Clinical Case Description
The case presented here is a male green macaw (Ara militaris mexicanus), approximately 15 years old, named Calvin. The patient arrived at the Benito Juárez Conservation Zoo in Morelia, Michoacan, in 2011 after being seized from illegal ownership. He already presented feather-plucking then, which made it impossible to establish a precise chronicity of the problem. The feather-pecking had continued until the time of the first CBD-oriented consultation in 2021. Calvin received periodic check-ups and other possible causes of feather-picking had already been ruled out, including external and internal parasites, primary or secondary malnutrition, fungal infections, and other organic causes. The patient was healthy, except for the plucking behavior, and his temperament, presenting as nervous, skittish, with excessive vocalizations, aggressive, and lashing out if anyone tried to touch him.
Reason for consultation
The patient presented a chronic severe pattern of feather-plucking spanning at least a decade, affecting several regions of his body: pectoral, cervical (dorsal, ventral and lateral), flanks, mantle and back, being practically devoid of feathers [
Figure 1]. He had paired with a female of his same species since 2017, and they had first reproduced in May 2021. He had begun to direct his feather-plucking behavior at his partner and chicks [
Figure 2]. The organic causes for the feather-picking had already been ruled out through annual tests (complete blood counts, metabolic panels, rectal swabs and enemas for infections and internal parasites, skin scraping and feather sampling for external parasites and fungal infections) returning no abnormal results.
Therapeutic approaches to the feather-picking had already been attempted with pharmacological treatments of tramadol and then haloperidol, however, no improvement was observed despite gradual increase in dosing, and administration became difficult due to the patient detecting the different drugs by taste, and finally these attempts at medication are abandoned due to staff rotation in the zoo.
Due to the lack of success with prior pharmacological treatments, the patient was only receiving environmental enrichment, and behavioral modification with positive reinforcement in 2021 showing a slight improvement, which suggested that the behavior may have originated from stress or anxiety. A presumptive diagnosis of psychogenic feather-plucking (with a possible Obsessive-Compulsive Disorder component) was established.
Since no significant improvement had been obtained using conventional treatments, the lead veterinarian in Calvin’s case sought to implement treatment with pharmaceutical-grade CBD isolate to reduce or eliminate the picking behavior in the patient, and improve his health and well-being.
Treatment and Evolution
The patient weighed 0.98 kg and had an active therapeutic plan at the time of consultation, including non-pharmacological treatment with the following: improvements in diet, environmental and social enrichment [
Figure 3], addition of a safe place, avoiding stressful situations, establishing a routine that provided comfort for the patient, and behavioral modification with positive reinforcement, through training with rewards and ignoring excessive vocalizations.
After the initial consultation in 2021, the ICAN veterinary team researched bibliographic references on the use of cannabinoids in birds, but no information was available at that time. The authors then sought to consult veterinary colleagues who could have had experience treating Psittacines with cannabinoids, interviewing Dr. Joli M. Jarboe (DVM, DACVIM Neurology) who had treated a scarlet macaw patient with hemp extract, but she did not have precise dosing data or follow-up on that case.
For these reasons, it was decided to start at a low dose of 0.25 mg/kg and to titrate up with gradual dose increases. A formulation was prescribed with 750 mg of CBD isolate in 30 ml of grapeseed oil, at a concentration of 25 mg/ml, formulated by a drug compounding laboratory in Mexico, BOTICAN®, with pharmaceutical-grade CBD isolate. During the first week of treatment, a dose of 0.25 mg/kg (0.25 mg total dose) was administered every 24 hours (SID). Since no changes were observed in the patient, the dose was increased to 0.5 mg/kg (0.5 mg total) SID during the second week of treatment. Thereafter, the dose was increased by an additional 0.25 mg/kg each week: week three at 0.75 mg/kg SID, week four at 1 mg/kg SID, week five at 1.25 mg/kg SID, and week six at 1.5 mg/kg SID.
Follow-up
Starting on the third day of treatment, a reduction in excessive vocalizations was observed. However, there seemed to be little improvement in the patient's feather-plucking during the first month and a half of treatment. Fortunately, no adverse effects or negative changes were observed when administered CBD isolate, so the dose continued to be increased at a rate of 0.25 mg/kg per week.
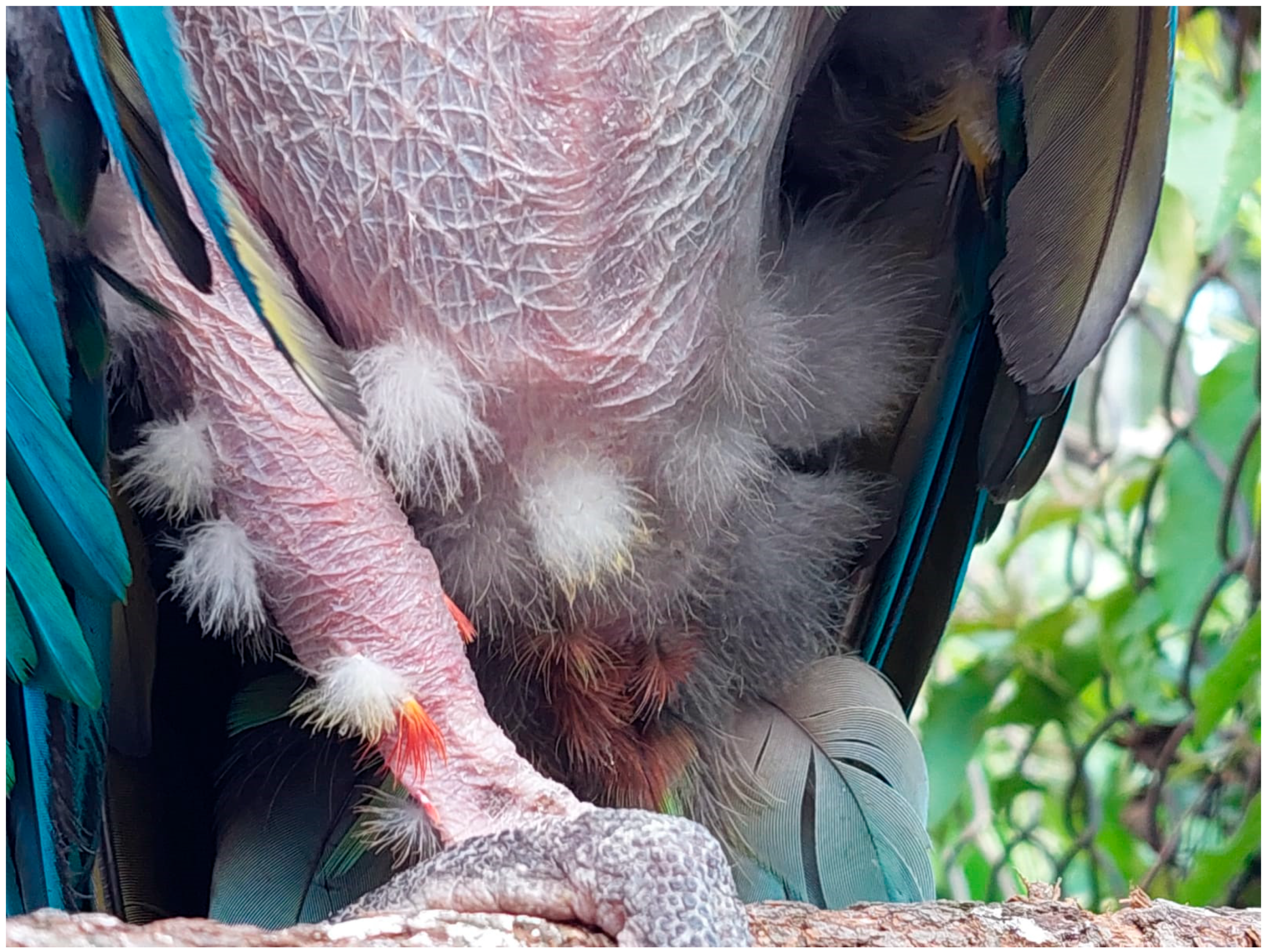
From week six of treatment, significant changes were seen in the patient; he showed a considerable decrease in excessive vocalizations, even stopping altogether for few days at a time. Additionally, the lead veterinarian and staff began to notice the patient was calmer, reducing aggressive behaviors, and showing greater tolerance to people outside his enclosure. The main improvement found at this dose was the reduction of the compulsive and repetitive feather-plucking, evidenced by the growth of filoplumes and down feathers (part of the birds' plumage cycle) [
Figure 4].
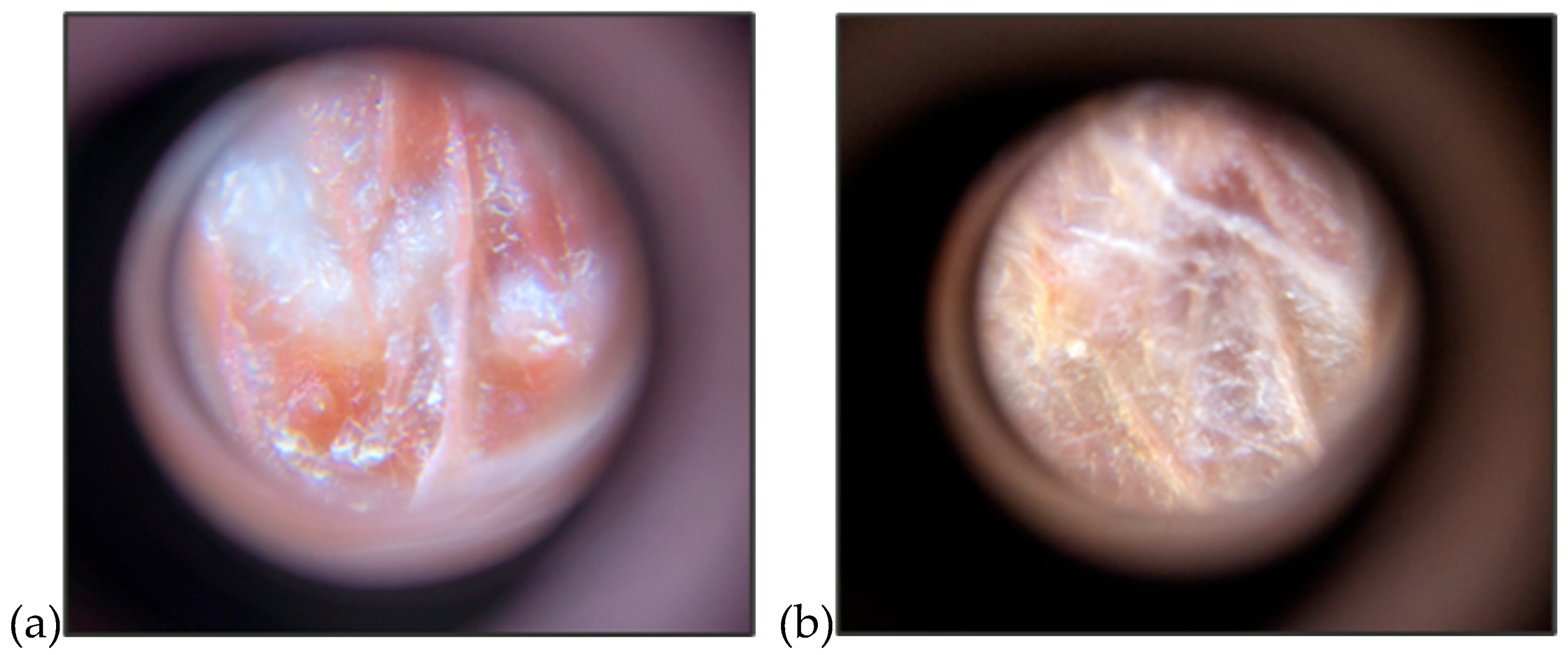
With these positive changes and no adverse effects, it was decided to continue increasing the dose weekly by 0.25 mg/kg SID, resulting in a dose of 1.75 mg/kg in week seven and 2 mg/kg in week eight. During these two weeks, the patient showed improvement in feather growth, a better condition of the skin with less redness and inflammation due to his prior excessive grooming [Figures 5a and 5b], and the growth of a greater number of filoplumes and down on the thighs, chest, and flanks [
Figure 6].
Figure 5.
Dermatological evaluation of the patient, showing red and inflamed skin before treatment (a) and healthier-looking skin after seven weeks of treatment (b).
Figure 5.
Dermatological evaluation of the patient, showing red and inflamed skin before treatment (a) and healthier-looking skin after seven weeks of treatment (b).
Dose increases of 0.25 mg/kg SID weekly continued for four additional weeks, until reaching a final dose of 3 mg/kg, a dose at which the patient remained behaviorally stable and without feather-plucking behaviors, although definitive plumage growth was not achieved possibly due to the long-term presentation of this problem (more than a decade).
The patient was kept at 3 mg/kg SID for several months and remained stable; however, due to internal changes in the zoo where he was kept, changes in the staff caused environmental and social fluctuations, disruption of the patient's schedules and routine, and intermittency in the administration of CBD, which caused the patient to relapse back into plucking behaviors, although not as severely as seen prior to CBD treatment. When CBD treatment was implemented by itself, without incorporating behavioral modification and environmental enrichment, a slight improvement was observed in the patient, whereas if a full behavioral treatment was provided together with CBD treatment, the patient showed significant improvement.
Unfortunately, the patient did not continue with his cannabinoid treatment during 2023 and his death was reported in mid-July after a medical complication not related to CBD.
Discussion
Feather-plucking, also known as feather-picking or feather pecking, is defined as a behavioral disorder based on the performance of repetitive and constant behavior without obvious function (stereotypy) that can evolve into a situation in which the affected avian performs the behavior persistently (and possibly becoming an obsessive-compulsive disorder). [
1] This behavior is considered one of the most frequent syndromes in Psittacines under human care, with an estimated prevalence between 10 and 17.5%. [
2,
3]
At the time when this patient began his CBD treatment, there was no published information on the use of cannabinoids in Psittacines, so this is one of the first reported clinical cases using cannabinoids in a macaw of the Psittacidae family. Although the CBD treatment did not result in the patient fully growing new feathers back, great improvement was observed in his mood, as well as in his way of relating to his macaw partner, macaw chicks, and the human staff who took care of him, in addition to improvement in the skin inflammation, most notably the pectoral area which was the most affected.
This behavioral problem has a wide range of possible causes and is known to affect other birds, such as laying hens. [
4] Evidence has been found that the serotonergic system is involved in this behavioral problem, showing improvement with dietary supplementation of tryptophan, an amino acid that serves as a serotonin precursor. [
4,
5]
Based on this information, it can be inferred that the various improvements seen in this patient derive from the anti-inflammatory effects of cannabidiol and its interaction with the endocannabinoid system (ECS), which exists in all vertebrates, [
6,
7] but also from the various interactions that CBD has with other receptors, such as anxiolytic effects partly due to activating the 5-HTA receptor, its antagonistic effect of the TRPV1 receptor, [
8] and its role in indirectly increasing the activation of cannabinoid 1 receptors (CB1R) [
9] through the inactivation of fatty acid amino hydrolase (FAAH), decreasing anandamide degradation and allowing it to bind to these receptors to decrease anxiety. [
10,
11,
12]
When analyzing the patient's responses according to the doses administered, there was no significant improvement at the beginning of CBD treatment, although decreased reactivity and aggressive behaviors were reported. However, as the dose was increased above 1.5 mg/kg, more positive changes were observed, such as decreased skin inflammation and reduction of feather-plucking. This may be related to the absence of CB2 receptors in Psittacines, [
13,
14] suggesting that this absence predisposes this avian family to suffer from neuroinflammation, [
14] and could have greater implications since this cannabinoid receptor is located in most of the immune system and is responsible for immunomodulation, [
15,
16,
17,
18] including the inflammatory response. Psittacines, lacking this receptor, could require higher doses of cannabinoids such as CBD to obtain positive results during treatment, and the responses related to this receptor may possibly be more modest.
The authors' clinical experience with avian patients appears to support the need for higher doses of CBD isolate in Psittaciformes than in patient cohorts such as dogs and cats. Psittacines make up nearly 89% of all avian clinical cases treated or supervised by ICAN veterinarians and have responded to doses between 0.25 and 3 mg/kg of cannabidiol isolate to achieve the desired therapeutic effects. The authors advise against the administration of clinical doses that may be too high for patients, emphasizing the importance of individualized dose titration to achieve therapeutic effects rather than extrapolating experimental dosages from research studies which might exceed the patient’s clinical needs.
Conclusions
As this is the first case published in macaws treated with cannabinoids in Mexico, it can be concluded that, despite seemingly requiring higher doses than mammalian species, Psittacines could benefit from therapy with legal and regulated cannabinoid medications. However, more research is needed to better understand the endocannabinoid system of Psittaciformes, clinical responses to different dosage ranges, as well as the pharmacokinetics and pharmacodynamics of cannabinoids in these avian species in order to offer them better therapeutic options and provide them with a better quality of life.
References
- Eeswara A, Pacheco-Spiewak A, Jergova S, Sagen J. Combined non-psychoactive Cannabis components cannabidiol and β-caryophyllene reduce chronic pain via CB1 interaction in a rat spinal cord injury model. PLoS One. 2023 Mar 13;18(3):e0282920.
- Langlois, I. Medical Causes of Feather Damaging Behavior. Vol. 24, Veterinary Clinics of North America - Exotic Animal Practice. W.B. Saunders; 2021. p. 119–52.
- van Zeeland YRA, Spruit BM, Rodenburg TB, Riedstra B, van Hierden YM, Buitenhuis B, et al. Feather damaging behaviour in parrots: A review with consideration of comparative aspects. Vol. 121, Applied Animal Behaviour Science. 2009. p. 75–95.
- Van Hierden YM, Koolhaas JM, Korte SM. Chronic increase of dietary L-tryptophan decreases gentle feather pecking behaviour. Appl Anim Behav Sci. 2004 Nov;89(1–2):71–84.
- de Haas EN, van der Eijk JAJ. Where in the serotonergic system does it go wrong? Unravelling the route by which the serotonergic system affects feather pecking in chickens. Neurosci Biobehav Rev. 2018 Dec 1;95:170–88.
- Atalay S, Jarocka-karpowicz I, Skrzydlewskas E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants. 2020;9(1):1–20.
- Robaina Cabrera CL, Keir-Rudman S, Horniman N, Clarkson N, Page C. The anti-inflammatory effects of cannabidiol and cannabigerol alone, and in combination. Pulm Pharmacol Ther. 2021 Aug 1;69.
- de Faria SM, de Morais Fabrício D, Tumas V, Castro PC, Ponti MA, Hallak JEC, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson’s disease. Journal of Psychopharmacology. 2020 Feb 1;34(2):189–96.
- Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015 Oct 1;172(20):4790–805.
- Wright M, Di Ciano P, Brands B. Use of Cannabidiol for the Treatment of Anxiety: A Short Synthesis of Pre-Clinical and Clinical Evidence. Vol. 5, Cannabis and Cannabinoid Research. Mary Ann Liebert Inc.; 2020. p. 191–6.
- Ortiz Rios FC, Dávila Ruiz IG, Sacal Dumani E. Cannabidiol as a personalized treatment for anxiety: clinical cases in Mexico. Drugs Context. 2022;11.
- Crippa JAS, Nogueira Derenusson G, Borduqui Ferrari T, Wichert-Ana L, Duran FLS, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. Journal of Psychopharmacology. 2011 Jan;25(1):121–30.
- Alonso-Ferrero ME, Paniagua MA, Mostany R, Pilar-Cuéllar F, Díez-Alarcia R, Pazos A, et al. Cannabinoid system in the budgerigar brain. Brain Res. 2006 May 4;1087(1):105–13.
- Divín D, Goméz Samblas M, Kuttiyarthu Veetil N, Voukali E, Ś Widerská Z, Krajzingrová T, et al. Cannabinoid receptor 2 evolutionary gene loss makes parrots more susceptible to neuroinflammation. Proceedings of the Royal Society B: Biological Sciences. 2022 Dec 7;289(1988).
- Cital S, Kramer K, Hughston L, Gaynor JS. Cannabis Therapy in Veterinary Medicine A Complete Guide [Internet]. 1st ed. Cital Stephen, Kramer Katherine, Hughston Liz, Gaynor James S., editors. Cham, Switzerland: Springer Nature Switzerland; 2021 [cited 2023 Sep 6]. 1–350 p. Available from: https://link.springer.com/book/10.1007/978-3-030-68317-7.
- Silver, RJ. The endocannabinoid system of animals. Vol. 9, Animals. MDPI; 2019.
- Howlett AC, Abood ME. CB1 and CB2 Receptor Pharmacology. In: Advances in Pharmacology. Academic Press Inc.; 2017. p. 169–206.
- Sánchez-Aparicio P, Florán B, Rodríguez Velázquez D, Ibancovichi JA, Varela Guerrero JA, Recillas S. Cannabinoids CB2 Receptors, One New Promising Drug Target for Chronic and Degenerative Pain Conditions in Equine Veterinary Patients. Vol. 85, Journal of Equine Veterinary Science. W.B. Saunders; 2020.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).