1. Introduction
Toxic-infectious shock (TIS) is a severe complication of the infectious process, manifesting as acute hemodynamic collapse caused by a massive influx of infectious agents and/or their toxic products into the bloodstream [
1,
2]. All microorganisms can cause toxic-infectious shock, but it is most commonly associated with Gram-negative and Gram-positive bacteria, both aerobic and anaerobic, as well as rickettsiae, viruses, fungi, and others [
1,
2]. Globally, there is an increasing incidence of this condition [
1,
2]. The reasons for this include the rising number of immunocompromised patients in combination with various chronic diseases such as diabetes, chronic cardiopathies, pneumopathies, nephropathies, and other systemic diseases [
1,
2]. Predisposing factors include neoplastic processes, cytostatics, glucocorticosteroids, alcoholism, smoking, extreme age groups, hospitalizations, and more [
1,
2]. Adding to the significance of the problem is the global increase in antimicrobial resistance, leading to therapeutic difficulties [
3].
Escherichia coli (
E. coli) is the most common pathogen causing bacterial enteritis in lambs and kids and poses a significant health risk to both animals and humans [
4]. Lambs infected with virulent strains of
E. coli, such as Shiga toxin-producing
Escherichia coli (STEC) and
Salmonella spp., and the presence of these microorganisms in lamb feces represent a veterinary and public health threat [
4].
E. coli O128 and O91 are the most frequently isolated strains from lamb chops [
5].
E. coli O128 is a common etiological agent of diarrhea, particularly in early childhood, with pathogenesis and clinical presentation linked to the production of toxins—enterotoxins or Shiga toxins [
6]. In the reported clinical case, enterotoxigenic
E. coli O128 strains with genes for enterotoxin production were isolated and confirmed by genetic and serological tests. These toxins lead to the secretion of electrolytes and water into the intestinal lumen: heat-labile (lt) and heat-stable (st) toxins, without genes for adherence (eae) [
6]. The importance of
Klebsiella oxytoca is increasing as a significant opportunistic pathogen causing nosocomial infections across all age groups [
7].
Klebsiella oxytoca is the causative agent of a wide spectrum of diseases. It is frequently isolated in respiratory and urinary infections, and in rare cases, it can cause diarrhea and infectious endocarditis [
7]. The spread of multidrug-resistant strains of
Klebsiella oxytoca is particularly concerning, as it is associated with fatal outcomes in immunocompromised patients and those with comorbidities [
7].
Streptococcus equi subsp. zooepidemicus is a pathogen primarily of veterinary-medical significance [
8].
Enterococcus durans is found in many animals with enteritis and is rarely detected in human fecal samples. Both microorganisms rarely cause diseases in humans but may be associated with the development of opportunistic invasive infections [
8].
2. Materials and Methods
The methods used include epidemiological and clinical analysis, clinical-laboratory studies, genetic and serological tests. Samples for microbiological examination from cadaveric material (spleen and intestine) were transported in Amies medium. Cultivation was performed on blood agar and Levine medium. For microbiological identification, automated systems Vitek-2 Compact and MALDI-TOF MS (bioMerieux, France) were used.
3. Case Presentation
This is a 65-year-old male, Y.I.P., Case No. 26392/2024, hospitalized in the Clinic of Infectious Diseases, St. George University Hospital, Plovdiv, with intestinal infection in a state of toxicoinfectious shock, presenting with an intense diarrheal syndrome of 1–2 days’ duration (about 10 liquid, light-brown stools daily without mucus or blood), general weakness, chest and abdominal pain, and fever. One day prior to the onset of complaints, he had consumed green salad and lamb meat.
The patient had comorbidities including chronic heart failure—arterial hypertension, two coronary stents; liver cirrhosis with complications—portal hypertension, esophageal varices; and anemia syndrome.
On admission, he was in moderately severe general condition, afebrile, conscious, psychomotorically agitated but adequate, with toxicoinfectious and diarrheal syndrome, moderately intoxicated, dehydrated grade I–II—pale, sweaty, cold skin with reduced turgor, petechiae, ecchymoses, and suffusions on the hands, dry tongue coated with a white deposit, arthralgias and myalgias, tachydyspnea 40/min, O₂ saturation 94% on room air, clear vesicular breath sounds. Heart rate was 98/min with arrhythmic cardiac activity, arterial blood pressure 114/75 mmHg, abdomen distended above the level of the thorax, diffusely tender but allowing deep palpation, increased intestinal peristalsis, lower limb edema, and normal neurological status.
Intravenous rehydration therapy was started immediately. During treatment, the condition rapidly deteriorated—the patient fell into a comatose state, Glasgow Coma Scale (GCS) = 3 points, with progressive tachydyspnea 40–45/min, lividity of the extremities, and absent circulation. Full cardiopulmonary resuscitation was promptly initiated in collaboration with an anesthesiologist but resulted in death.
Hematological investigations (
Table 1) revealed a mild anemia syndrome (hemoglobin [Hgb] 129 g/L, reference values 140–180 g/L; red blood cells [RBC] 3.67 T/L, reference values 4.5–6 T/L) and thrombocytopenia (platelets [Plt] 90 G/L, reference values 140–400 G/L), probably due to hemorrhagic diathesis and/or consumptive coagulopathy, elevated erythrocyte sedimentation rate (ESR 33 mm/h, reference values 2–25 mm/h), and differential blood count showing neutrophilia and lympho-monocytopenia (neutrophils [Neu] 87%, reference 42–70%; lymphocytes [Ly] 9%, reference 22–48%; monocytes [Mo] 2%, reference 6–12%).
The elevation of D-dimer (DD) in the patient (DD >35 mg/L, reference value 0–0.50 mg/L) (
Table 2) is an indicator of thrombosis or risk of thrombosis in a latent condition, most commonly venous thrombosis (thrombophlebitis, pulmonary embolism) and disseminated intravascular coagulation (DIC) syndrome.
From the biochemical investigations (
Table 3), notable changes in the reference values of serum bile pigments and liver enzymes were observed: a mild increase in total serum bilirubin (T BIL) 56.7 μmol/L (reference value 3.4–21 μmol/L) with direct bilirubin (D BIL) 18.7 μmol/L (reference value 0.8–8.5 μmol/L); decreased serum cholinesterase (CHE) activity 2000 U/L (reference value 3600–11,500 U/L), indicating reduced hepatic synthetic function; and mildly to moderately elevated aminotransferases: alanine aminotransferase (ALT) 75 U/L (reference value 0–50 U/L) and aspartate aminotransferase (AST) 542 U/L (reference value 0–50 U/L).
Changes were also detected in clinical laboratory markers of acute myocardial ischemia and ischemic heart disease: elevated creatine kinase MB fraction (CK-MB) 478 U/L (reference value 0–22 U/L) and troponin (TnI) 0.08 ng/mL (reference value 0.0–0.04 ng/mL). These biochemical alterations could be associated with the known comorbidities—chronic cardiac and hepatic insufficiency—exacerbated during the course of the infectious process.
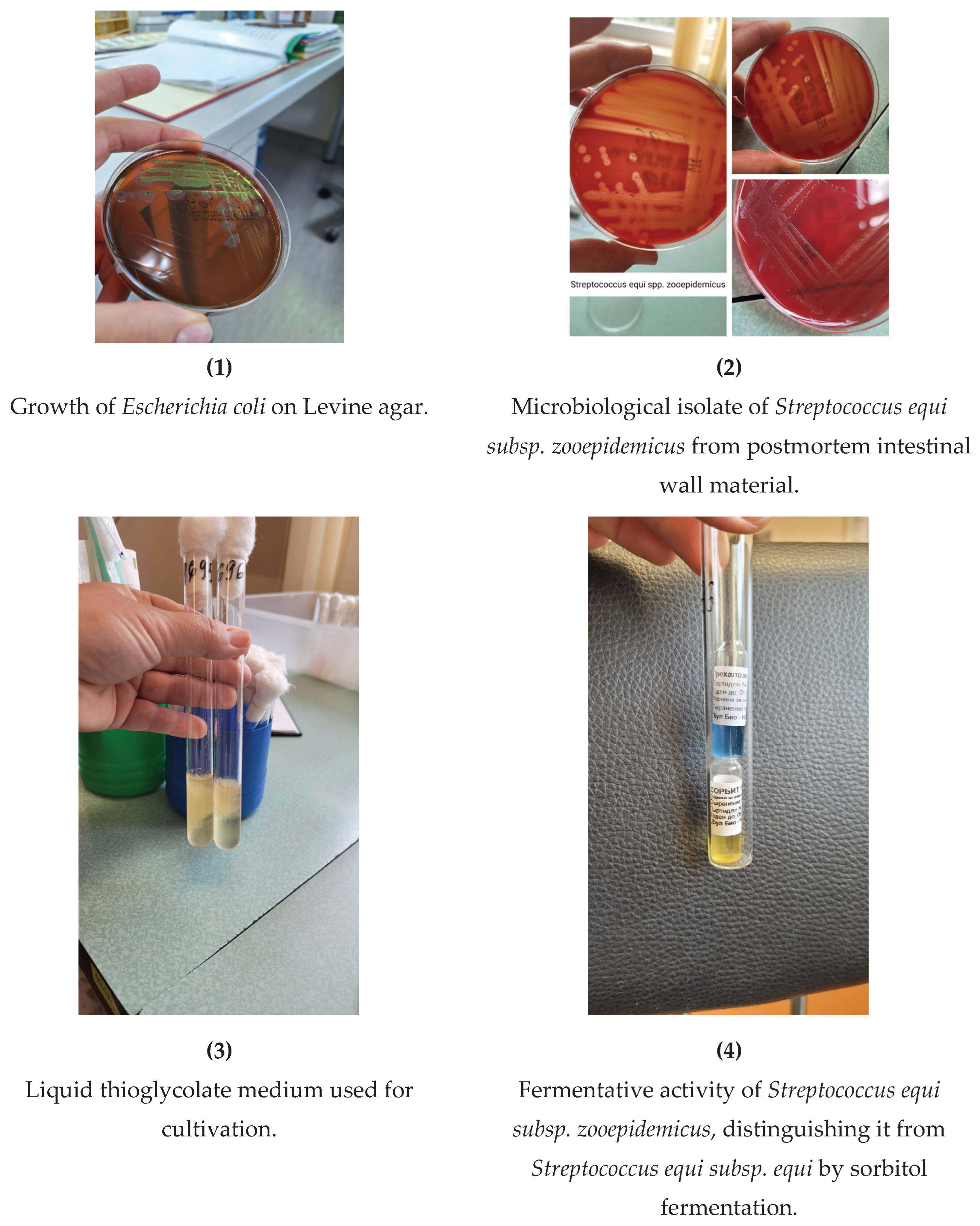
From the microbiological examination of postmortem intestinal wall material, pure cultures of
Escherichia coli (
Figure 1) were isolated, sensitive according to the antibiogram to Ampicillin, Piperacillin, Cefoxitin, Cefotaxime, Ceftriaxone, Amoxicillin-clavulanic acid, Amikacin, Ciprofloxacin, Trimethoprim/sulfamethoxazole, Levofloxacin, Meropenem, Imipenem, Cefepime, and Tigecycline; and
Streptococcus equi subsp. zooepidemicus (
Figure 2,
Figure 3,
Figure 4), sensitive according to the antibiogram to Penicillin, Ceftriaxone, Cefepime, Vancomycin, Teicoplanin, Trimethoprim/sulfamethoxazole, Tigecycline, Linezolid, Moxifloxacin, Amoxicillin-clavulanic acid, Cefuroxime (Axetil), and Rifampin; resistant to Erythromycin, Clindamycin, and Tetracycline; with intermediate susceptibility to Levofloxacin (
Table 4).
From the microbiological examination of postmortem spleen material, pure cultures of
Enterococcus durans were isolated, sensitive according to the antibiogram to ampicillin, ciprofloxacin, norfloxacin, gentamicin, vancomycin, teicoplanin, linezolid, tigecycline, amoxicillin-clavulanic acid, piperacillin, levofloxacin, and ampicillin-sulbactam; and
Klebsiella oxytoca, sensitive according to the antibiogram to cefoxitin, cefotaxime, ceftriaxone, amoxicillin-clavulanic acid, amikacin, ciprofloxacin, trimethoprim/sulfamethoxazole, levofloxacin, meropenem, imipenem, cefepime, piperacillin-tazobactam, colistin, and ceftazidime/avibactam; resistant to ampicillin and piperacillin (
Table 5).
From biopsy material, enterotoxigenic E. coli O128 were isolated and confirmed by genetic and serological tests, carrying genes for the production of heat-labile (lt) and heat-stable (st) toxins, without adhesion (eae) genes.
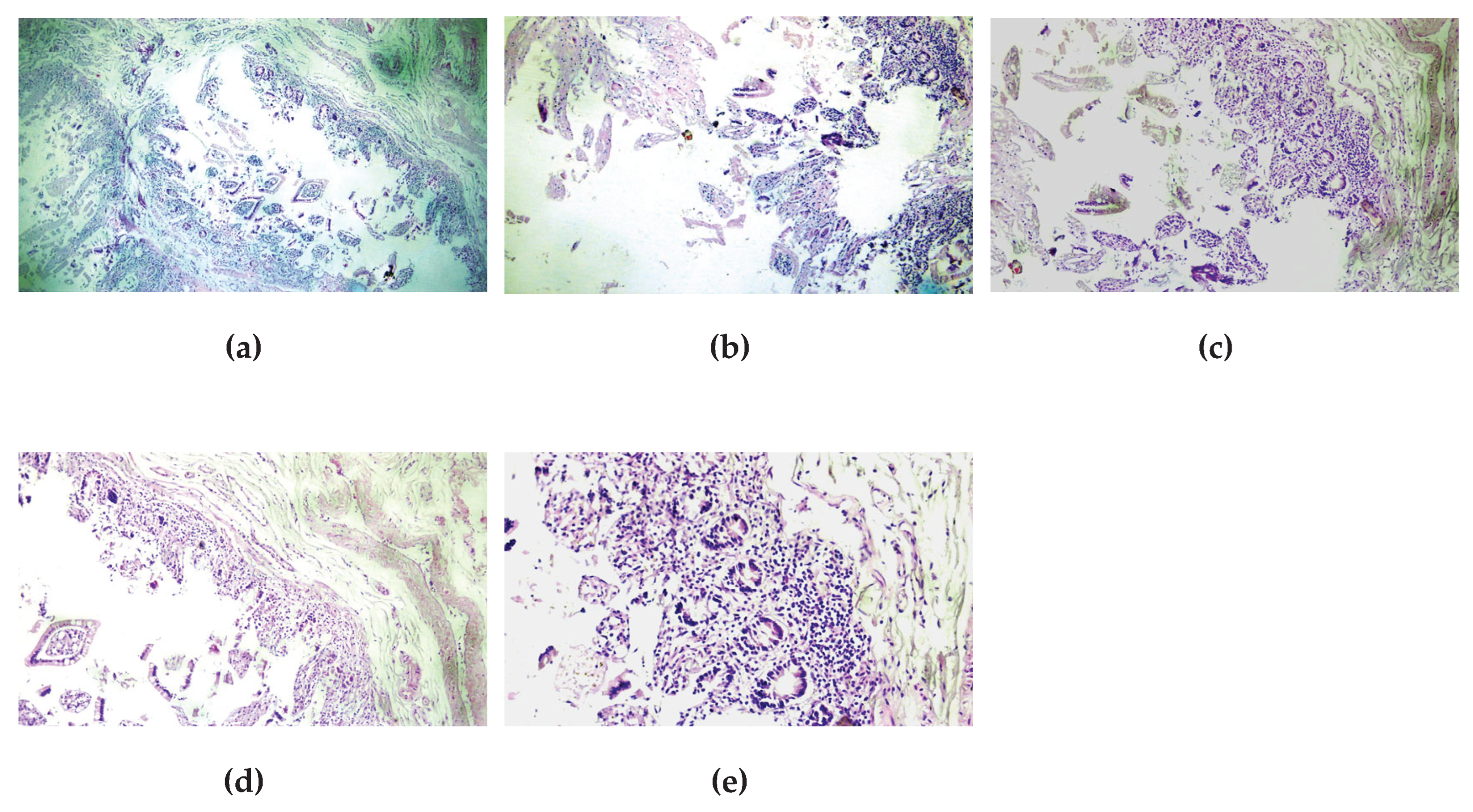
During the autopsy, notable findings included fibrinous deposits on the serosa of the small intestine, with edematous mucosa, as well as atherosclerotic changes (stage 3 of the aorta) leading to chronic ischemic heart disease (CIHD) and subsequent pulmonary edema. The leading changes for thanatogenesis were in the liver—micronodular cirrhosis with esophageal varices and gastric hemorrhage. Histological examination confirmed the catarrhal enteritis caused by the zoonotic infection, with characteristic changes—fibrinous plaques, edema of the wall and mucosa, and inflammatory infiltrate (
Figure 5).
4. Discussion
In the present study, we describe a rare clinical case of a 65-year-old man with severe toxicoinfectious shock and diarrheal syndrome, resulting from a mixed infection with four pathogens. The first pathogen was Escherichia coli O128, identified as an enterotoxigenic strain with genes for heat-labile (lt) and heat-stable (st) toxins, without the adhesion (eae) gene. The second pathogen was Streptococcus equi subsp. zooepidemicus, isolated from the intestinal wall, with a specific antibiogram (susceptible to Penicillin, Ceftriaxone, etc.). The third pathogen was Klebsiella oxytoca, isolated from the spleen, with a profile characteristic of multidrug-resistant opportunistic strains (resistant to Ampicillin and Piperacillin). The fourth pathogen was Enterococcus durans, also isolated from the spleen, with susceptibility to most of the commonly used antibiotics.
The patient had concomitant chronic diseases—chronic heart failure (with two stents and hypertension) and liver cirrhosis with complications (portal hypertension, esophageal varices)—which contributed to the severity of the clinical course and the fatal outcome. Laboratory findings showed significant abnormalities. First, mild anemia (Hgb 129 g/L, RBC 3.67 T/L) and thrombocytopenia (Plt 90 G/L) were established. Second, impaired hemostasis was found, with decreased prothrombin time (PT 27.2%) and markedly elevated D-dimer (>35 mg/L, compared to the normal 0–0.50 mg/L). There were significant biochemical changes with increased total bilirubin (56.7 μmol/L), direct bilirubin (18.7 μmol/L), mildly elevated ALT (75 U/L) and markedly elevated AST (542 U/L), as well as elevated markers of myocardial ischemia (CK-MB and troponin).
Histological examination of the intestinal wall revealed catarrhal enteritis with characteristic fibrinous plaques, edema, and inflammatory infiltrate, confirming the severe inflammatory damage.
Reporting of mixed infection with four pathogens in humans is extremely rare, as the literature usually describes cases with mono- or dual infections. Escherichia coli O128 is most often associated with diarrhea in early childhood, and its pathogenicity in adults is uncommon. Klebsiella oxytoca is increasingly reported as a cause of nosocomial infections, especially in immunocompromised patients, highlighting the trend of multidrug-resistant strains. Streptococcus equi subsp. zooepidemicus and Enterococcus durans are mainly of veterinary significance, with zoonotic infections caused by them remaining exceptionally rare.
These differences underscore the uniqueness of the present case and demonstrate that the combined action of several pathogens may lead to synergistic effects accelerating progression to toxicoinfectious shock.
The mixed infection was likely the result of the combined influence of several factors. Chronic diseases—cardiac and hepatic insufficiency—significantly increase the risk of severe infectious complications by reducing immune response. The pathogenic characteristics of the microorganisms—the presence of enterotoxins in E. coli O128 leads to massive secretion of electrolytes and water in the intestines, which, together with the possible synergy between the different microorganisms, accelerates the onset of toxicoinfectious shock. Hematological and biochemical abnormalities—high D-dimer, decreased prothrombin time, and elevated liver enzymes—indicate systemic dysfunction, probably resulting from the interaction of bacterial toxins with the pre-existing chronic conditions.
This clinical case highlights several important aspects. First, the need for rapid diagnosis—the use of molecular and serological techniques for pathogen identification may be crucial, especially in high-risk patients. Second, an integrated approach to treatment—assessment of laboratory parameters such as hemostasis, liver enzymes, and cardiac markers should be part of the standard protocol when mixed infections are suspected. Third, prevention and control of nosocomial infections—the increasing importance of multidrug-resistant strains such as Klebsiella oxytoca requires optimization of antimicrobial therapy and strict infection control protocols.
As a single case, this report has limitations regarding the generalization of the results. The absence of a comparative group makes it difficult to draw broad conclusions. Limited monitoring of the dynamics of the infection and potential methodological limitations in pathogen isolation may affect data interpretation.
Based on the obtained results, the following directions for future research are proposed: 1) Systematic investigation of mixed bacterial infections—collection of a larger number of cases to analyze common features and risk factors; 2) Molecular mechanisms of synergy—study of interactions between different pathogens and their impact on cellular and systemic immune responses; 3) Optimization of diagnostic methods—development and implementation of rapid, highly specific tests to allow timely identification of rare and combined infections; 4) Antimicrobial resistance—in-depth study of the resistance profiles of pathogens to ensure adequate and targeted therapy.
5. Conclusions
The present clinical case demonstrates that combined infection with four pathogens—E. coli O128, Streptococcus equi subsp. zooepidemicus, Klebsiella oxytoca, and Enterococcus durans—in patients with risk factors predisposes to a severe disease course, with rapid deterioration in general condition and even fatal outcome. The results emphasize the need for the introduction of highly specific and rapid diagnostic methods, which are crucial for timely diagnosis and the initiation of adequate targeted therapy. Serological and molecular techniques are essential for the detection and characterization of pathogenic bacteria and virulence markers associated with bacterial enteritis. This report highlights new aspects in the pathogenesis of mixed infections and provides a foundation for future research in the field of infectious diseases.
Author Contributions
Conceptualization, P.V. and M.S.; methodology, P.V., A.I., V.G. and Y.K, .; validation, P.V., Y.K. and A.I.; formal analysis, P.V.; investigation, P.V.; resources, P.V., Y.K.,A.I.; data curation, P.V., A.I., Y.K.; writing—original draft preparation, P.V, Y.K..; writing—review and editing, P.V., Y.K., M.S.; visualization, P.V., Y.K., A.I., V.G.; supervision, M.S.; project administration, Y.K., P.V.; funding acquisition, Y.K. All authors have read and agreed to the pulished version of the manuscript.
Funding
This study was funded by the European Union-Next Generation EU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0007-C01 “Strategic Research and Innovation Program for the Development of MU-PLOVDIV–(SRIPD-MUP)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The patient signed an informed consent form for the provision of hospital care and the application of diagnostic and therapeutic procedures upon admission. Patient consent was waived due to the postmortem nature of the study.
Data Availability Statement
Data supporting this study are available upon request from the corresponding author.
Acknowledgments
The authors acknowledge the technical support of the Department of Medical Microbiology and Department of General and Clinical Pathology, MU-Plovdiv.”
Conflicts of Interest
The authors declare no conflict of interest.
References
- Durmishev, A; Iliev, B; Denchev, V; Mitov, G; Radev, M; Gancheva, C; Baev, V; Angelov, L; Ilieva, P; Miteva, R; Durmishev, L. Infectology, 1st ed.; Publisher: Prof. Marin Drinov Publishing House of BAS, Sofia, Bulgaria, 2001; p. 65.
- Dikov, I; Stoyanova, D; Kaneva, J; Nenova, M; Kostadinova, P. Infectious diseases, 3rd ed.; Publisher: Znanie, Sofia, Bulgaria, 2006; p. 282.
- Kasper, D; Fauci, A; Hauser, S; Longo, D; Jameson, L; Loscalzo, J. Harrison's Infectious diseases, 3rd ed.; Publisher: McGraw-Hill Education, New York, United States, 2017; p. 507.
- Mokhbatly, A.A.; Elsheikh, N.; Ghazy, E.W.; Elgamal, A.M.; Hegazy, Y.M.; Assar, D.H. Prevalence of Shiga toxin-producing Escherichia coli and Salmonellae and some associated hematologic and biochemical profile alterations in lambs. Vet Res Forum. 2022, 2, 155–162. [Google Scholar] [CrossRef]
- Barlow, R.S.; Gobius, K.S.; Desmarchelier, P.M. Shiga toxin-producing Escherichia coli in ground beef and lamb cuts: results of a one-year study. Int J Food Microbiol. 2006, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scotland, S.M.; Smith, H.R.; Rowe, B. Escherichia coli O128 strains from infants with diarrhea commonly show localized adhesion and positivity in the fluorescent-actin staining test but do not hybridize with an enteropathogenic E. coli adherence factor probe. Infect Immun. 1991, 4, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Neog, N.; Phukan, U.; Puzari, M.; et al. Klebsiella oxytoca and Emerging Nosocomial Infections. Curr Microbiol, 2021, 78, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Argirova, P.; Kalchev, Y.; Baltadzhiev, I.; Stoycheva, M.; Murdjeva, M. Streptococcus zooepidemicus Meningitis in an HIV-Positive Horse Breeder Patient: A Case Study and Literature Review. Infect. Dis. Rep. 2023, 15, 527–534. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).