Submitted:
30 October 2025
Posted:
31 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Gut–Heart Axis
2.1. Microbiomes
2.2. Metabolites
3. Gut-Brain Axis
3.1. Microbiomes
3.2. Metabolites
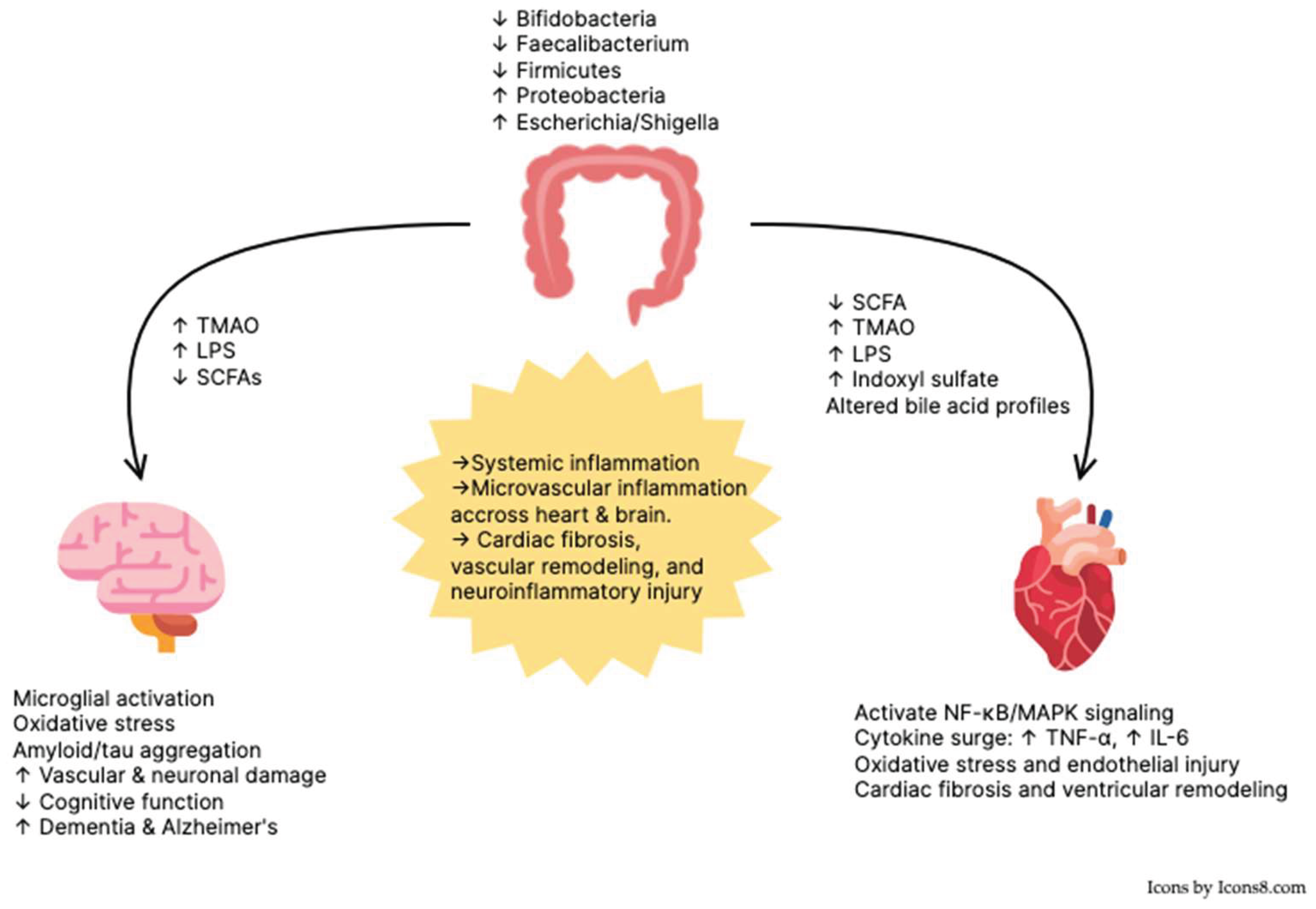
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| AD | Alzheimer’s Disease |
| BNP | B-type Natriuretic Peptide |
| CRP | C-Reactive Protein |
| CSF | Cerebrospinal Fluid |
| HF | Heart Failure |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| HR | Hazard Ratio |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MCI | Mild Cognitive Impairment |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| NF-κB | Nuclear Factor Kappa B |
| NLRP3 | NLR Family Pyrin Domain Containing 3 (Inflammasome) |
| PGC-1α | Peroxisome Proliferator–Activated Receptor Gamma Coactivator 1-Alpha |
| SCFA | Short-Chain Fatty Acid |
| TLR4 | Toll-Like Receptor 4 |
| TMAO | Trimethylamine-N-oxide |
| TNF-α | Tumor Necrosis Factor-Alpha |
References
- Ran, J.; et al. Global, regional, and national burden of heart failure and its underlying causes, 1990–2021: results from the global burden of disease study 2021. Biomarker Research 2025, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; et al. HF STATS 2025: Heart Failure Epidemiology and Outcomes Statistics An Updated 2025 Report from the Heart Failure Society of America. Journal of Cardiac Failure.
- Martin, S.S.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2025, 151, e41–e660. [Google Scholar]
- Goh, F.Q.; et al. Cognitive Impairment in Heart Failure-A Review. Biology (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Goyal, P.; et al. Cognitive Impairment in Heart Failure: A Heart Failure Society of America Scientific Statement. J Card Fail 2024, 30, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Huynh, Q.; Potter, E.L. Cognitive Dysfunction in Heart Failure: Pathophysiology and Implications for Patient Management. Curr Heart Fail Rep 2022, 19, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Liori, S.; et al. Cognitive impairment in heart failure: clinical implications, tools of assessment, and therapeutic considerations. Heart Fail Rev 2022, 27, 993–999. [Google Scholar] [CrossRef]
- Cannon, J.A.; et al. Cognitive impairment and heart failure: systematic review and meta-analysis. Journal of cardiac failure 2017, 23, 464–475. [Google Scholar] [CrossRef]
- Testai, F.D.; et al. Cardiac Contributions to Brain Health: A Scientific Statement From the American Heart Association. Stroke 2024, 55, e425–e438. [Google Scholar] [CrossRef]
- Adelborg, K.; et al. Heart failure and risk of dementia: a Danish nationwide population-based cohort study. Eur J Heart Fail 2017, 19, 253–260. [Google Scholar] [CrossRef]
- Jefferson, A.L.; et al. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation 2015, 131, 1333–1339. [Google Scholar] [CrossRef]
- Miao, F.; et al. Cognitive impairment in young and middle-aged patients with acute heart failure. ESC Heart Fail 2024, 11, 2977–2985. [Google Scholar] [CrossRef]
- Vishwanath, S.; et al. Cognitive Decline and Risk of Dementia in Individuals With Heart Failure: A Systematic Review and Meta-analysis. J Card Fail 2022, 28, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Epelde, F. The Role of the Gut Microbiota in Heart Failure: Pathophysiological Insights and Future Perspectives. Medicina (Kaunas) 2025, 61. [Google Scholar] [CrossRef]
- Tooley, K.L. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; et al. Gut-Heart Axis: The Role of Gut Microbiota and Metabolites in Heart Failure. Circulation Research 2025, 136, 1382–1406. [Google Scholar] [CrossRef] [PubMed]
- Kondapalli, N.; et al. Microbiota in Gut-Heart Axis: Metabolites and Mechanisms in Cardiovascular Disease. Compr Physiol 2025, 15, e70024. [Google Scholar] [CrossRef]
- Shen, S.; et al. Heart Failure and Gut Microbiota: What Is Cause and Effect? Research 2025, 8, 0610. [Google Scholar] [CrossRef]
- Matacchione, G.; et al. The role of the gut microbiota in the onset and progression of heart failure: insights into epigenetic mechanisms and aging. Clinical Epigenetics 2024, 16, 175. [Google Scholar] [CrossRef]
- Loh, J.S.; et al. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduction and Targeted Therapy 2024, 9, 37. [Google Scholar] [CrossRef]
- Jimenez-García, A.M.; Villarino, M.; Arias, N. A systematic review and meta-analysis of basal microbiota and cognitive function in Alzheimer’s disease: A potential target for treatment or a contributor to disease progression? Alzheimers Dement (Amst) 2024, 16, e70057. [Google Scholar] [CrossRef]
- Manfredi, J.N.; et al. Gut microbiota dysbiosis in Alzheimer’s disease (AD): Insights from human clinical studies and the mouse AD models. Physiol Behav 2025, 290, 114778. [Google Scholar] [CrossRef]
- Gallo, A.; et al. The Gut in Heart Failure: Current Knowledge and Novel Frontiers. Med Princ Pract 2022, 31, 203–214. [Google Scholar] [CrossRef]
- Polsinelli, V.B.; Sinha, A.; Shah, S.J. Visceral Congestion in Heart Failure: Right Ventricular Dysfunction, Splanchnic Hemodynamics, and the Intestinal Microenvironment. Curr Heart Fail Rep 2017, 14, 519–528. [Google Scholar] [CrossRef]
- Santilli, A.; Stefanopoulos, S.; Cresci, G.A.M. The gut barrier and chronic diseases. Curr Opin Clin Nutr Metab Care 2022, 25, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front Immunol 2020, 11, 594150. [Google Scholar] [CrossRef] [PubMed]
- Boulet, J.; et al. Inflammation in heart failure: pathophysiology and therapeutic strategies. Inflammation Research 2024, 73, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; et al. The role of gut microbiota in myocardial ischemia-reperfusion injury. LID - 1625299. (2297-055X (Electronic)).
- Colella, M.; et al. Microbiota revolution: How gut microbes regulate our lives. World J Gastroenterol 2023, 29, 4368–4383. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; et al. The Human Microbiome and Its Impacts on Health. Int J Microbiol 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- Rahman, M.M.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front Cell Infect Microbiol 2022, 12, 903570. [Google Scholar] [CrossRef]
- Tang, W.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nature Reviews Cardiology 2019, 16, 137–154. [Google Scholar] [CrossRef]
- Wang, L.; et al. The role of the gut microbiota in health and cardiovascular diseases. Mol Biomed 2022, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Simadibrata, D.M.; et al. The Gut Microbiota Profile in Heart Failure Patients: A Systematic Review. J Gastrointestin Liver Dis 2023, 32, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; et al. The Gut Microbiome of Heart Failure With Preserved Ejection Fraction. Journal of the American Heart Association 2021, 10, e020654. [Google Scholar] [CrossRef] [PubMed]
- Katsimichas, T.; et al. Non-Ischemic Heart Failure With Reduced Ejection Fraction Is Associated With Altered Intestinal Microbiota. Circulation Journal 2018, 82, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; et al. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC: Heart Failure 2016, 4, 220–227. [Google Scholar] [CrossRef]
- Wang, Z.; et al. The Correlation between Gut Microbiota and Serum Metabolomic in Elderly Patients with Chronic Heart Failure. Mediators Inflamm 2021, 2021, 5587428. [Google Scholar] [CrossRef]
- Kummen, M.; et al. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. Journal of the American College of Cardiology 2018, 71, 1184–1186. [Google Scholar] [CrossRef]
- Desai, D.; et al. Re-defining the Gut Heart Axis: A Systematic Review of the Literature on the Role of Gut Microbial Dysbiosis in Patients With Heart Failure. Cureus 2023, 15, e34902. [Google Scholar] [CrossRef]
- Dai, H.; et al. Causal relationships between the gut microbiome, blood lipids, and heart failure: a Mendelian randomization analysis. European Journal of Preventive Cardiology 2023, 30, 1274–1282. [Google Scholar] [CrossRef]
- Lu, X.; et al. Microbial metabolites and heart failure: Friends or enemies? Front Microbiol 2022, 13, 956516. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. Excli j 2021, 20, 301–319. [Google Scholar]
- Facchin, S.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life (Basel) 2024, 14. [Google Scholar] [CrossRef]
- Tang, W.W.; et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. Journal of the American College of Cardiology 2014, 64, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; et al. Trimethylamine N-oxide and prognosis in acute heart failure. Heart 2016, 102, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; et al. Trimethylamine <i>N</i>-Oxide and Related Gut Microbe-Derived Metabolites and Incident Heart Failure Development in Community-Based Populations. Circulation: Heart Failure 2024, 17, e011569. [Google Scholar] [PubMed]
- Li, X.; et al. Trimethylamine N-Oxide in Heart Failure: A Meta-Analysis of Prognostic Value. Front Cardiovasc Med 2022, 9, 817396. [Google Scholar] [CrossRef]
- Marques, F.Z.; et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Zuo, K.; et al. Commensal microbe-derived SCFA alleviates atrial fibrillation via GPR43/NLRP3 signaling. Int J Biol Sci 2022, 18, 4219–4232. [Google Scholar] [CrossRef]
- Yukino-Iwashita, M.; et al. Short-Chain Fatty Acids in Gut-Heart Axis: Their Role in the Pathology of Heart Failure. J Pers Med 2022, 12. [Google Scholar] [CrossRef]
- Shulpekova, Y.; et al. The Role of Bile Acids in the Human Body and in the Development of Diseases. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Molinero, N.; et al. Intestinal Bacteria Interplay With Bile and Cholesterol Metabolism: Implications on Host Physiology. Front Physiol 2019, 10, 185. [Google Scholar] [CrossRef]
- Mayerhofer, C.C.K.; et al. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. Journal of Cardiac Failure 2017, 23, 666–671. [Google Scholar] [CrossRef]
- Desai, M.S.; et al. Bile acid excess induces cardiomyopathy and metabolic dysfunctions in the heart. Hepatology 2017, 65, 189–201. [Google Scholar] [CrossRef]
- Mohr, A.E.; et al. Lipopolysaccharide and the gut microbiota: considering structural variation. FEBS Lett 2022, 596, 849–875. [Google Scholar] [CrossRef]
- Fountoulakis, P.N.; et al. Gut Microbiota in Heart Failure-The Role of Inflammation. Biomedicines 2025, 13. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; et al. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: Effect of adherence to mediterranean diet. Journal of the American Heart Association 2017, 6, e005784. [Google Scholar] [CrossRef]
- Yoshida, N.; et al. Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar]
- Yuzefpolskaya, M.; et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. The Journal of Heart and Lung Transplantation 2020, 39, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; et al. Gut-microbiota-derived indole sulfate promotes heart failure in chronic kidney disease. Cell Host & Microbe 2025, 33, 1715–1730.e5. [Google Scholar]
- Gao, K.; et al. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv Nutr 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Lekawanvijit, S.; et al. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? European Heart Journal 2010, 31, 1771–1779. [Google Scholar] [CrossRef]
- Cao, X.-S.; et al. Association of Indoxyl Sulfate with Heart Failure among Patients on Hemodialysis. Clinical Journal of the American Society of Nephrology 2015, 10, 111–119. [Google Scholar] [CrossRef]
- Imazu, M.; et al. Plasma indoxyl sulfate levels predict cardiovascular events in patients with mild chronic heart failure. Scientific Reports 2020, 10, 16528. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr Med (Encinitas) 2018, 17, 28–32. [Google Scholar]
- Zou, B.; et al. Gut Microbiota is an Impact Factor based on the Brain-Gut Axis to Alzheimer’s Disease: A Systematic Review. Aging Dis 2023, 14, 964–1678. [Google Scholar] [CrossRef]
- González Cordero, E.M.; et al. Relationship Between the Gut Microbiota and Alzheimer’s Disease: A Systematic Review. J Alzheimers Dis 2022, 87, 519–528. [Google Scholar] [CrossRef]
- Sheng, C.; et al. Altered Gut Microbiota in Adults with Subjective Cognitive Decline: The SILCODE Study. J Alzheimers Dis 2021, 82, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Angoorani, P.; et al. Is There Any Link between Cognitive Impairment and Gut Microbiota? A Systematic Review. Gerontology 2022, 68, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Kossowska, M.; et al. The Interplay between Gut Microbiota and Cognitive Functioning in the Healthy Aging Population: A Systematic Review. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Gyriki, D.; et al. The gut microbiota and aging: interactions, implications, and interventions. Front Aging 2025, 6, 1452917. [Google Scholar] [CrossRef]
- Mukherjee, U.; Reddy, P.H. Gut-brain relationship in dementia and Alzheimer’s disease: Impact on stress and immunity. Ageing Research Reviews 2025, 111, 102843. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Y.N.; et al. Microbiome Gut-Brain-Axis: Impact on Brain Development and Mental Health. Mol Neurobiol 2025, 62, 10813–10833. [Google Scholar] [CrossRef]
- Lei, W.; et al. Gut microbiota-driven neuroinflammation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Front Immunol 2025, 16, 1582119. [Google Scholar] [CrossRef]
- Liu, P.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Vogt, N.M.; et al. Gut microbiome alterations in Alzheimer’s disease. Scientific Reports 2017, 7, 13537. [Google Scholar] [CrossRef]
- Zhuang, Z.-Q.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. Journal of Alzheimer’s Disease 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Saji, N.; et al. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: a cross-sectional study conducted in Japan. Scientific Reports 2019, 9, 19227. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiology of Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Li, B.; et al. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement 2019, 15, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Jemimah, S.; et al. Gut microbiome dysbiosis in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. PLoS One 2023, 18, e0285346. [Google Scholar] [CrossRef]
- Fan, K.C.; et al. Compositional and functional gut microbiota alterations in mild cognitive impairment: links to Alzheimer’s disease pathology. Alzheimers Res Ther 2025, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- L, K.; et al. Gut-brain axis through the lens of gut microbiota and their relationships with Alzheimer’s disease pathology: Review and recommendations. Mechanisms of Ageing and Development 2023, 211, 111787. [Google Scholar] [CrossRef]
- Schaible, P.; Henschel, J.; Erny, D. How the gut microbiota impacts neurodegenerative diseases by modulating CNS immune cells. Journal of Neuroinflammation 2025, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; et al. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Lee, B.; et al. Gut Microbiota Metabolite Messengers in Brain Function and Pathology at a View of Cell Type-Based Receptor and Enzyme Reaction. Biomol Ther (Seoul) 2024, 32, 403–423. [Google Scholar] [CrossRef]
- Brunt, V.E.; et al. The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. Geroscience 2021, 43, 377–394. [Google Scholar] [CrossRef]
- Caradonna, E.; et al. Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health. Metabolites 2025, 15. [Google Scholar] [CrossRef]
- Martelli, A.; et al. Trimethylamine N-Oxide (TMAO) Acts as Inhibitor of Endothelial Nitric Oxide Synthase (eNOS) and Hampers NO Production and Acetylcholine-Mediated Vasorelaxation in Rat Aortas. Antioxidants (Basel) 2025, 14. [Google Scholar] [CrossRef]
- Su, M.L.; et al. Trimethylamine-N-oxide damages astrocytes and lymphatic endothelial cells in the cerebral lymphatic system. IBRO Neurosci Rep 2025, 19, 614–623. [Google Scholar] [CrossRef]
- Ren, Z.; Mo, L. Association between levels of trimethylamine N-oxide and cognitive dysfunction: a systematic review and meta-analysis. PeerJ 2025, 13, e20000. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Long, C.; et al. Association of trimethylamine oxide and its precursors with cognitive impairment: a systematic review and meta-analysis. Frontiers in Aging Neuroscience 2024, 16, 1465457. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; et al. The Role of Gut Microbiota-Derived Trimethylamine N-Oxide in the Pathogenesis and Treatment of Mild Cognitive Impairment. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Dalile, B.; et al. The role of short-chain fatty acids in microbiota–gut–brain communication. Nature Reviews Gastroenterology & Hepatology 2019, 16, 461–478. [Google Scholar]
- van de Wouw, M.; et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Gao, C.; et al. Early changes of fecal short-chain fatty acid levels in patients with mild cognitive impairments. CNS Neurosci Ther 2023, 29, 3657–3666. [Google Scholar] [CrossRef]
- Marizzoni, M.; et al. Circulating short chain fatty acids in Alzheimer’s disease: A cross-sectional observational study. J Alzheimers Dis 2025, 106, 38–43. [Google Scholar] [CrossRef]
- Ho, L.; et al. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother 2018, 18, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host-Microbe Interplay. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Nho, K.; et al. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimers Dement 2019, 15, 232–244. [Google Scholar] [CrossRef] [PubMed]
- MahmoudianDehkordi, S.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimers Dement 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Varma, V.R.; et al. Bile acid synthesis, modulation, and dementia: A metabolomic, transcriptomic, and pharmacoepidemiologic study. PLoS Med 2021, 18, e1003615. [Google Scholar] [CrossRef]
- Mulak, A. Bile Acids as Key Modulators of the Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J Alzheimers Dis 2021, 84, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.C. LPS and neuroinflammation: a matter of timing. Inflammopharmacology 2016, 24, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. Journal of Neuroinflammation 2019, 16, 180. [Google Scholar] [CrossRef]
- Zhao, J.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Scientific Reports 2019, 9, 5790. [Google Scholar] [CrossRef]
- Haider, D.G.; et al. C-reactive protein is expressed and secreted by peripheral blood mononuclear cells. Clin Exp Immunol 2006, 146, 533–539. [Google Scholar] [CrossRef]
- Walker, K.A.; et al. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology 2019, 92, e1256–e1267. [Google Scholar] [CrossRef]
- Kotulla, S.; et al. Does Human Experimental Endotoxemia Impact Negative Cognitions Related to the Self? Front Behav Neurosci 2018, 12, 183. [Google Scholar] [CrossRef]
- Kaplin, A.; et al. IL-6 release by LPS-stimulated peripheral blood mononuclear cells as a potential biomarker in Alzheimer’s disease. Int Psychogeriatr 2009, 21, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; et al. Economic Issues in Heart Failure in the United States. J Card Fail 2022, 28, 453–466. [Google Scholar] [CrossRef]
- Jutkowitz, E.; et al. Risk factors associated with cognitive, functional, and behavioral trajectories of newly diagnosed dementia patients. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 2017, 72, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; et al. Cognitive Impairment in Heart Failure: Landscape, Challenges, and Future Directions. Front Cardiovasc Med 2021, 8, 831734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; et al. Gut microbiota dysbiosis exacerbates heart failure by the LPS-TLR4/NF-κB signalling axis: mechanistic insights and therapeutic potential of TLR4 inhibition. J Transl Med 2025, 23, 762. [Google Scholar] [CrossRef]
- Mekhora, C.; Lamport, D.J.; Spencer, J.P.E. An overview of the relationship between inflammation and cognitive function in humans, molecular pathways and the impact of nutraceuticals. Neurochemistry International 2024, 181, 105900. [Google Scholar] [CrossRef]
- Shoukry, A.E.A.; Rahhal, A.; Constantinou, C. The role of the gut microbiota and metabolites in heart failure and possible implications for treatment. Heart Fail Rev 2025. [Google Scholar] [CrossRef]
- Ji, X.; et al. Gut microbial metabolites and the brain-gut axis in Alzheimer’s disease: A review. Biomol Biomed 2025, 26, 240–250. [Google Scholar] [CrossRef]
- Lupu, V.V.; et al. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12. [Google Scholar] [CrossRef]
- Liang, Y.; et al. The link between gut microbiome and Alzheimer’s disease: From the perspective of new revised criteria for diagnosis and staging of Alzheimer’s disease. Alzheimers Dement 2024, 20, 5771–5788. [Google Scholar] [CrossRef]
- Liu, S.; et al. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol Neurobiol 2020, 57, 5026–5043. [Google Scholar] [CrossRef] [PubMed]
- Abeltino, A.; et al. Unraveling the Gut Microbiota: Implications for Precision Nutrition and Personalized Medicine. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Mamic, P.; Snyder, M.; Tang, W.H.W. Gut Microbiome-Based Management of Patients With Heart Failure. JACC 2023, 81, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
| FEATURE | HEART FAILURE | COGNITIVE IMPAIRMENT |
|---|---|---|
|
OVERALL DIVERSITY |
↓α-diversity in HFpEF/HFrEF | ↓α-diversity in AD/MCI |
|
DOMINAN PHYLA |
↓Firmicutes; Proteobacteria, Actinobacteria |
↓Firmicutes, Bifidobacterium, Bacteroidetes & Proteobacteria |
|
PATHOBIONT ENRICHMENT |
↑Enterococcus, Streptococcus, Escherichia/Shigella, Klebsiella |
↑Escherichia/Shigella, Bacteroides |
|
LOSS OF BENEFICIAL TAXA |
↓SCFA producers: Faecalibacterium, Eubacterium, Ruminococcaceae |
↓SCFA producers: Eubacterium, Bifidobacterium |
|
FUNCTIONAL IMPLICATION |
barrier dysfunction, cardiac inflammation |
Blood brain barrier disruption, microglial activation, cognitive decline |
| METABOLITE | HEART FAILURE | COGNITIVE IMPAIRMENT |
|---|---|---|
| TMAO | Elevated; predicts mortality and incident HF; promotes myocardial fibrosis. |
Elevated; crosses/modulates blood brain barrier; linked to neuroinflammation and higher risk of cognitive decline. |
| SCFAS | Depleted; especially butyrate; lower levels associate with inflammation and worse cardiovascular outcomes; fiber/acetate can be protective in models. | Reduced; imbalances correlate with amyloid deposition and neurodegeneration biomarkers. |
| BILE ACIDS | Altered profiles; mechanistic links to cardiac metabolic stress. | Altered profiles; higher gut-derived bile acids ratios associate with lower CSF Amyloid-beta, cortical atrophy, and worse cognition. |
| LPS | Translocation from leaky gut; associates with systemic inflammation and vascular dysfunction. | Potent driver of neuroinflammation; promotes amyloid/tau pathology and cognitive deficits in models. |
| INDOXYL SULFATE | Elevated; pro-fibrotic/hypertrophic; predicts HF events and rehospitalization, especially with renal dysfunction. | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





