1. Introduction
Parotid tumours represent about 3% of all head and neck neoplasms [
1]. Approximately 80% are benign, the Pleomorphic Adenoma (PA) or mixed benign tumour being the most common in the parotid [
2,
3], while the most frequent malign is the mucoepidermoid carcinoma [
4,
5]. The PA mainly affects middle aged women. It is a tumour that grows slowly, there is usually only one and it is asymptomatic for years [
6,
7] so they are usually large at the time of diagnosis. The rapid growth, the appearance of local symptoms (like pain, facial paralysis and ulceration) and the existence of enlarged lymph nodes indicate the possibility of malignity. Treatment of PA is surgical, although this surgery may not be as aggressive as it is for malign tumours and be restricted to a partial parotidectomy or local excision of the tumour but with conservation of the facial nerve [
1,
8].
Imaging techniques play an important part in the diagnosis in order to implement an appropriate surgical planning. Although there are various techniques available, such as the ultrasound or a CT Scan, Magnetic Resonance (MRI scan), which achieves an excellent anatomical definition and a delimitation of the contours of the lesion that is much better than other imaging techniques, also allowing an assessment of the perineural and intra-cranial extent of the tumour. The introduction of advanced MRI sequences such as diffusion sequences (DWI) and the perfusion curve after contrast is added have resulted in a higher diagnostic accuracy in conventional diagnosis sequences of pleomorphic adenoma [
9,
10,
11].
Qualitative MRI features combined with quantitative ADC values offer a non-invasive, accurate approach for diagnosing PA [
12]. A retrospective study including patients with parotid masses who underwent surgical resection and preoperative MRI. Patient and MRI characteristics, including quantitative apparent diffusion coefficient (ADC) values, were analyzed to identify predictors of PA. Among 157 patients, 86 (55%) had PA. MRI sensitivity and specificity for PA were 56% and 96%, respectively. Key predictors of PA included higher ADC values, T2 hyperintense signal, lobulated tumor contour, and absence of dumbbell shape. [
12].
MRI - diffusion-weighted imaging findings and ADC also contribute to diagnosis of parotid tumours [
13]. Patients with parotid masses diagnosed using histopathology and/or cytology were enrolled in this retrospective study. MRI findings and ADC values were compared between benign-malignant groups and pleomorphic adenoma
vs Warthin's tumor groups. Sixty tumors (48 benign, 12 malignant) were evaluated in a total of 60 patients. The mean lesion size was 26 (±10, 11-61) mm. T2 dominantly hyperintense/with hypointensity signal was seen in 87% of pleomorphic adenomas and T2 dominantly hypointense/with hyperintesity signal was encountered in 64% of all Warthin's tumors. Seven (28%) Warthin's tumors were misdiagnosed as pleomorphic adenomas and two others (8%) as malignant tumors. The commonly used mean ADC value was 1.6 ± 0.6 × 10
-3 mm
2 s
-1 for benign tumors, 0.8 ± 0.3 × 10
-3 mm
2 s
-1 for malign tumors, 1 (0.9-1.8) × 10
-3 mm
2 s
-1 for Warthin's tumors, and 1.9 ± 0.3 × 10
-3 mm
2 s
-1 for pleomorphic adenomas. In addition to benign-malignant differentiation, the added ADC measurement may also be useful for differentiating Warthin's tumors from pleomorphic adenomas [
13].
The aim of this study was to investigate the relationship between histopathological characteristics of PA and MRI - diffusion-weighted imaging findings and contribution of apparent diffusion coefficient (ADC) to diagnosis.
2. Materials and Methods
This retrospective study, which was approved by the Ethics Committee of Universitary Hospital Puerta del Mar of Faculty of Medicine of Cádiz. The inclusion criteria applied to patients over the age of 18, who had undergone a parotid MRI between January 2016 and March 2025, with radiological suspicion of PA.
All studies were carried out with the same MRI (Philips Healthcare 1.5 Tesla). The MRI protocol included enhanced sequences in T1 (T1W), T2 (T2W) diffusion study (DWI) and enhanced sequences in T1 with fat saturation (T1W FS) after injecting the contrast. The contrast used was gadolinio (1 mmol/ml in injectable solution, with a dosage of 0.2 ml/kg of weight) administered with an injection pump that made a total of 8 measurements (every 30 seconds). Demographic variables (age and sex) were collected and analysed, as well as the clinic and the type of surgical treatment given.
A radiologist (JPE with 10 years of experience in head and neck MRI) and an expert in oral pathology (JLL with over 20 years of experience in radiology and oral pathology) did an independent assessment of the parotid MRI images, masking both the radiological case report and the histopathological results. The following variables were recorded:
- Precontrast study: Location (superficial, or deep lobule, or both), number of lesions; morphology (oval, lobulated or irregular); signal intensity (homogeneous or heterogeneous); margins (well defined, not well defined or spiculated); capsule presence (complete or incomplete), extra-glandular invasion and the presence of adenopathies.
- Diffusion sequence (DWI): The Apparent Diffusion Coefficient (ADC) indicates the cellular degree of the tumour. A low ADC value indicates high cellularity. I.
- Post-contrast sequences: Curves A, B and D are related to benignity while C (maximum contrast before 120’’ and washout higher than 30%) is related to malignancy.
The diagnosis of radiological suspicion was verified with the histopathological analysis, thus being the standard reference.
The statistical analysis of the numerical data was performed with the SPSS v 24.0 programme (IBM Corp). The average and the range of continuous quantitative variables and the percentage of the frequencies of other variables were calculated.
3. Results
During the analysed period, parotid MRI was performed on 113 patients, who all had signs and symptoms of a palpable nodule. The radiological diagnosis of PA was established in 56 patients. 13 of the 56 cases were excluded: one because of having a previous histological PA result by fine needle aspiration puncture (PAAF) of the lesion, which affected the study and 12 patients due to no histological confirmation being available or not require surgery. Of the 43 operated patients, the PA diagnosis was confirmed for 39 with the removed surgical piece (90.1%). Basal cell adenoma was the histopathology of the 4 cases with no concordance.
The average age of the 39 confirmed PA cases was 43 years (22 - 62 years) 17 of which were women and 22 were men. They all had only one lesion, seventeen on the left side of the parotid gland and twenty-two on the right. Twenty six (66.7%) of them affected the superficial lobe, six the deep lobe and seven affected both.
Table 1 summarizes the radiological characteristics of the series with the number of cases in each one.
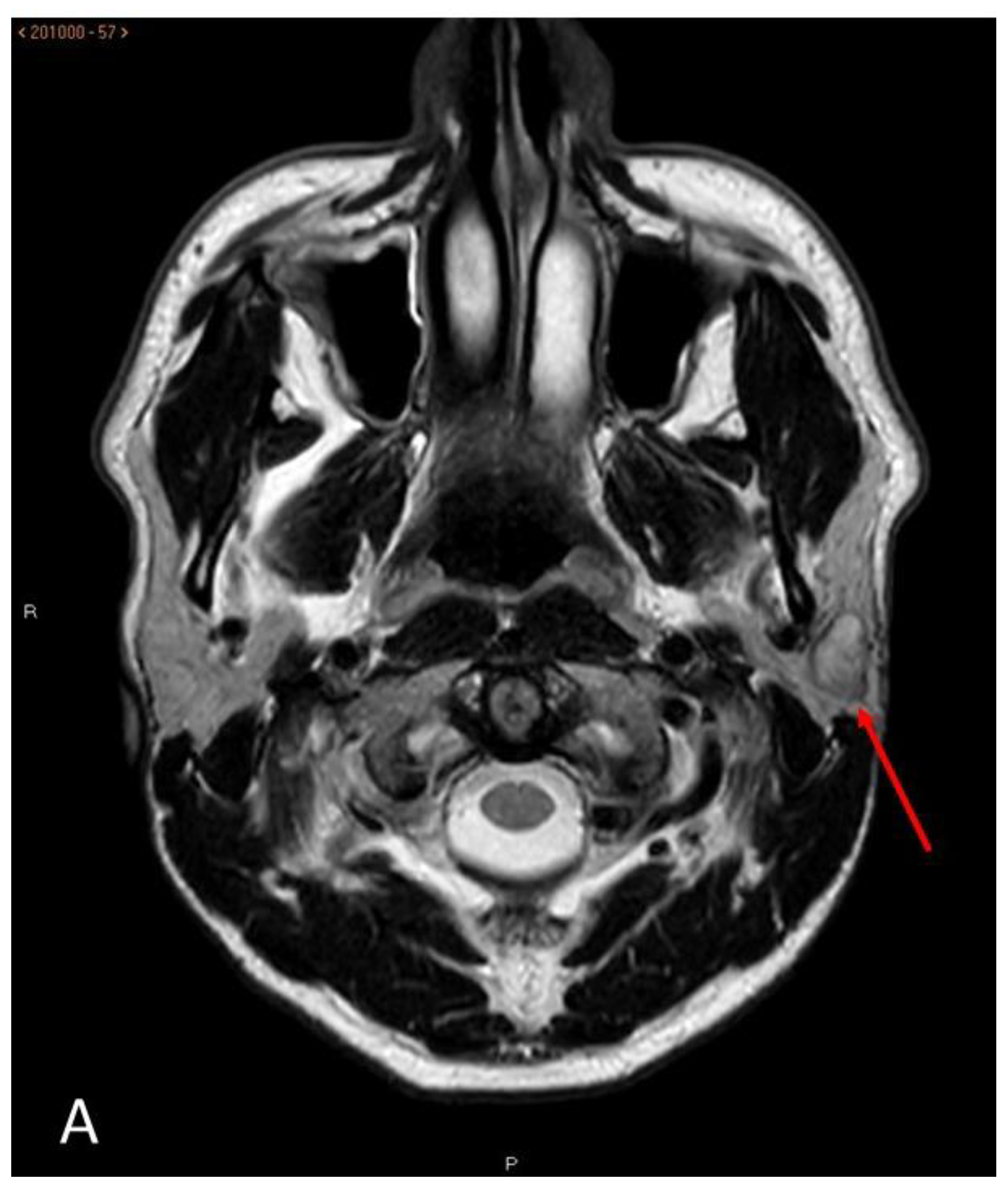
Morphologically, these were oval or lobulated contour lesions all encapsulated with well-defined margins. More than half (23/39) had homogeneous signal strength. The other sixteen had a slightly heterogeneous signal strength, indicating a mixed component (
Figure 1). None of them presented extra capsular invasion data, signs of infiltration of neither the facial nerve nor adenopathies suspected of cervical chain malignancy.
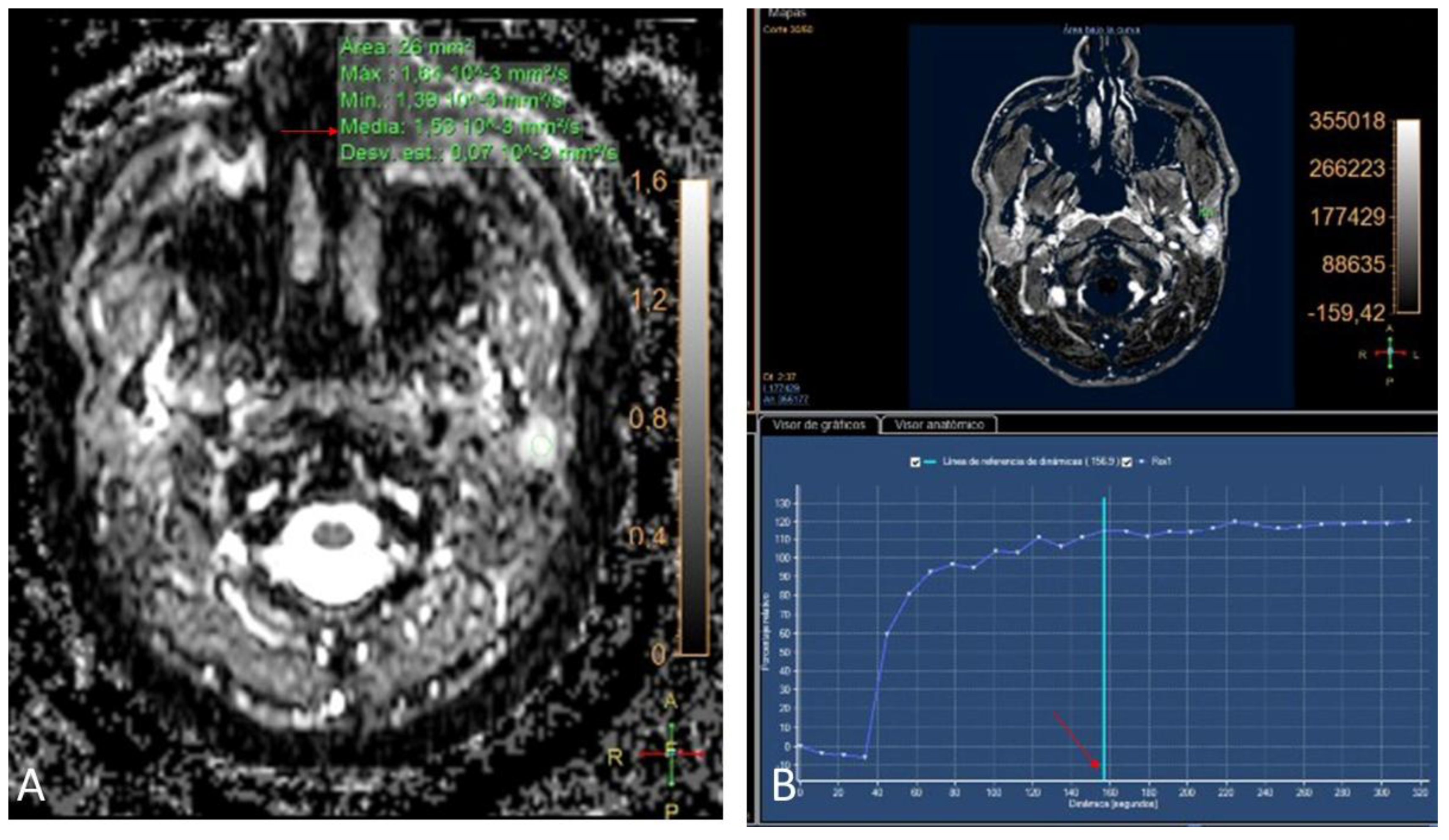
In the DWI sequence, the nodules presented an average ADC value of 1.85 x 10-3 mm2/s (1.26 - 2.59) (
Figure 2, image A). After the administration of the contrast, all the lesions presented a progressive enhancement reaching 120” with a maximum setting and with no subsequent washing (type A curve, with benign behaviour) (
Figure 2, image B). The interobserver concordance was 100% in the final PA diagnosis.
Surgical treatment was given in all of the cases. Ten patients with full parotidectomy, while 29 preferred conservative surgery with lumpectomy [
8] or partial parotidectomy [
10].
4. Discussion
The PA is the most frequent of either major or minor salivary gland tumours and is the most frequent parotid gland tumour [
14,
15]. Due to its benign behaviour, it is very important to make a correct preoperative diagnosis in order to consider all surgical options or to also opt for monitoring those patients with surgical risk. PAs have been morphologically described as masses with well-defined borders surrounded by a complete or incomplete capsule, with no infiltration data from neighbouring structures and with homogeneous signal intensity [
16,
17]. Among our patients, 23 lesions had all the benignity criteria, while 16 had a heterogeneous signal intensity, which could create doubts regarding the PA diagnosis. It should be remembered that heterogeneous enhancement, along with other parameters such as a low apparent diffusion coefficient or a high signal intensity ratio on T1-weighted images with gadolinium and fat suppression, are signs of a worse prognosis in parotid gland neoplasms [
18].
The PA in DWI sequences will have a high ADC due to its low cellularity. One of the first original works that studied the behaviour of PA in diffusion sequences was that done by Yabuuchi et al [
10] where they established an ADC diagnostic value for PA > 1.4 x 10-3 mm2/s. In a later work, Matsusue et al. [
19] established the same ADC threshold value for the PA diagnosis. Both are retrospective studies. In our series, 79,5% of the lesions (31 of 39) had an ADC ≥ 1.40 x 10-3 mm2/s. The eight cases that were below this value had an ADC between 1.3-1.39 x 10-3 mm2/s, which is still above the threshold value established by both authors [
10,
20] for the Warthin tumour and those malign lesions which ADC set at <1 x 10-3 mm2/s. These eigth lesions morphologically correspond to the most heterogeneous lesions. The only work published on this subject with prospective recruitment, is that done by Milad et al. Only 8 of the 46 parotid tumours analysed are PAs. They establish an ADC value of 1.67 x 10-3 mm2/s, although that is the average for all the benign lesions (including cysts and adenopathies), not only for PAs. However, nineteen (48.7%) of our lesions are also above this threshold level. Our work is the only prospective design, which exclusively studies PA behaviour.
The use of contrast in the diagnosis of parotid lesions has also been the object of a number of investigations [
10,
18,
19,
21,
22,
23]. All agree that benign lesions have a homogeneous enhancement. The perfusion curve morphology is also a characteristic Curves A, B and D indicate benignancy, curve A being more indicative of PA. All our cases showed a type A enhancement curve. Some authors such as Stefanovic et al. [
21], reached the conclusion that the type of curve is decisive for the correct relation n of the lesions in specific cases that had an ADC between 1.2 and 1.4 x 10-3 mm2/s. Yabucchi et al. [
10,
19] studied this same aspect, coming to the conclusion that in lesions that showed washing (types B and C), resorting to the interpretation of the diffusion study is required, whilst the A and D curves are diagnoses of benign lesions. Following the protocols that interpret all of the advanced MRI sequences together, all our lesions are conclusively PAs.
Four cases of the 43 initially reviewed, which radiologically appeared to be PA, were finally diagnosed as monomorphic or basal cell adenoma, with a concordance greater than 90.6%. This kind of tumour is not very frequent; also being a benign type [
23,
24]. The main difference with the PA is the higher recurrence rate. The radiological findings are indistinguishable from the PA. Due to the low prevalence, there are not any studies related to the behaviour of these tumours neither in advanced MRI sequences nor about the existence of any difference in behaviour with the PAs.
MRI is a very effective imaging method in the diagnosis of PA described for years [
25,
26,
27], the morphological image together with the functional one allowed to establish the preoperative diagnosis in 90.7% of the patients in our series. MRI is the first choice technique for the evaluation of PA, having surpassed other diagnostic imaging methods, such as ultrasound or CT, in the diagnosis of salivary tumor pathology. MRI helps in the differential diagnosis of PA with other parotid tumors, benign and malignant, by means of the morphological and fundamentally functional image, as it presents different ADC values and perfusion curves [
28,
29,
30]. And the technique has improved in recent years not only in diagnosis but also in treatment, through 3D reconstruction and fusion with computed tomography (CT) [
31].
The main limitation of this study is that it was designed as a diagnostic and follow-up case series study, rather than a blinded clinical trial. Furthermore, only cases with suspected plemorphic adenoma after MRI were evaluated.
Our study is restricted to studying the behaviour of PAs only, without comparing them with other types of tumours, such as the Warthin tumour or malign types (mucoepidermoid or metastasis among others). The next step will be to compare the behavior of our series of PAs with other tumors, but we need the analysis of more cases to obtain statistically relevant differences. This is also the only study among observers with a varied range of experience (one expert in pa-rotid MRI and another only expert in oral patholoogy), 100% agreement. This fact shows that the PA diagnosis is easy with basic knowledge of morphology and behavior in the diffusion study in-cluding contrast.
5. Conclusions
The Pleomorphic Adenoma are the most common benign tumours in the parotid gland. They are morphologically well defined lesions, without any infiltration from neighbouring, homogeneous structures. Advanced MRI sequences play the most importatan rol in imaging diagnostic an important role in their diagnosis. The lack of washing after administration of contrast and a >1.4 x 10-3 mm2/s ADC are PA diagnostics.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used Conceptualization, J.P-E.; J.L.L. and E.V-O.; Methodology, J.P-E.; E.V-O. and J.L-L.; Validation, E.V-O. and J.L-L.; Formal analysis, J.P-E. and C.G-V.; Investigation, J.P-E. and C.G-V.; Resources, J.P-E.; I.O.-G.; L.M.-F.; and A.J.-G.; Data Curation, J.P-E.; E.V-O.; A.J.-G. and J.L-L.; Writing – Original Draft Preparation, J.P-E. and E.V-O.; Writing – Review & Editing, J.P-E.; E.V-O.; A.J.--G. and J.L-L.; Visualization, E.V-O., I.O.-G.; L.M.G. and J.L-L.; Supervision, E.V-O. and J.L-L.; Project Administration, J.P-E.; E.V-O. and J.L-L..
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations.
The following abbreviations are used in this manuscript:
| MRI scan |
Image Magnetic Resonance |
| CT |
Computed tomography |
| DWI |
Diffusion sequence |
| ADC |
Apparent Diffusion Coefficient |
References
- Tartaglione, T.; Botto, A.; Sciandra, M.; Gaudino, S.; Danieli, L.; Parrilla, C.; et al. Differential diagnosis of parotid gland tumours: Which magnetic resonance findings should be taken in account? Acta Otorhinolaryngol Ital 2015, 35, 314–320. [Google Scholar] [CrossRef]
- Chakrabarty, N.; Pai, P.; Sahu, A.; Chowdhury, O.R.; Kandalgaonkar, P.; Dadlani, T.; Menon, M.; Ankathi, S.K. MRI Signatures of Parotid Tumours Impacting Management Decisions: A Retrospective Study With Radiology and Pathology Correlation. J Med Imaging Radiat Oncol 2025, 69, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Hernández Sandemetrio, R.; Armengot Carceller, M.; Alba García, JR.; Taleb, C.; Jiménez Roig, J.P.; Frías Moya-Angeler, S.; et al. Adenoma pleomorfo del lóbulo profundo de la parótida: A propósito de un caso. Acta Otorrinolaringol Esp 2006, 57, 56–58. [Google Scholar] [CrossRef]
- Collazo-Fernández, L.; Campo-Trapero, J.; Cano-Sánchez, J.; García-Martín, R.; Ballestín-Carcavilla, C. Retrospective study of 149 cases of salivary gland carcinoma in a Spanish hospital population. Med Oral Patol Oral Cir Bucal 2017, 22, e207–e213. [Google Scholar] [CrossRef]
- Orhan Soylemez, U.P.; Atalay, B. Differentiation of Benign and Malignant Parotid Gland Tumors with MRI and Diffusion Weighted Imaging. Medeni Med J 2021, 36, 138–145. [Google Scholar] [CrossRef]
- Gaurang, V.; Shah, M. MR Imaging of salivary glands. Magn Reson Imaging Clin N Am 2002, 10, 631–632. [Google Scholar] [CrossRef]
- Kim, Y.; Song, J.S.; Choi, S.H.; Nam, S.Y.; Cho, K.J. Association Between the Histological Subtypes, Anatomical Locations, and MAML2 Rearrangement of Head and Neck Mucoepidermoid Carcinoma. Head Neck Pathol 2025, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Chulam, T.C.; Noronha Francisco, A.; Goncalves Filho, J. Warthin’s tumour of the parotid gland: Our experience. Acta otorhinolaryngol Ital 2013, 33, 393–397. [Google Scholar] [PubMed]
- Nayak, G.K.; Hagiwara, M. Imaging of salivary gland pathology. Operative Techniques in Otolaryngology 2018, 29, 116–128. [Google Scholar] [CrossRef]
- Yabuuchi, H.; Matsuo, Y.; Kamitani, T.; Setoguchi, T.; Okafuji, T.; Soeda, H.; et al. Parotid gland tumors: Can addition of diffusion-weighted MR imaging to Dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology 2008, 249, 909–916. [Google Scholar] [CrossRef]
- Fathi Kazerooni, A.; Nabil, M.; Alviri, M.; Koopaei, S.; Salahshour, F.; Assili, S.; Saligheh Rad, H.; Aghaghazvini, L. Radiomic Analysis of Multi-parametric MR Images (MRI) for Classification of Parotid Tumors. J Biomed Phys Eng 2022, 12, 599–610. [Google Scholar] [CrossRef]
- Abend, A.; Keir, G.; Krishnan, K.; Kim, J.; Strauss, S.; Kutler, D. Parotid Pleomorphic Adenoma and Apparent Diffusion Coefficient: A Novel Clinical Prediction Tool. Laryngoscope 2025, 135, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Karaman, C.Z.; Tanyeri, A.; Özgür, R.; Öztürk, V.S. Parotid gland tumors: Comparison of conventional and diffusion-weighted MRI findings with histopathological results. Dentomaxillofac Radiol 2021, 50, 20200391. [Google Scholar] [CrossRef]
- Kakimoto, N.; Gamoh, S.; Tamaki, J.; Kishino, M.; Murakami, S.; Furukawa, S. CT and MR images of pleomorphic adenoma in major and minor salivary glands. Eur J Radiol 2009, 69, 464–672. [Google Scholar] [CrossRef]
- Kato, H.; Kawaguchi, M.; Ando, T.; Mizuta, K.; Aoki, M.; Matsuo, M. Pleomorphic adenoma of salivary glands: Common and uncommon CT and MR imaging features. Jpn J Radiol 2018, 36, 463–471. [Google Scholar] [CrossRef]
- Ginat, D.T. Imaging of benign neoplastic and nonneoplastic salivary gland tumors. Neuroimaging Clin N Am 2018, 159–169. [Google Scholar] [CrossRef]
- Gökçe, E.; Beyhan, M. Advanced magnetic resonance imaging findings in salivary gland tumors. World J Radiol 2022, 14, 256–271. [Google Scholar] [CrossRef]
- Ando, T.; Kato, H.; Shibata, H.; Ogawa, T.; Noda, Y.; Hyodo, F.; Matsuo, M. Prognostic Factors of Pretreatment Magnetic Resonance Imaging for Predicting Clinical Outcome in Patients With Parotid Gland Cancer. J Comput Assist Tomogr 2023, 47, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Matsusue, E.; Fujihara, Y.; Matsuda, E.; Tokuyasu, Y.; Nakamoto, S.; Nakamura, K.; et al. Differentiating parotid tumors by quantitative signal intensity evaluation on MR imaging. Clin Imagin 2017, 46, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Milad, P.; Elbegiermy, M.; Shokry, T.; Mahmoud, H.; Kamal, I.; Taha, M.S.; et al. The added value of pretreatment DW MRI in characterization of salivary glands pathologies. Am J Otolaryngol 2017, 38, 13–20. [Google Scholar] [CrossRef]
- Stefanovic, X.; Tabaa, Y.; Gascou, G.; Lacombe, S.; Auge, Y.; Delort, P.; et al. Magnetic Resonance Imaging of Parotid Gland Tumors: Dynamic Contrast-Enhanced Sequence Evaluation. J Comput Assist Tomogr 2017, 41, 541–546. [Google Scholar] [CrossRef]
- Yabuuchi, H.; Fukuya, T.; Tajima, T.; Hacitanda, Y.; Tomita, K.; Koga, M. Salivary gland tumors: Diagnostic value of gadolinium-enhanced Dynamic MR imaging with histopathologic correlation. Radiolog 2003, 226, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiang, M.; Wu, L.; Tao, X. Differential diagnostic value of diffusion-weighted and dynamic contrast-enhanced MR imaging in non-cystic lesions in floor of the mouth. Dentomaxillofac Radiol 2019, 48, 20180240. [Google Scholar] [CrossRef] [PubMed]
- Fantasia, J.E.; Neville., B.W. Basal cell adenomas of the minor salivary glands. A clinicopathologic study of seventeen new cases and review of the literarture. Oral Surg. 1980, 50, 433–440. [CrossRef]
- Som, P.M.; Shugar, J.M.; Sacher, M.; Stollman, A.L.; Biller, H.F. Benign and malignant parotid pleomorphic adenomas: CT and MR studies. J Comput Assist Tomogr 1988, 12, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Mirich, D.R.; McArdle, C.B.; Kulkarni, M.V. Benign pleomorphic adenomas of the salivary glands: Surface coil MR imaging versus CT. J Comput Assist Tomogr 1987, 11, 620–623. [Google Scholar] [CrossRef]
- Paz-Exposito, J.; Velasco-Ortega, E.; Manso-Garcia, F.; Bullon-Fernandez, P. Magnetic resonance imaging in the diagnosis of parotid pleomorphic adenomas. Med Oral. 1997, 2, 271–282. [Google Scholar] [PubMed]
- Alrohaimi, F.A.; Alanazi, F.M.; Almousa, H.M.; Almutairi, A.B.; Alqahtani, S.M. Basal Cell Adenoma of the Minor Salivary Glands in the Buccal Mucosa: A Rare Entity Arising in an Unusual Location. Cureus 2023, 15, e36580. [Google Scholar] [CrossRef]
- Zouhair, N.; Mallouk, S.; Oukessou, Y.; Rouadi, S.; Abada, R.L.; Roubal, M.; Mahtar, M. Corrélation entre l’imagerie par résonnance magnétique, l’extemporanée et l’histologie définitive des tumeurs parotidiennes: Série de cas [Correlation between magnetic resonance imaging and extemporaneous and definitive histological examination of parotid gland tumors: A case series]. Pan Afr Med J 2020, 37, 80, French. [Google Scholar] [CrossRef]
- Sreecharan, V.R.; Naik, S.; Deep, N.; Adhya, A.K.; Chappity, P.; Mohakud, S.; Nayak, M.K.; Patel, R.K.; Tripathy, T. Multiparametric MRI in Diagnosis of Parotid Gland Tumor: An Observational Study in 3-T MRI. Indian J Radiol Imaging 2024, 35, 402–410. [Google Scholar] [CrossRef]
- Yue, L.; Yao, Y.; Zunan, T.; Leihao, H.; Wenbo, Z.; Xin, P. Three-dimensional reconstruction based on computed tomography/magnetic resonance imaging multimodal image fusion for parotid gland tumor diagnosis and treatment. J Craniomaxillofac Surg 2025, 53, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).