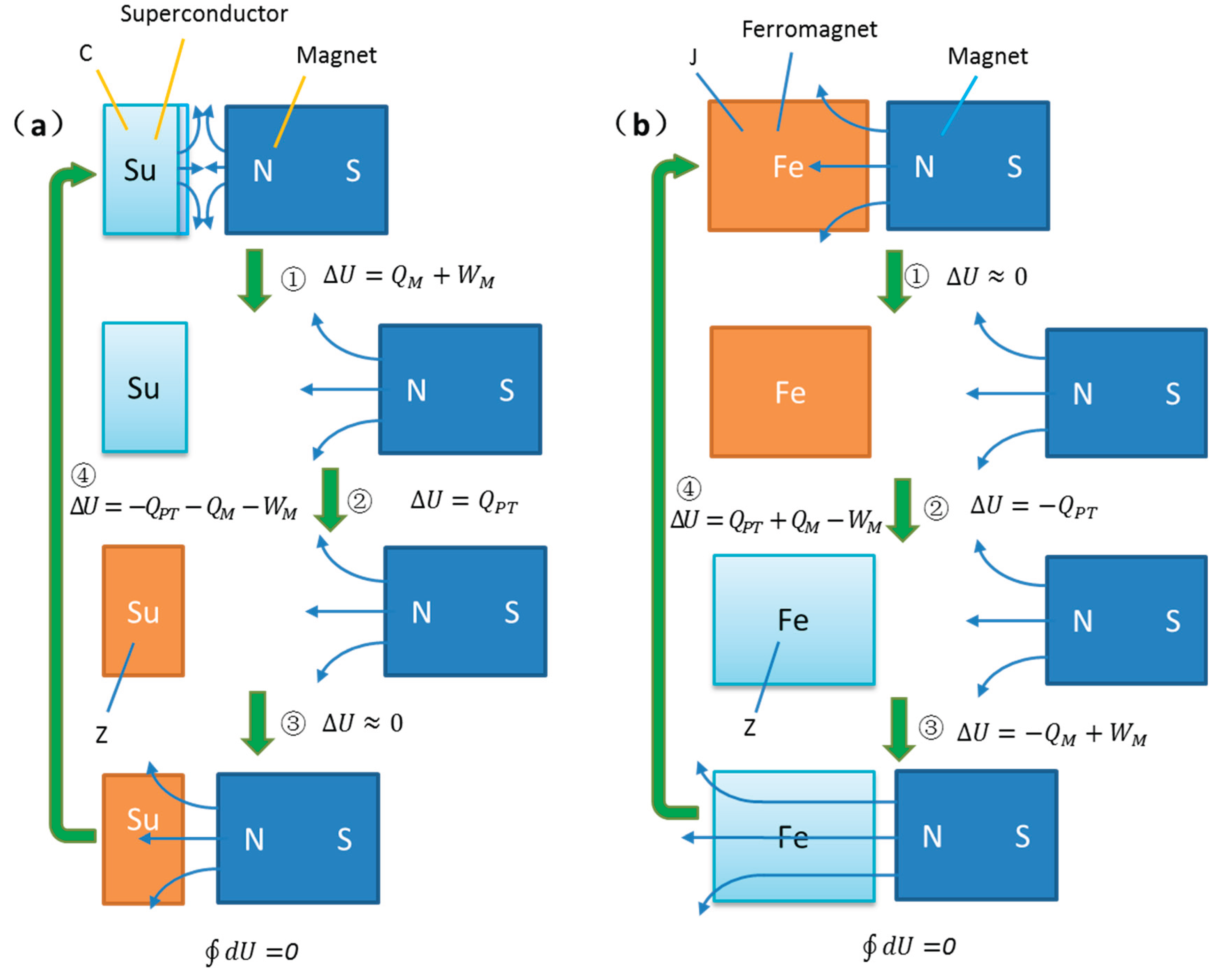
Figure 3 combines
Figure 1 and
Figure 2, analyzing the phase transition object placed in the adiabatic chamber of
Figure 2. It presents a slightly modified thermodynamic model of the magnetic field phase transition cycle based on
Figure 1. Unlike
Figure 1, during the phase transition, the permanent magnet’s magnetic field approaches or recedes accordingly, further analyzing the heat changes in the phase transition object itself and the variations in magnetization work.
- (a)
Thermodynamic Analysis of Superconducting Phase Transition
The phase transition object is placed in the adiabatic chamber of
Figure 2, indicating an adiabatic environment for the entire process. The required temperature changes for phase transition are achieved by compressing the gas, visually demonstrating that the process does not truly consume energy. In
Figure 3, characters identical to those in
Figure 1 have the same meanings. Additionally,
represents the heat absorbed during the phase transition of the superconductor without a magnetic field,
is the heat absorbed after the superconductor is magnetized by the field, and
is the total heat absorbed during the phase transition in the magnetic field, thus:
When the magnetic field is removed in the superconducting state, although the supercurrent vanishes without energy consumption, the absence of magnetic field inhibition causes the energy levels of the superconductor to tend toward a lower state, with an increased energy gap. The superconductor “relaxes” from a higher-energy excited state spectrum to a lower-energy, more stable ground state spectrum. The superconductor would normally cool down, but in an adiabatic environment, it absorbs heat.
It can be seen that Step ④ in
Figure 3a is identical to Step ② in
Figure 1a, while Steps ①, ②, and ③ together equivalent to Step ① in
Figure 1a. In Steps ①, ②, and ③, the superconductor starts in the superconducting state. First, the permanent magnet moves away, resulting in positive magnetization work
, accompanied by a decrease in energy levels, absorbing heat in an isothermal environment. Then, it transitions to the normal state in a zero-field condition, with energy levels rising due to phase transition, necessarily absorbing heat from the environment
. Finally, in the normal state, the magnetic field is applied, with minimal effect on the paramagnet
. In Step ④, the superconductor transitions back to the initial superconducting state in the magnetic field. Here, it experiences negative magnetization work and phase transition simultaneously, both causing a decrease in energy levels and releasing heat to the environment
. Due to phase transition reversibility, this step is entirely the inverse of the first three steps.
Analysis shows that after a complete cycle, the cumulative work done on the superconductor is zero, and the cumulative heat transfer is zero, conserving its internal energy. Assuming the cumulative work done by the piston on the adiabatic chamber is also zero, the internal energy of the gas inside is conserved, and the entire adiabatic phase transition system conserves internal energy.
According to the first law of thermodynamics, is a state function. Regardless of how work and heat transfer vary during the superconducting phase transition, as long as the initial state is restored, the cumulative work and heat exchange with the environment are zero, internal energy is conserved, verifying the correctness of the derivation.
- (b)
Thermodynamic Analysis of Curie Phase Transition and Comparison with Superconducting Phase Transition
For the Curie phase transition, applying a magnetic field to a ferromagnet orders the magnetic domains, lowering the free energy and producing a magnetocaloric effect where the object releases heat and temperature rises. Conversely, demagnetization increases free energy; if the ferromagnet is in an adiabatic chamber, the temperature inside decreases due to heat absorption by the ferromagnet to raise its free energy, known as the adiabatic demagnetization cooling effect.
It can be seen that Step ④ in
Figure 3b is identical to Step ② in
Figure 1b, while Steps ①, ②, and ③ together equivalent to Step ① in
Figure 1b. In Steps ①, ②, and ③, the Curie ferromagnet starts in the normal state. First, the permanent magnet moves away, with minimal effect on the paramagnet in the normal state
. Then, in the zero-field condition, the ferromagnet transitions to the ferromagnetic state, with energy levels decreasing due to phase transition, necessarily releasing heat to the environment
. Next, in the ferromagnetic state, the magnetic field is applied, resulting in positive magnetization work, domain ordering, further lowering of energy levels, and heat release
. In Step ④, the ferromagnet transitions to the normal state in the magnetic field, with domain disordering raising energy levels, while simultaneously experiencing negative magnetization work, both causing heat absorption from the environment
. Due to phase transition reversibility, this step is entirely the inverse of the first three steps.
Analysis shows that after a complete cycle, the cumulative work done on the ferromagnet is zero, and the cumulative heat transfer is zero, conserving its internal energy. Assuming the cumulative work done by the piston on the adiabatic chamber is also zero, the internal energy of the gas inside is conserved, and the entire adiabatic phase transition system conserves internal energy.
This aligns with the results derived from the state function of thermodynamics.
This indicates that the two phase transitions in a magnetic field are highly similar: both involve magnetization work, but the direction of magnetization work is opposite—positive for the ferromagnet and negative for the diamagnet—due to their inherent properties. The difference lies in the fact that the superconducting phase transition in a magnetic field involves latent heat of phase transition, with abrupt heat release, classifying it as a first-order phase transition; whereas the Curie phase transition, with or without a magnetic field, is a second-order phase transition with no latent heat and continuous heat changes.
5. Causality Between Magnetization Work and Internal Energy Change in Phase Transitions
The analysis above shows that when a superconductor in the superconducting state has the magnetic field removed, it gains positive magnetization work and absorbs heat. Conversely, when transitioning from the normal to superconducting state in a magnetic field, it gains negative work and releases heat due to magnetization work. This differs from the Curie phase transition in ferromagnets, where gaining positive magnetization work in the ferromagnetic state releases heat, and gaining negative work during demagnetization absorbs heat. However, the commonality between the two phase transitions is that whenever the phase transition object gains magnetization work (whether positive or negative), it induces heat absorption or release reactions. Thus, excluding the internal energy change due to the phase transition itself, magnetization work is the fundamental cause of internal energy changes in the phase transition object. Additionally, based on the analysis of the process in
Figure 3, after a complete phase transition cycle where the object returns to its initial state, the cumulative magnetization work is zero, the cumulative energy exchange with the environment is zero, and internal energy is conserved. Assuming the cumulative work done by the external environment on the adiabatic chamber is zero over one cycle, the internal energy of the gas inside is also conserved, and the entire adiabatic phase transition system conserves internal energy.
6. Impact of Practical Factors like Hysteresis on Energy
When a superconductor transitions to the superconducting state, becoming diamagnetic, removing the magnetic field may cause hysteresis effects. However, upon heating to the normal state, all hysteresis disappears. In practice, remnant magnetism in superconductors is eliminated by heating-induced quench. Volume changes during phase transitions do not exchange work with the environment, constituting virtual work, and after a cycle, the cumulative volume work is zero. Hysteresis effects in Curie phase transitions for ferromagnets are identical to those in superconductors—no cumulative effects remain after one cycle. Thus, these factors need not be considered and do not affect the analysis.
7. Conclusions
This paper analyzes the heat absorption/release characteristics by comparing Curie phase transitions in ferromagnets and superconducting phase transitions, revealing the commonalities and differences in energy conversion during these processes. The commonalities demonstrate the universality of basic thermodynamic principles, while the differences reflect how distinct phase transitions influence energy pathways, fundamentally accounting for their different thermodynamic effects. More importantly, the analysis concludes that magnetization work during phase transitions has a causal relationship with heat absorption/release. After a complete phase transition cycle, if the cumulative magnetization work is zero, the object not only conserves internal energy but also has zero cumulative heat exchange with the environment.
References
- Zhang Yuheng. Superconductivity Physics. Hefei: University of Science and Technology of China Press, 2009, pp. 31–36, 42–43.
- Cui Haining. Theory of Thermodynamic Systems. Changchun: Jilin University Press, 2009, pp. 274–275.
- Michael Tinkham,Introduction to Superconductivity,Dover Publications (section 2), 1996, Chapter 2, 19-21.
- Vladimir Kozhevnikov,Thermodynamics of Magnetizing Materials and Superconductors,CRC Press,2019, Chapter 2, 52-54.
- J. M. D. Coey,Magnetism and Magnetic Materials, Cambridge University Press, 2010 50-56.
- Vladimir Kozhevnikov, Thermodynamics of Magnetizing Materials and Superconductors,CRC Press,2019, Chapter 1, 6-7.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).