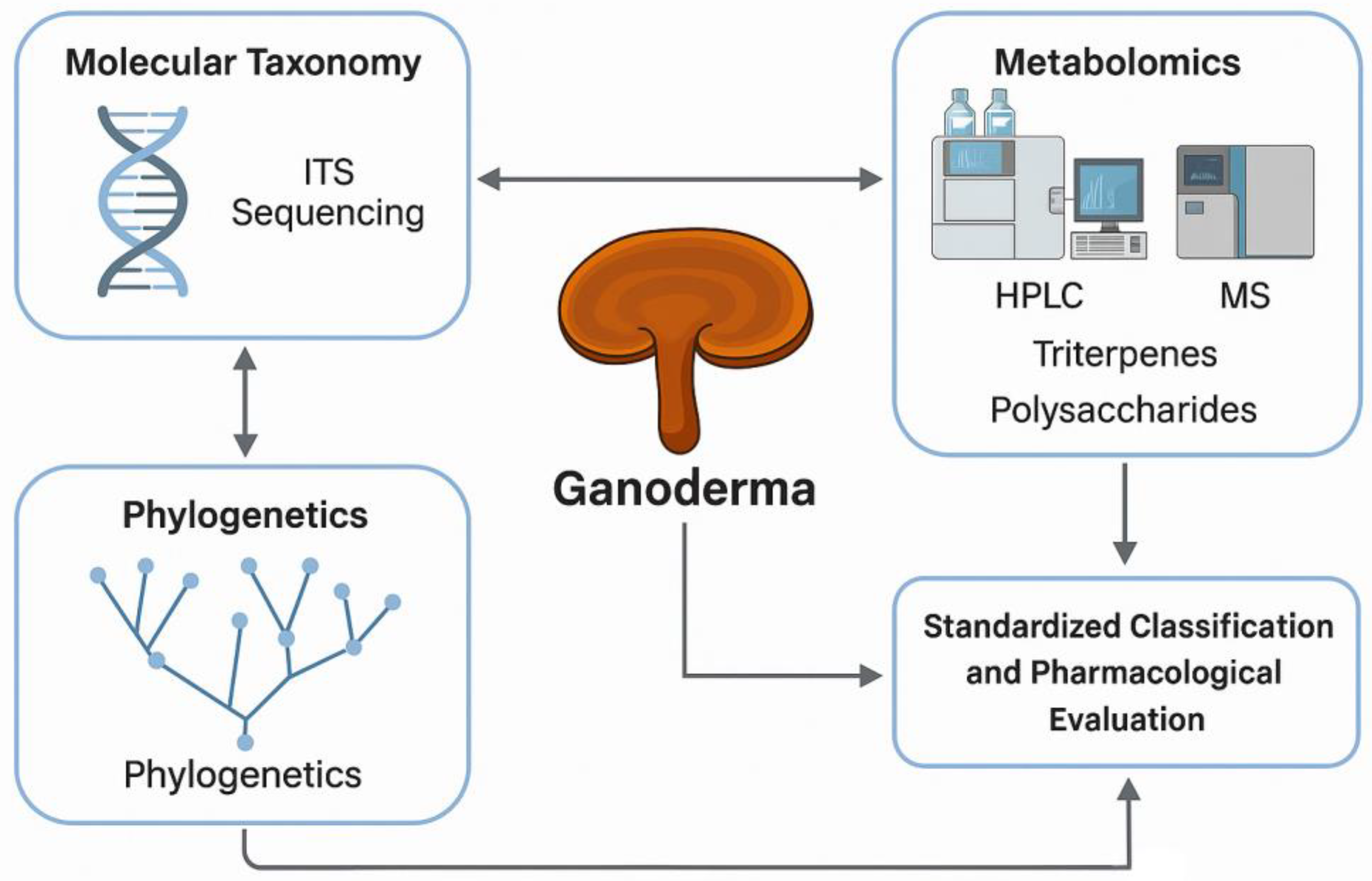
1. Introduction
Ganoderma is a genus of medicinal fungi historically used in traditional Eastern medicine, and is particularly common in China and Japan, where it is esteemed for its immunomodulatory, hepatoprotective, anticancer, and antiinflammatory properties [
1,
2]. Among its bioactive constituents, triterpenes, polysaccharides, glucans, and glycoproteins are consistently associated with these pharmacological effects [
2,
3]. Increasing global demand for complementary and alternative therapies has driven the incorporation of
Ganoderma-derived compounds into nutraceuticals, functional foods, and pharmaceuticals [
4].
Research on
Ganoderma has significantly increased, over the last few decades, well beyond its therapeutic potential to encompass its genetic, taxonomic, and metabolic diversity. Accurate species identification and characterization have become critical to ensuring the consistency, efficacy, and safety of
Ganoderma-based products. In the past, scientists classified species within the
Ganoderma genus primarily by looking at physical traits, like the size of the basidiospores, or the shape and structure of the cap (pileus), as well as the texture of the spore walls. However, these features can vary significantly depending on factors such as the specimen’s age, the type of substrate it grows on, and the surrounding environmental conditions. Because of this variability, relying solely on morphology has proven problematic when trying to accurately distinguish between species [
5,
6]. Consequently, ambiguity and misclassification have hindered
Ganoderma taxonomy.
The emergence of molecular phylogenetics has significantly transformed fungal taxonomy, allowing for more accurate species identification through the use of genetic barcoding. The internal transcribed spacer (ITS) region of ribosomal RNA has become a widely recognized barcode for fungi identification because of its high variability between species and its ease of amplification [
7]. In addition to ITS, other genetic markers, such as the nuclear large subunit (nLSU), small subunit (nSSU), translation elongation factor 1-alpha (EF1-α), and RNA polymerase II subunit (RPB2), have further improved phylogenetic resolution and identification due to their high interspecific variability and ease of amplification [
8]. Additional genetic markers, including the nLSU, nSSU, EF1-α, and RPB2, have further enhanced phylogenetic resolution [
9].
In parallel, metabolomics has become essential for elucidating the biochemical composition of
Ganoderma. Analytical platforms such as high-performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) spectroscopy are commonly employed to identify and quantify key secondary metabolites, including ganoderic acids and β-glucans [
10]. These compounds play a central role in the pharmacological activity of
Ganoderma, contributing to its immunostimulatory, antioxidant, and anticancer effects [
11,
12]. Comprehensive metabolite profiling is therefore crucial for product standardization, quality assurance, and validation of therapeutic efficacy (
Figure 1).
While recent progress has expanded our understanding of
Ganoderma, notable gaps still exist, especially in underexplored areas like sub-Saharan Africa. While several species from this region have been described, studies into the molecular or metabolomic profiles are limited [
12]. Moreover, this geographic underrepresentation has hindered global efforts to catalog biodiversity and develop regionally tailored therapeutics.
This review provides an integrative synthesis of the state-of-the-art on Ganoderma systematics, phylogenetics, and metabolomics with an emphasis on the application of molecular identification techniques. Specifically, we explore the phylogenetic relationships within the genus and highlight advances in metabolite profiling using modern analytical tools. Methodological limitations, regional research gaps, and future priorities are also discussed, with particular focus on Africa, where emerging studies offer promising new directions.
2. Methodology
We used a systematic approach to identify, select and integrate peer-reviewed literature as it pertains to systematics, phylogenetics and metabolomics of Ganoderma species. In this review, we endeavored to ensure that a comprehensive coverage of both foundational studies and recent advancements across these thematic domains was achieved.
2.1. Data Sources and Search Strategy
Literature searches were conducted using five major academic databases: PubMed, Web of Science, Science Direct, Springer Link, and Google Scholar. These platforms were selected based on their broad coverage of molecular biology, pharmacognosy, mycology, and analytical sciences. PubMed was primarily utilized to retrieve studies on pharmacological and biomedical aspects, while Web of Science and ScienceDirect were targeted for literature on fungal taxonomy, molecular identification, and phylogenetic techniques. SpringerLink and Google Scholar were used to supplement the primary search with additional articles, reviews, and relevant book chapters.
The search was restricted to publications from 1990 to 2025, and only literature published in English was considered. Key search terms included:
"Ganoderma taxonomy"
"Ganoderma phylogenetics"
"Molecular identification of Ganoderma"
"ITS region in Ganoderma"
"Ganoderma metabolomics"
"Triterpene analysis in Ganoderma"
"Bioactive compounds in Ganoderma"
"Molecular barcoding of Ganoderma species"
Boolean operators ("AND," "OR") and truncation symbols were employed to refine and expand search results. Reference lists of relevant articles were also reviewed to identify additional sources not captured during the initial database search.
2.2. Inclusion and Exclusion Criteria
Studies were included if they met one or more of the following criteria:
Utilized molecular techniques for species identification (e.g., ITS, nLSU, EF1-α, RPB2)
Conducted phylogenetic analyses based on DNA sequencing
Performed metabolomic profiling using advanced analytical platforms (e.g., LC-MS,
HPLC, NMR)
Investigated the pharmacological relevance of Ganoderma bioactive compounds
Contributed data from underexplored regions, particularly sub-Saharan Africa
Studies were excluded if they:
Relied exclusively on morphological taxonomy without molecular validation
Focused solely on cultivation methods without molecular or biochemical analysis
Were not peer-reviewed (e.g., theses, conference abstracts, editorials, or opinion pieces)
2.3. Data Extraction and Synthesis
Data from the included studies were extracted and organized into three thematic categories:
Each study was evaluated for its methodological approach, molecular markers utilized, analytical techniques employed, taxonomic resolution achieved, and geographical coverage. A thematic analysis was conducted to identify prevailing trends, technical challenges, and gaps in the literature—particularly those concerning African Ganoderma species. Methodological strengths and limitations were critically assessed, and findings were synthesized to construct an integrated narrative that informs future research priorities in Ganoderma systematics and pharmacological evaluation.
3. Molecular Taxonomy and Systematics
Systematics of Ganoderma with Emphasis on Molecular Identification Methods
The genus
Ganoderma, recognized for both its medicinal significance and ecological functions, has long presented taxonomic challenges. Early classification systems relied primarily on morphological traits, including basidiospore shape and size, pileus coloration, and spore ornamentation. However, these features exhibit considerable phenotypic plasticity and are influenced by environmental conditions, developmental stage, and substrate specificity, resulting in frequent misidentifications [
6]. Even basidiospore morphology—once considered diagnostic—has shown limited interspecific variation and reduced discriminatory power [
13]. As a result, the taxonomy of
Ganoderma has been in a state of considerable ambiguity.
The advent of molecular systematics has substantially improved species delineation by providing objective, high-resolution tools that are less susceptible to environmental variability. Among these, the ITS region of the nuclear ribosomal RNA operon has become the standard barcode for fungal identification, including within
Ganoderma [
8,
14]. ITS sequencing has facilitated the resolution of cryptic species, clarified evolutionary relationships, and enhanced the accuracy of species-level classification.
Multiple studies have supported the use of the ITS region in conjunction with other loci, such as the nLSU and nSSU rRNA genes, to improve phylogenetic resolution [
15,
16]. The high interspecific variability of the ITS region has made it especially valuable for distinguishing morphologically similar taxa [
17]. However, ITS-based identification has limitations; in some species complexes, ITS sequences alone do not provide sufficient resolution, necessitating supplementary genetic markers [
18].
The ITS2 subregion has gained attention as a potentially superior barcode due to its shorter sequence length, ease of amplification, and high discriminatory power [
19]. Despite these advantages, its widespread adoption has been limited by inconsistent amplification success and the lack of universally effective primers [
20]. While the full ITS region and ITS1 subregion may offer broader coverage, ITS2 remains a useful tool for fine-scale resolution among closely related species when the full-length ITS is not available. [
21].
The need for molecular tools is especially pressing in biodiverse but undercharacterized regions such as sub-Saharan Africa. In many parts of the continent,
Ganoderma species have been identified almost exclusively through macroscopic traits, with minimal molecular confirmation [
22]. For example, taxonomic surveys in Ghana have largely relied on morphology alone, raising concerns about the reliability of these identifications [
12]. Incorporating molecular barcoding and phylogenetic analysis, particularly polymerase chain reaction (PCR)-based techniques, markedly improve the accuracy of species identification and biodiversity assessments in these regions.
Beyond taxonomy, molecular identification methods have practical applications in agriculture and forestry. Several
Ganoderma species are pathogenic to economically important crops such as cocoa, cashew, and coffee. Molecular diagnostics provide rapid, cost-effective, and accurate tools for early detection and species-level identification, which are essential for the timely management of plant disease outbreaks [
13].
In summary, while traditional morphological approaches have laid the foundation for understanding Ganoderma diversity, they are increasingly being supplanted by molecular methods that offer greater precision, reproducibility, and scope. The combined use of ITS, ITS2, and additional loci constitutes a robust framework for resolving taxonomic ambiguities and uncovering cryptic species. As molecular technologies become more widely accessible, they are expected to play a pivotal role in advancing both fundamental mycological research and the applied sciences related to Ganoderma, particularly in regions where research remains limited but potential biodiversity is high.
4. Phylogenetic Advances in Ganoderma Research
4.1. Ganoderma Phylogenetics: Molecular Insights and Advancements
Over the past several decades, phylogenetic studies of Ganoderma have undergone a significant transformation, driven by advances in molecular biology. Traditional taxonomy, based on macroscopic and microscopic features, has proven inadequate for resolving closely related species within the genus. As a result, researchers have increasingly adopted DNA sequencing and molecular phylogenetic tools to clarify species boundaries and evolutionary relationships.
4.2. The Role of ITS and rRNA Genes in Ganoderma Phylogenetics
One of the earliest pivotal studies in
Ganoderma phylogeny was conducted by Moncalvo et al. [
22], who analyzed sequences from the ITS and the 25S rRNA gene. Their work demonstrated that the ITS region could effectively differentiate most species, though it was insufficient to resolve the
G. tsugae complex. The D2 domain of the 25S rRNA gene proved useful at higher taxonomic levels, and its combination with ITS sequences supported the monophyly of the subgenus
Elfvingia. This study laid the groundwork for multilocus approaches in
Ganoderma systematics.
Subsequent research has confirmed that ITS sequencing remains a foundational tool for species delimitation, especially when combined with multiple loci. Zhang and colleagues conducted a multilocus phylogenetic analysis on 32 collections of the
G. lucidum complex using ITS, tef1α, rpb1, and rpb2. The study identified three distinct genetic clades (A, B, and C) within the complex, none of which corresponded strictly to geographic origin [
23]. These findings underscore the extensive taxonomic complexity in
Ganoderma, revealing that morphological classification alone often fails to accurately distinguish genetically divergent taxa due to overlapping features. A complementary ITS-focused study further demonstrated that ITS1 provided better geographic clustering than ITS2 among
G. lucidum strains from global origins [
24]. However, ITS2 alone was insufficient for fine-scale resolution, highlighting ITS limitations in detecting closely related but distinct lineages.
4.3. Multilocus Phylogenetics and Species Delimitation
Although ITS remains a core barcode for fungal systematics, it does not always provide sufficient resolution for species delimitation in complex groups. Multilocus sequencing, typically combining ITS with loci such as translation elongation factor 1-alpha (EF1-α) and RNA polymerase II subunit (RPB2), has emerged as a more robust approach.
Xing et al. employed multilocus sequencing on specimens collected in South Africa and identified a distinct lineage within the
G. lucidum complex, ultimately describing a new species,
G. aridicola [
25]. Similarly, He et al. used multilocus phylogenetics in Yunnan Province to describe two novel species,
G. dianzhongense and
G. esculentum, based on their genetic divergence from known taxa [
26]. These examples demonstrate the power of multilocus strategies for uncovering cryptic diversity and refining species concepts.
4.4. Phylogenetic Tools for Product Authentication
Molecular phylogenetics has also become essential for verifying the identity of commercial
Ganoderma products. Gunnels et al. used ITS-based phylogenetic analysis to confirm the presence of
G. lingzhi in dietary supplements, supporting the use of this marker for quality assurance in the nutraceutical sector [
27]. Liao et al. further employed DNA barcoding to assess strain-level variation in cultivated
G. lucidum from China and Europe [
28]. Their findings revealed significant genetic divergence, suggesting that some commercial
G. lucidum may consist of multiple cryptic species, a concern with implications for product efficacy and standardization.
4.5. Regional phylogenetic studies: expanding global taxonomy
Regional molecular studies continue to expand the known diversity of
Ganoderma, particularly in previously understudied areas. Kinge and others employed ITS and mtSSU sequencing to characterize Cameroonian isolates, identifying eight phylogenetic species, three of which were assigned to named taxa (
G. ryvardense,
G. cupreum and
G. weberianum) while the remaining five lineages did not match any described species [
29]. In Egypt, El-Fallal et al. identified
G. resinaceum and a distinct lineage referred to as
Ganoderma sp. EGDA from fig and citrus trees using ITS and 5.8S rRNA data [
30]. These studies emphasize the importance of applying molecular tools globally to revise classifications and uncover undocumented fungal diversity.
4.6. Recent Advances and the Reclassification of G. lingzhi
A recent revision of
Ganoderma systematics by Du et al. resolved a long-standing nomenclatural ambiguity by demonstrating that
G. lingzhi is a later synonym of
G. sichuanense [
31]. Their study, based on phylogenetic comparisons of type specimens, exemplifies the need for molecular verification in taxonomic revision. Sun et al. further employed a six-locus dataset, including ITS, nLSU, EF1-α, and RPB2, to clarify relationships within the
Ganodermataceae [
16]. Their work showed that multilocus data outperform ITS alone in resolving polytomies and identifying novel species, advocating for broader use of these techniques in future studies.
In summary, molecular phylogenetics has become central to Ganoderma research, informing taxonomy, product authentication, and biodiversity discovery. While ITS remains a foundational marker, multilocus sequencing offers superior resolution, particularly in species complexes and cryptic groups. Continued adoption of these tools is expected to drive a comprehensive reevaluation of Ganoderma taxonomy worldwide.
5. Metabolomic Profiling and Bioactive Compounds
5.1. Ganoderma Metabolomics: Analytical Approaches for Characterization and Quality Control
The growing global demand for Ganoderma-based products has spurred interest in profiling its bioactive constituents. Metabolomics, defined as the comprehensive analysis of metabolites within a biological system, has become a critical tool for elucidating the therapeutic potential and ensuring quality control of Ganoderma species. Advanced analytical platforms such as liquid chromatography–mass spectrometry (LC-MS), high-performance liquid chromatography (HPLC), and nuclear magnetic resonance (NMR) spectroscopy allow for the precise identification and quantification of secondary metabolites, particularly triterpenes and polysaccharides.
5.2. LC-MS-Based Triterpene Profiling
Triterpenes, especially ganoderic acids, are among the most pharmacologically active compounds in
Ganoderma. In 2012 Qi et al. described an LC-MS method for the simultaneous detection of 14 ganoderic acids that utilized previously described analytes as standards [
32]. This targeted profiling approach is valuable for quality control in commercial production, enabling verification of triterpene composition in raw materials, extracts, and finished products. However, its focus on known compounds highlights the need for untargeted metabolomics capable of detecting novel triterpenoids. Adotey et al. applied LC-MS and multivariate statistical analysis to distinguish
Ganoderma species from Ghana’s Lower Volta River region [
33]. Partial least squares–discriminant analysis (PLS-DA) of total ion chromatograms (TICs) separated the samples into three species:
G. enigmaticum,
G. resinaceum, and
G. weberianum–sichuanense. Heatmap visualization further revealed interspecific differences in metabolite composition, suggesting potential variation in bioactivity. This study illustrates the dual utility of metabolomics for both taxonomic resolution and therapeutic evaluation.
The metabolite profile of
Ganoderma changes significantly with developmental stage. A recent metabolomics–proteomics study of
G. lucidum demonstrated that triterpenoid content, including ganoderenic acids E, H, and I, is highest at the budding stage, followed by a decline through maturation, implying that harvest timing critically influences therapeutic compound yield [
34]. Earlier work by Chen & Chen (2003) further characterized multiple ganoderic acids (A, C, D, E, G) and ganoderenic acid D in
G. tsugae fruiting bodies using HPLC and NMR, reinforcing the link between developmental stage and triterpenoid accumulation [
35].
Zhang et al. employed ultra-high performance liquid chromatography coupled with Orbitrap high-resolution mass spectrometry (UPLC–Orbitrap–HRMS) to comprehensively profile metabolites in
G. lucidum [
36]. Their analysis identified 95 compounds, including ganoderic acids (e.g., A, B, C2, D, H, Y), ganoderenic acids (e.g., A, D, G), and additional bioactives such as kaempferol, genistein, and ergosterol. These compounds have been associated with a range of pharmacological activities, including antioxidant, antiinflammatory, and anticancer properties. This integrative approach supports structure–activity relationship studies and the development of standardized therapeutic formulations.
Expanding the geographic scope of metabolomics, Wongkhieo et al. analyzed mycelial extracts of wild
G. australe collected in Thailand using LC-MS/MS [
37]. Their chemical profiling revealed the presence of lovastatin plus tentative identification of p-coumaric acid, nicotinamide, γ-aminobutyric acid (GABA), choline, nucleosides, amino acids, and saccharides. The detection of lovastatin, a known cholesterol-lowering compound, highlights the pharmaceutical and functional food potential of this underexplored
Ganoderma species.
In a follow-up study, Adotey et al. examined
Ganoderma mycelial cultures from Ghana and identified four lanostanoid triterpenes, ganoderenic acid A, ganoderenic acid D, ganoderic acid C6, and ganoderic acid G, through LC-MS-based dereplication [
33]. Two additional compounds, ganoderic acid AM1 and ganoderenic acid K, were tentatively identified based on retention time and MS fragmentation. These findings suggest that local
Ganoderma strains may possess distinct metabolic signatures, offering opportunities for region-specific product development.
Indeed, metabolomic profiling has become integral to the chemical characterization and quality assurance of Ganoderma products. Techniques such as LC-MS and NMR not only facilitate the quantification of known compounds but also support the discovery of novel metabolites. When integrated with taxonomic and ecological data, metabolomics enhances product standardization, species authentication, and pharmacological validation. Future efforts should prioritize untargeted analyses and expanded geographic sampling to fully explore the therapeutic potential of this medicinal genus.
6. Integrative Discussion and Regional Perspectives
The integration of molecular systematics, phylogenetics, and metabolomics has significantly advanced the study of
Ganoderma, enabling more accurate species delimitation, discovery of cryptic taxa, and detailed profiling of pharmacologically relevant metabolites. While morphology-based taxonomy laid the groundwork, it cannot meet the demands of taxonomic resolution, pharmaceutical validation, or product authentication. Molecular tools, especially ITS barcoding [
8] and robust multilocus analysis using EF1-α and RPB2 alongside ITS and nLSU [
13] provide essential accuracy for species-level identification.
ITS has been widely adopted as the primary fungal barcode due to its high interspecific variability, amplification success, and broad representation in public databases [
8]. However, ITS alone often fails to resolve closely related
Ganoderma species; supplementary markers such as EF1-α, RPB2, and nLSU significantly improve phylogenetic resolution and reduce misidentification [
13]. Multilocus approaches have revealed cryptic species and enabled taxonomic revision, with implications for conservation, biotechnology, and pharmacognosy.
Phylogenetic analyses have revealed geographically structured clades within
Ganoderma, underscoring the importance of regional molecular studies for mapping global diversity [
13]. Yet, substantial taxonomic uncertainty remains in Africa, where many species, such as
G. resinaceum and
G. weberianum are still identified solely by morphological features. Expanding the application of molecular barcoding and multilocus phylogenetics in African contexts will enhance our understanding of
Ganoderma evolution and distribution while supporting the sustainable development of endemic fungal resources.
Concurrently, metabolomics has emerged as a powerful tool for characterizing
Ganoderma bioactive molecules. Triterpenes, polysaccharides, nucleosides, and other secondary metabolites, linked to immunomodulatory, antiinflammatory, and anticancer effects, have been comprehensively profiled using LC-MS, LC-MS/MS, and NMR in multiple species [
33,
37,
38]. This biochemical diversity reinforces the therapeutic and commercial potential of
Ganoderma, especially strains from underexplored regions.
Metabolite profiles vary with species, developmental stage, and cultivation conditions. For example, triterpenoid accumulation peaks during the primordia or budding stage and declines in mature fruiting bodies [
34]. Regional metabolomics research, such as that conducted in Ghana, revealed distinct interspecific profiles among local strains, suggesting geography-dependent pharmacological variation [
33].
Despite these advances, several critical challenges remain. First, the limited availability of complete genome sequences and curated metabolomic datasets for many Ganoderma species hampers the development of integrative identification frameworks. Second, the synergistic or antagonistic interactions among multiple bioactive compounds in crude extracts are not well understood. Although individual metabolites have demonstrated therapeutic potential, their combined effects on efficacy and toxicity require systematic study using integrated “omics” platforms and functional assays.
Third, variation in cultivation, extraction, and analytical protocols continues to impede product standardization. Environmental factors such as substrate composition, humidity, and light exposure can substantially influence metabolite production. Future work should prioritize the optimization and harmonization of these variables to ensure reproducibility and commercial quality control.
Remaining challenges include limited available genomes and curated metabolite datasets, uncharted synergy among bioactive compounds in crude extracts, and inconsistency in cultivation and analytical protocols affecting reproducibility and quality control. In particular, sub-Saharan African Ganoderma research is often descriptive and lacks molecular or chemical validation. Coordinated genomics and metabolomics efforts in Africa could uncover novel bioactive molecules and foster medicinal innovation. Long-term investment and international collaborative networks are essential to build research capacity.
In this review, we have endeavored to consolidate current advances in Ganoderma systematics, phylogenetics, and metabolomics, while identifying key priorities for future research. A unified, multidisciplinary framework that integrates molecular identification, phylogenetic analysis, and metabolite profiling is essential for unlocking the full therapeutic and commercial potential of this medicinally important genus, particularly in biogeographic regions that remain underexplored.
7. Conclusions and Future Directions
The integration of molecular identification, phylogenetic analysis, and metabolomic profiling has significantly advanced the study of Ganoderma, transitioning it from a genus primarily defined by morphology to one characterized by genomic and biochemical precision. Advances in DNA barcoding, particularly ITS and multilocus sequencing have enabled more accurate species delimitation and revealed substantial cryptic diversity. Concurrently, metabolomic analyses have provided detailed insights into the chemical complexity that underlies Ganoderma's pharmacological properties.
In this review, we have attempted to synthesize these interdisciplinary developments and proposes a unified framework for taxonomic classification, biochemical characterization, and quality control of Ganoderma species. It underscores the essential role of molecular tools in overcoming the limitations of traditional taxonomy and highlights the utility of metabolomics in supporting both pharmacological validation and commercial standardization.
Despite substantial progress, key challenges remain, particularly in underexplored regions such as sub-Saharan Africa, where species diversity is insufficiently documented and largely unsupported by molecular or metabolomic data. Future efforts should prioritize the comprehensive genomic and metabolomic profiling of regional Ganoderma strains, the implementation of standardized analytical workflows, and the investigation of compound interactions that contribute to bioactivity.
Advancing these priorities through integrated, multidisciplinary research will facilitate a more complete understanding of Ganoderma as a medicinal resource, supporting biodiversity conservation, scientific innovation, and the development of evidence-based therapeutic applications.
Author Contributions
Conceptualization, G.A. and V.C.L.; methodology, G.A., V.C.L., A.Q. and M.A.G.; validation, G.A., V.C.L., A.K.A., L.K.N.O. and W.S.K.G.; formal analysis, G.A., V.C.L. and P.O.; investigation, G.A., V.C.L., P.Y., M.A.G. and P.O.; resources, G.A., V.C.L., A.K.A., L.K.N.O., W.S.K.G. and J.C.H.; data curation, G.A., V.C.L., A.Q. and P.Y.; writing—original draft preparation, G.A., V.C.L., A.Q. and M.A.G.; writing—review and editing, G.A., V.C.L. and J.C.H.; visualization, G.A., V.C.L., P.O. and P.Y.; supervision, G.A. and V.C.L.; project administration, G.A. and V.C.L.; funding acquisition, V.C.L. and G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by V.C.L. and G. A.
Acknowledgments
The authors express their sincere gratitude to Accra Technical University and the University of Nevada, Reno School of Medicine, for institutional support. Special thanks are extended to colleagues at the Noguchi Memorial Institute for Medical Research and the Department of Biochemistry, Cell and Molecular Biology, University of Ghana, for their contributions to scholarly dialogue. The authors also acknowledge the constructive use of generative AI (ChatGPT, GPT-5, OpenAI) during the drafting of sections of this manuscript, as well as grammatical editing and confirming the accuracy of references. The authors have reviewed and edited the output and take full responsibility for the content.
Conflicts of Interest
John C. Holliday, is the director of research and chief scientific officer of Aloha Medicinals, which produces and markets various Ganoderma products. The authors declare no other conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| DNA – Deoxyribonucleic Acid |
| EF1-α – Translation Elongation Factor 1-Alpha |
| HPLC – High-Performance Liquid Chromatography |
| HRMS – High-Resolution Mass Spectrometry |
| ITS – Internal Transcribed Spacer |
| ITS1 – Internal Transcribed Spacer 1 subregion |
| ITS2 – Internal Transcribed Spacer 2 subregion |
| LC-MS – Liquid Chromatography–Mass Spectrometry |
| LC-MS/MS – Liquid Chromatography–Tandem Mass Spectrometry |
| NMR – Nuclear Magnetic Resonance |
| NMIMR – Noguchi Memorial Institute for Medical Research |
| nLSU – Nuclear Large Subunit ribosomal RNA gene |
| nSSU – Nuclear Small Subunit ribosomal RNA gene |
| PCR – Polymerase Chain Reaction |
| PLS-DA – Partial Least Squares–Discriminant Analysis |
| rDNA – Ribosomal Deoxyribonucleic Acid |
| rRNA – Ribosomal Ribonucleic Acid |
| RPB1 – RNA Polymerase II Subunit 1 |
| RPB2 – RNA Polymerase II Subunit 2 |
| TIC – Total Ion Chromatogram |
| UPLC – Ultra-Performance Liquid Chromatography |
| UPLC–Orbitrap–HRMS – Ultra-Performance Liquid Chromatography–Orbitrap High-Resolution Mass Spectrometry |
References
- Andrejc, D.C.; Knez, Z.; Marevci, M.K. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front Pharmacol 2022, 13. ARTN 934982. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.Q.; Zhang, J.; Li, Z.M.; Liu, H.G.; Wang, Y.Z. Traditional uses, chemical components and pharmacological activities of the genus P. Karst.: a review. Rsc Adv 2020, 10, 42084–42097. [Google Scholar] [CrossRef]
- Tsai, C.C.; Yang, F.L.; Huang, Z.Y.; Chen, C.S.; Yang, Y.L.; Hua, K.F.; Li, J.; Chen, S.T.; Wu, S.H. Oligosaccharide and peptidoglycan of Ganoderma lucidum activate the immune response in human mononuclear cells. J Agric Food Chem 2012, 60, 2830–2837. [Google Scholar] [CrossRef]
- El Sheikha, A.F. Nutritional Profile and Health Benefits of Ganoderma lucidum "Lingzhi, Reishi, or Mannentake" as Functional Foods: Current Scenario and Future Perspectives. Foods 2022, 11. [Google Scholar] [CrossRef]
- He, J.; Han, X.; Luo, Z.L.; Li, E.X.; Tang, S.M.; Luo, H.M.; Niu, K.Y.; Su, X.J.; Li, S.H. Species diversity of Ganoderma (Ganodermataceae, Polyporales) with three new species and a key to Ganoderma in Yunnan Province, China. Front Microbiol 2022, 13, 1035434. [Google Scholar] [CrossRef] [PubMed]
- Mardones, M.; Carranza-Velazquez, J.; Mata-Hidalgo, M.; Amador-Fernandez, X.; Urbina, H. Taxonomy and phylogeny of the genus Ganoderma (Polyporales, Basidiomycota) in Costa Rica. MycoKeys 2023, 100, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cheng, Y.; Wang, Y.; Zhang, G.; Gao, R.; Xiang, C.; Feng, J.; Lou, D.; Liu, Y. Investigation of the fungal community structures of imported wheat using high-throughput sequencing technology. PLoS One 2017, 12, e0171894. [Google Scholar] [CrossRef] [PubMed]
- He, J., Luo, Z.-L., Tang, S.-M., Li, Y.-J., Li, S.-H., & Su, H.-Y. (2021). Phylogenetic analyses and morphological characters reveal two new species of Ganoderma from Yunnan province, China. MycoKeys, 84, 141–162. [CrossRef]
- Yang, H.D.; Ding, Y.; Wen, T.C.; Hapuarachchi, K.K.; Wei, D.P. Ganodermaovisporum sp. nov. (Polyporales, Polyporaceae) from Southwest China. Biodivers Data J 2022, 10, e80034. [Google Scholar] [CrossRef]
- Liu, C., Chen, F., Fan, X., Liu, B., Chai, X., He, S., Huang, T., Wang, X., Liu, L., Liu, H., Zeng, D., Jiang, B., Zhang, X., & Liu, M. (2024). Combined NMR and MS-based metabonomics and real-time PCR analyses reveal dynamic metabolic changes of Ganoderma lucidum during fruiting body growing. Food research international (Ottawa, Ont.), 180, 114056. [CrossRef]
- Liu, Y., Ren, S., Sang, Q., Cheng, X., & Bi, Y. (2025). Potential Active Compounds of Ganoderma lucidum and Their Anticancer Effects: A Comprehensive Review. Food science & nutrition, 13(8), e70741. [CrossRef]
- Obodai, M.; Mensah, D.L.; Fernandes, A.; Kortei, N.K.; Dzomeku, M.; Teegarden, M.; Schwartz, S.J.; Barros, L.; Prempeh, J.; Takli, R.K.; et al. Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Jargalmaa, S.; Eimes, J.A.; Park, M.S.; Park, J.Y.; Oh, S.Y.; Lim, Y.W. Taxonomic evaluation of selected Ganoderma species and database sequence validation. PeerJ 2017, 5, e3596. [Google Scholar] [CrossRef]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J Nat Prod 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Adotey, G., Alolga, R. N., Quarcoo, A., Yerenkyi, P., Otu, P., Anang, A. K., Okine, L. K. N., Gbewonyo, W. S. K., Holliday, J. C., & Lombardi, V. C. (2023). Molecular Identification and Characterization of Five Ganoderma Species from the Lower Volta River Basin of Ghana Based on Nuclear Ribosomal DNA (nrDNA) Sequences. Journal of fungi (Basel, Switzerland), 10(1), 6. [CrossRef]
- Sun, Y.F.; Xing, J.H.; He, X.L.; Wu, D.M.; Song, C.G.; Liu, S.; Vlasak, J.; Gates, G.; Gibertoni, T.B.; Cui, B.K. Species diversity, systematic revision and molecular phylogeny of Ganodermataceae (Polyporales, Basidiomycota) with an emphasis on Chinese collections. Stud Mycol 2022, 101, 287–415. [Google Scholar] [CrossRef]
- Gunnels, T., Creswell, M., McFerrin, J., & Whittall, J. B. (2020). The ITS region provides a reliable DNA barcode for identifying reishi/lingzhi (Ganoderma) from herbal supplements. PloS one, 15(11), e0236774. [CrossRef]
- Gazis, R., Rehner, S., & Chaverri, P. (2011). Species delimitation in fungal endophyte diversity studies and its implications in ecological and biogeographic inferences. Molecular ecology, 20(14), 3001–3013. [CrossRef]
- Han, J.; Zhu, Y.; Chen, X.; Liao, B.; Yao, H.; Song, J.; Chen, S.; Meng, F. The short ITS2 sequence serves as an efficient taxonomic sequence tag in comparison with the full-length ITS. Biomed Res Int 2013, 2013, 741476. [Google Scholar] [CrossRef]
- Wang, X.C.; Liu, C.; Huang, L.; Bengtsson-Palme, J.; Chen, H.; Zhang, J.H.; Cai, D.; Li, J.Q. ITS1: a DNA barcode better than ITS2 in eukaryotes? Mol Ecol Resour 2015, 15, 573–586. [Google Scholar] [CrossRef]
- Yang, R.H.; Su, J.H.; Shang, J.J.; Wu, Y.Y.; Li, Y.; Bao, D.P.; Yao, Y.J. Evaluation of the ribosomal DNA internal transcribed spacer (ITS), specifically ITS1 and ITS2, for the analysis of fungal diversity by deep sequencing. PLoS One 2018, 13, e0206428. [Google Scholar] [CrossRef]
- Moncalvo, J.M.; Wang, H.H.; Hseu, R.S. Phylogenetic-Relationships in Ganoderma Inferred from the Internal Transcribed Spacers and 25s Ribosomal DNA-Sequences. Mycologia 1995, 87, 223–238. [Google Scholar] [CrossRef]
- Zhou, L.W.; Cao, Y.; Wu, S.H.; Vlasak, J.; Li, D.W.; Li, M.J.; Dai, Y.C. Global diversity of the Ganoderma lucidum complex (Ganodermataceae, Polyporales) inferred from morphology and multilocus phylogeny. Phytochemistry 2015, 114, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Z.; Pei, H.; Chen, Z.; Tan, X.; Hu, J.; Yang, B.; Sun, J. Intraspecific Variation and Phylogenetic Relationships Are Revealed by ITS1 Secondary Structure Analysis and Single-Nucleotide Polymorphism in Ganoderma lucidum. PLoS One 2017, 12, e0169042. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.H.; Song, J.; DeCock, C.; Cui, B.K. Morphological characters and phylogenetic analysis reveal a new. Phytotaxa 2016, 266. [Google Scholar] [CrossRef]
- He, J.; Luo, Z.L.; Tang, S.M.; Li, Y.J.; Li, S.H.; Su, H.Y. Phylogenetic analyses and morphological characters reveal two new species of Ganoderma from Yunnan province, China. MycoKeys 2021, 84, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Gunnels, T.; Creswell, M.; McFerrin, J.; Whittall, J.B. The ITS region provides a reliable DNA barcode for identifying reishi/lingzhi (Ganoderma) from herbal supplements. PLoS One 2020, 15, e0236774. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Chen, X.; Han, J.; Dan, Y.; Wang, L.; Jiao, W.; Song, J.; Chen, S. Identification of commercial Ganoderma (Lingzhi) species by ITS2 sequences. Chin Med 2015, 10, 22. [Google Scholar] [CrossRef]
- Kinge, T.R.; Mih, A.M.; Coetzee, M.P.A. Phylogenetic relationships among species of <i>Ganoderma</i> (Ganodermataceae, Basidiomycota) from Cameroon. Australian Journal of Botany 2012, 60, 526–538. [Google Scholar] [CrossRef]
- El-Fallal, A.A.; El-Sayed, A.K.A.; El-Esseily, S.R. First record of two species from North East Nile Delta-Egypt. Mycosphere 2015, 6, 248–259. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Wang, X.C.; Wang, K.; Yao, Y.J. Re-Examination of the Holotype of Ganoderma sichuanense (Ganodermataceae, Polyporales) and a Clarification of the Identity of Chinese Cultivated Lingzhi. J Fungi (Basel) 2023, 9. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, L.; Sun, H.H. Development of a rapid and confirmatory method to identify ganoderic acids in Ganoderma mushrooms. Front Pharmacol 2012, 3, 85. [Google Scholar] [CrossRef]
- Adotey, G.; Alolga, R.N.; Quarcoo, A.; Gedel, M.A.; Anang, A.K.; Holliday, J.C. Ultra Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry (UPLC-Q-TOF-MS)-based metabolomic analysis of mycelial biomass of three Ganoderma isolates from the Lower Volta River Basin of Ghana. J Pharm Biomed Anal 2021, 205, 114355. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Huo, H.; Bao, H.; Wang, J.; Gao, D. Changes of Active Substances in Ganoderma lucidum during Different Growth Periods and Analysis of Their Molecular Mechanism. Molecules 2024, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.H.; Chen, W.K.D. Determination of ganoderic acids in triterpenoid constituents of. J Food Drug Anal 2003, 11, 195–200. [Google Scholar]
- Zhang, J.; Li, S.Y.; Luo, W.H.; Wang, J.; Beng, S.J.; Han, H.L.; Xiong, Q.P.; Peng, D.Y.; Peng, C. [Chemical composition analysis of Ganoderma lucidum based on UPLC-Orbitrap-HRMS and virtual screening of FXR activators for treatment of liver fibrosis]. Zhongguo Zhong Yao Za Zhi 2024, 49, 3804–3817. [Google Scholar] [CrossRef]
- Wongkhieo, S.; Tangmesupphaisan, W.; Siriwaseree, J.; Aramsirirujiwet, Y.; Wiriyajitsomboon, P.; Kaewgrajang, T.; Pumloifa, S.; Paemanee, A.; Kuaprasert, B.; Choowongkomon, K.; et al. In vitro cholesterol lowering activity of Ganoderma australe mycelia based on mass spectrometry, synchrotron Fourier-transform infrared analysis and liver-spheroid bioactivity. Sci Rep 2023, 13, 13619. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, C.; Wu, F.; Zhao, S.; Chen, T.; You, H.; Lin, Y.; Zou, X. Integrated Transcriptomic and Targeted Metabolomic Analysis Reveals the Key Genes Involved in Triterpenoid Biosynthesis of Ganoderma lucidum. J Fungi (Basel) 2025, 11. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).